Abstract
Introduction
Patients diagnosed with cancer have an increased risk both for myelodysplastic syndromes (MDS) and for acute myeloid leukemia (AML) following treatment.
Methods
Using SEER-Medicare data, we selected patients aged 66 years and older who completed systemic therapy between 2002 and 2014 for breast (stage I–III), lung (stage I–III), or prostate (stage I–IV) cancer. For each cancer, we estimated the risk of a composite endpoint of MDS or AML in patients receiving granulocyte colony-stimulating factor (G-CSF) vs. not.
Results
The 10-year cumulative risk difference (granulocyte colony-stimulating factor [G-CSF] − no G-CSF) for MDS-AML was 0.45% (95% CI 0.13–0.77%) in breast cancer and 0.39% (95% CI 0.15–0.62%) in lung cancer. G-CSF use was associated with a hazard ratio of 1.60 (95% CI 1.07–2.40) in breast cancer and 1.50 (95% CI 0.99–2.29) in lung cancer. Filgrastim use was associated with a hazard ratio of 1.01 (95% CI 1.00–1.03) per administration in breast cancer and 1.02 (95% CI 0.99–1.05) per administration in lung cancer. Pegfilgrastim was associated with a hazard ratio of 1.08 (95% CI 1.01–1.15) per administration in breast cancer and 1.12 (95% CI 1.00–1.25) per administration in lung cancer. Analyses in prostate cancer were limited because of the low number of events.
Conclusions
The use of G-CSF in patients diagnosed with breast and lung cancer is associated with an increased risk of MDS-AML. However, the MDS-AML absolute risk difference is very low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study |
Patients with cancer have an increased risk for myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) following systemic therapy; it is biologically plausible for granulocyte colony-stimulating factor (G-CSF) to increase the risk of these myeloid disorders |
The purpose of this study was to compare the risk of MDS or AML between those receiving G-CSF versus not receiving G-CSF, in patients treated with systemic therapy for breast, lung, or prostate cancer |
What was learned from this study |
The risk of MDS or AML was low regardless of whether G-CSF was used with a 10-year cumulative probability of 1.24% in breast cancer, 0.84% in lung cancer, and 0.67% in prostate cancer |
The relative risk of MDS or AML was 1.5 to 1.6-fold higher for patients receiving G-CSF in breast and lung cancer, but was not elevated in prostate cancer |
The 10-year absolute risk difference (G-CSF–no G-CSF) was less than 0.5% in the breast and lung cancer cohorts |
Introduction
Patients diagnosed with cancer have an increased risk both for myelodysplastic syndromes (MDS) and for acute myeloid leukemia (AML) following treatment [1,2,3,4,5,6,7]. The MDS and AML that develops after cancer treatment accounts for approximately 10–20% of cases of MDS-AML [7]. These patients typically have poorer survival than patients with de novo MDS-AML [6]. Both radiation therapy and systemic therapy are established risk factors for secondary MDS and AML [5, 8,9,10]. In addition to radiation and systemic therapy, patients receiving myelosuppressive systemic therapy have a risk of febrile neutropenia that increases with dose intensity, and commonly receive granulocyte colony-stimulating factor (G-CSF, including filgrastim and pegfilgrastim) for febrile neutropenia treatment and prophylaxis [11].
Because G-CSF induces proliferation of myeloid progenitor cells, which are especially sensitive to myelosuppressive systemic therapy drugs, it is biologically plausible for G-CSF to increase the risk of myeloid disorders such as secondary MDS and AML [12, 13]. In particular, clonal hematopoiesis appears to have a role in the development of therapy-induced MDS and AML following cytotoxic therapy [14, 15]. In a recent systematic review and meta-analysis of 25 clinical trials that randomly assigned patients to receive G-CSF (n = 6058) or no-G-CSF (n = 6746) and had at least 2 years of follow-up, the authors observed that both risk of MDS-AML and intensity of systemic therapy were increased in patients receiving G-CSF support [1]. However, the authors were unable to differentiate between the causal effect of G-CSF and the causal effect of dose-intensified systemic therapy since patients with G-CSF support are more likely to receive intense systemic therapy.
In two previous SEER-Medicare observational studies of patients receiving systemic therapy for breast cancer, one reported no association of G-CSF with risk of AML (hazard ratio 1.14; 95% CI 0.67, 1.92) [10] and another reported an increased risk for MDS-AML following G-CSF use in women receiving chemotherapy (hazard ratio 2.14; 95% CI 1.12, 4.08) [16]. A recent study by Calip et al. [2] in patients with breast cancer found that, compared to women who did not receive chemotherapy, MDS-AML risk was associated with anthracycline/cyclophosphamide-containing regimens (hazard ratio 1.86, 95% CI 1.33–2.61) and filgrastim (hazard ratio 1.47, 95% CI 1.05–2.06), but not pegfilgrastim (hazard ratio 1.10, 95% CI 0.73–1.66). The differences between filgrastim and pegfilgrastim risk could be related to differences in pharmacokinetics, differences in the administration frequency of filgrastim (approximately 10 administrations per cycle) compared to pegfilgrastim (one administration per cycle) [17,18,19], or to the statistical methods used in the analyses.
To investigate the effect of G-CSF on the risk of MDS-AML further, the primary objective of this study was to compare the risk of MDS or AML between those receiving G-CSF versus not receiving G-CSF, in patients aged 66 years and older treated with systemic therapy for breast (stage I–III), lung (stage I–III), or prostate (stage I–IV) cancer. These populations were selected because of the large sample sizes needed to identify rare outcomes [20] and because G-CSF is relatively commonly used in these cancers [20,21,22].
Methods
Study Design
This retrospective, observational cohort study used data from the National Cancer Institute (NCI) SEER cancer registry linked with Medicare enrollment and claims data from 1 January 2000 through 31 December 2015 [23]. It was conducted among patients diagnosed with first primary breast, lung, or prostate cancer who completed first-line outpatient systemic therapy. The study protocol was reviewed by Advarra and received an exemption determination on 24 May 2019. The authors received permission to access and use the USRDS data from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Study Data Source
This study is based on cancer incidence data from the SEER-18 registry which covers approximately 28% of the US population [24]. The SEER program collects data on incident cancer for persons diagnosed with a new primary cancer who reside in one of the SEER geographic areas. The SEER data include patient demographic information and tumor characteristics (e.g., stage, grade, and histology). Medicare is the federally funded program in the USA that provides health insurance for 97% of individuals age 65 years and over, as well as coverage for people with end-stage renal disease or a qualifying disability independent of age.
In the SEER-Medicare data, cancer registry data are linked to Medicare enrollment and claims records. In this study, de-identified, linked Medicare claims for calendar years 2000 to 2015 were used to identify comorbid diagnoses and treatments, including medications, surgery, and radiation. Claims records for this study were derived from the hospital facility, outpatient facility, hospice facility, physician, and durable medical equipment files; mortality information was derived from Medicare enrollment files. Home health files, and Medicare Part D files (containing oral medications with no intravenous equivalent) were not used in this study.
Study Observation Period
The completion of first-line outpatient systemic therapy was defined as an interval of 60 days with no outpatient chemotherapy after the first chemotherapy dose. Because patients had to survive for 60 days to identify the end of first-line systemic therapy, the study index date that defined the start of the risk period was the first day after the 60-day gap to avoid immortal time bias (Supplementary Fig. 1) [25]. The baseline period comprised of all time prior to and including the index date. The follow-up period began the day after the index date and continued to the end of observation. Observation ended at the earliest of the following events: death, MDS-AML diagnosis, switch to managed care coverage, or the end of the SEER follow-up period (31 December 2015). The study observation period allowed for the possibility of at least 1 year of follow-up for all patients.
Inclusion Criteria
Only patients whose index date was between 1 January 2002 and 31 December 2014 were included. Patients were required to have a stage I–III breast cancer diagnosis within 18 months prior to their index date, stage I–III lung cancer diagnosis within 1 year prior to their index date, or stage I–IV prostate cancer at any time prior to their index date. The breast, lung, and prostate cancers were required to be first primary cancers for each person and were required to be microscopically confirmed. Patients were included if they were age 66 years or older at the index date. Patients must have had at least 12 continuous months of Medicare Part A and B with no HMO coverage prior to their index date.
Exclusion Criteria
Patients diagnosed with stage 0 or unknown stage, as well as those diagnosed at autopsy, were excluded. Male patients with breast cancer were also excluded. Patients diagnosed with a subsequent primary malignancy or end-stage renal disease prior to the index date were excluded. And finally, patients with a history of sargramostim use were excluded because it is different from G-CSF but could potentially affect MDS-AML incidence [26].
Study Variables
All G-CSF, systemic therapy, and radiation therapy use was assessed during the baseline period and throughout the follow-up period. Patients were considered to be exposed to G-CSF if there was at least one claim with a Healthcare Common Procedure Coding System (HCPCS) code for filgrastim or pegfilgrastim in the baseline period. Each calendar date with at least one G-CSF administration was considered to be an administration.
Systemic therapy was defined using HCPCS codes for infused agents, as well as National Drug Codes (NDC) for oral agents with intravenous equivalents. Both chemotherapy and immunotherapy were defined as systemic on the basis of the SEER*Rx category in the NCI Oncology Toolbox [27]. Hormonal therapies were not included. We used all Medicare claims in the baseline period to identify the initial use of systemic therapy. The date of the first claim indicated the beginning of therapy. Categories of myelosuppressive systemic therapy included alkylating drugs, anthracycline drugs, enzyme inhibitor drugs, antimetabolite drugs, antineoplastic antibiotic drugs, mitotic inhibitor drugs, platinum drugs, monoclonal antibody drugs, taxane drugs, and topoisomerase inhibitor drugs. To be consistent with the definition of G-CSF exposure, a single calendar date with at least one administration was considered to be an exposure.
Radiation use was based on HCPCS and Current Procedural Terminology (CPT) codes, revenue codes, and ICD-9-CM procedure and diagnosis codes used in published literature, as well as a manual review of HCPCS and CPT codes [28, 29]. The date of the first claim for radiation was used to indicate the beginning of radiation therapy.
Cancer-directed surgery was determined in the baseline period using HCPCS and International Classification of Diseases, Clinical Modification, 9th Edition (ICD-9) procedure codes identified from published studies of breast, prostate, and lung cancer using SEER-Medicare linked data [28, 30,31,32].
For patients with prostate and lung cancer, stage was defined using AJCC 6th Edition or AJCC 3rd Edition depending on the year of diagnosis. For breast cancer, we used the version of AJCC 6th Edition staging provided by SEER that harmonizes the 3rd and 6th editions. The National Cancer Institute (NCI) comorbidity index was defined using guidelines provided by the NCI [33]. The index includes 16 comorbid conditions, each of which is given a different weight, with higher weight corresponding to higher risk of death.
MDS-AML
MDS and AML were identifiable in two ways for this study. The first was based on subsequent primary malignancy data (MDS or AML) reported by the SEER program. The second was as diagnosis codes reported in Medicare claims data. Published validated algorithms were used to identify MDS and AML diagnoses in Medicare claims data [34, 35]. The first occurrence of SEER-reported or Medicare claims reported MDS or AML defined an MDS-AML event.
Statistical Methods
Population characteristics were described by G-CSF use for each cancer cohort. Categorical variables were summarized using counts and proportions. Continuous variables were summarized using means and standard deviations. The 10-year cumulative incidence (probability) of MDS-AML and 95% confidence interval were reported for each cancer group accounting for the competing risk of death.
Factors associated with MDS or AML were evaluated using Cox proportional hazard models. We selected covariates for the model using a directed acyclic graph (DAG) [36] to ensure that important covariates were included (Supplementary Fig. 2). According to the DAG, both radiation and systemic therapy were essential in the model in order to estimate the effect of G-CSF on MDS-AML. The most common categories of systemic therapy were included, along with radiation therapy (yes/no). We also included age because of its potential as a confounder and its association with the outcome, and we included calendar year of diagnosis (per year as a continuous variable) to capture possible systematic temporal effects.
In Cox proportional hazards models of MDS-AML risk, G-CSF and systemic therapy were treated as binary exposures (i.e., exposed or unexposed). In sensitivity analyses, filgrastim and pegfilgrastim were included separately on the basis of cumulative administrations (i.e., the number of unique days with an administration) during the baseline period. This facilitated the analyses of patients who received both agents. Systemic therapy was encoded similarly by using administrations. These models censored follow-up at new occurrences of radiation, G-CSF use, or myelosuppressive systemic therapy use. We conducted sensitivity analyses that censored follow-up after 3 years of observation. We conducted different sensitivity analyses that used time-dependent covariates for radiation, G-CSF use, and myelosuppressive chemotherapy use, allowing patients to change exposure groups over time and avoiding censoring during the follow-up period. We also estimated variance inflation factors for our primary models to evaluate collinearity between our primary G-CSF exposure and other exposures. As a result of the relatively small number of events, we did not evaluate interactions among covariates.
Selected results are presented for the prostate cancer cohort because there were so few events; additional results for this cohort are included in Supplementary Materials.
Jigsaw software (Outcomes Insights, Inc., Agoura Hills, CA) was used to create and implement algorithms for extracting data and creating analysis data sets. The final analysis data sets and all analyses were created in R (version 3.4.4) [37].
Results
See Supplementary Tables 1–3 for attrition tables for the creation of each cohort.
Treatment Patterns
In the overall breast cancer cohort of 23,340 patients at baseline (Table 1), 96.2% received at least one myelosuppressive systemic agent, 96.6% received surgery, and 57.7% received radiation. The most common myelosuppressive agents were alkylating agent (79.9% of the cohort), taxane (64.5%), and anthracycline (43.8%). There were 15,626 (66.9%) who received G-CSF during the baseline period; of these, 1898 (12.1%) received both filgrastim and pegfilgrastim. The mean number of administrations during the baseline period was 12.9 for those who received filgrastim and 4.4 for those who received pegfilgrastim. In the G-CSF group, 15.7% received radiation during the follow-up period. In the no G-CSF group, 5.8% received G-CSF during follow-up and 15.2% received radiation during follow-up.
In the lung cancer cohort of 24,495 patients at baseline (Table 2), 97.4% received at least one myelosuppressive systemic agent, 27.2% received surgery, and 66.8% received radiation. The most common myelosuppressive agents were platinum (93.4% of the cohort), taxane (57.6%), and topoisomerase (25.8%). There were 10,503 (42.9%) who received G-CSF during the baseline period; of these, 1255 (11.9%) received both filgrastim and pegfilgrastim. The mean number of administrations was 8.6 for those who received filgrastim and 3.0 for those who received pegfilgrastim. In the G-CSF group, 12.1% received radiation during the follow-up period. In the no G-CSF group, 9.3% received G-CSF during follow-up and 9.2% received radiation during follow-up.
In the prostate cancer cohort of 7534 patients at baseline (Supplementary Table 4), 47.8% received at least one myelosuppressive systemic agent, 14.6% received surgery, and 61.5% received radiation. Of note, in those with prior radiation exposure, the mean time from radiation initiation to the index date was 43 months. There were 1326 (17.6%) who received G-CSF during the baseline period; of these, 157 (11.8%) received both filgrastim and pegfilgrastim. The mean number of administrations during the baseline period was 9.7 for those who received filgrastim and 5.3 for those who received pegfilgrastim. In the G-CSF group, 9.4% received radiation during the follow-up period. In the no G-CSF group, 7.9% received G-CSF during follow-up and 7.9% received radiation during follow-up.
Outcomes
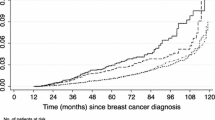
In the breast cancer cohort, mean follow-up was 5.7 years from the index date for G-CSF patients and 6.6 years for no G-CSF patients. The proportion of patients who died during follow-up was 24.1% for G-CSF and 35.6% for no G-CSF. Unadjusted mortality rates per 1000 person-years were 42.5 (95% CI 41.2–43.9) in the G-CSF group and 53.7 (95% CI 51.6–55.7) in the no G-CSF group. There were 193 total MDS-AML events, 140 in the G-CSF group and 53 in the no G-CSF group. The unadjusted incidence rate of MDS-AML per 1000 person-years of follow-up was 1.6 (95% CI 1.3–1.8) in the G-CSF group and 1.0 (95% CI 0.8–1.3) in the no G-CSF group. The 10-year cumulative probability of MDS-AML was 1.24% (95% CI 1.04–1.49) in the G-CSF group and 0.79% (95% CI 0.59–1.06) in the no G-CSF group, accounting for the competing risk of death (Fig. 1a). At 10 years, the difference (G-CSF − no G-CSF) in the cumulative probability was 0.45% (95% CI 0.13–0.77%; Fig. 1b).
In the primary breast cancer analysis, G-CSF use (i.e., filgrastim or pegfilgrastim) was associated with a hazard ratio of 1.60 (95% CI 1.07–2.40) versus no G-CSF (Table 3) for MDS-AML. Fitting the model with separate effects for filgrastim and pegfilgrastim, filgrastim use was associated with a hazard ratio of 1.01 (95% CI 1.00–1.03) per administration and pegfilgrastim was associated with a hazard ratio of 1.08 (95% CI 1.01–1.15) per administration (Table 4). Restricting these analyses to 3 years of follow-up (Supplementary Table 5) generally showed stronger associations; in particular, the filgrastim hazard ratio was 1.03 (95% CI 1.01–1.05) and the pegfilgrastim hazard ratio was 1.15 (95% CI 1.05–1.26; Supplementary Tables 6–7). Supplementary Table 8 shows the results using time-dependent covariates.
In the lung cancer cohort, mean follow-up was 2.6 years from the index date for G-CSF patients and 2.6 years for no G-CSF patients. The proportion of patients who died during follow-up was 75.3% for G-CSF and 76.8% for no G-CSF. Unadjusted mortality rates per 1000 person-years were 289 (95% CI 283–295) in the G-CSF group and 301 (295–307) in the no G-CSF group. There were 128 total MDS-AML events, 75 in the G-CSF group and 53 in the no G-CSF group. The unadjusted incidence rate of MDS-AML per 1000 person-years of follow-up was 2.7 (95% CI 2.1–3.4) in the G-CSF group and 1.5 (95% CI 1.1–1.9) in the no G-CSF group. The 10-year cumulative probability of MDS-AML was 0.84% (95% CI 0.66–1.05) in the G-CSF group and 0.45 (95% CI 0.34–0.60) in the no G-CSF group, accounting for the competing risk of death (Fig. 1c). At 10 years, the difference (G-CSF − no G-CSF) in the cumulative probability was 0.39% (95% CI 0.15–0.62%; Fig. 1d).
In the primary analysis, G-CSF use (i.e., filgrastim or pegfilgrastim) was associated with a hazard ratio of 1.50 (95% CI 0.99–2.29) versus no G-CSF (Table 3) for MDS-AML. Fitting the model with separate effects for filgrastim and pegfilgrastim (Table 4), filgrastim use was associated with a hazard ratio of 1.02 (95% CI 0.99–1.05) per administration and pegfilgrastim was associated with a hazard ratio of 1.12 (95% CI 1.00–1.25) per administration. Restricting these analyses to 3 years of follow-up (Supplementary Table 9) generally showed similar associations. In particular, the filgrastim hazard ratio was 1.02 (95% CI 0.99–1.05) and the pegfilgrastim hazard ratio was 1.12 (95% CI 1.00–1.25; Supplementary Tables 10–11). Supplementary Table 12 shows the results using time-dependent covariates.
In the prostate cancer cohort, mean follow-up was 1.8 years from the index date for G-CSF patients and 3.3 years for no G-CSF patients. The proportion of patients who died during follow-up was 83.0% for G-CSF and 46.8% for no G-CSF. Unadjusted mortality rates per 1000 person-years were 470.2 (95% CI 442.4–497.9) in the G-CSF group and 142.7 (95% CI 137.6–147.9) in the no G-CSF group. There were 50 total MDS-AML events in the total cohort. The unadjusted incidence rate of MDS-AML per 1000 person-years of follow-up was 1.7 (95% CI 0.0–3.4) in the G-CSF group and 2.3 (95% CI 1.6–2.9) in the no G-CSF group. The 10-year cumulative probability of MDS-AML was 0.67% (95% CI 0.19–2.43) in the G-CSF group and 1.21% (95% CI 0.87–1.69) in the no G-CSF group, accounting for the competing risk of death. See Supplementary Tables 13–14 and Supplementary Figs. 3–4 for prostate cancer analyses similar to those conducted in the breast and lung cancer cohorts.
Finally, we assessed variance inflation factors to assess multicollinearity in the primary analyses for the breast and lung cancer cohorts. None exceeded 2.3, with most less than 2.0 (data not shown).
Discussion
This study summarizes the effect of G-CSF on the risk of MDS-AML in cohorts of patients with breast, lung, and prostate cancer aged 66 and older who completed first-line systemic therapy between 2002 and 2014. In general, MDS-AML is rare. In the breast, lung, and prostate cohorts the unadjusted 10-year cumulative incidence of MDS-AML in patients receiving G-CSF as part of their initial systemic therapy regimen is 1.2%, 0.8%, and 0.7% compared to 0.8%, 0.5%, and 1.2% in patients not receiving G-CSF. The differences between the G-CSF and no G-CSF groups at 10 years were 0.5%, 0.4%, and − 0.5%, respectively, for these cancers.
Comparing to other studies, the relative hazard of MDS-AML for G-CSF was between 1.50 (lung) to 1.60 (breast), which is consistent with, but less than, the hazard ratio of 1.92 from the meta-analysis by Lyman et al. [1] which compared the G-CSF-treated arm with the control-treated arm across 25 randomized controlled studies. In a study of breast cancer based on SEER-Medicare data by Calip et al. the association between G-CSF and MDS-AML risk in patients receiving anthracycline–cyclophosphamide regimens was 1.78 and was between 0.98 and 1.37 in other regimen groups [2].
Radiation therapy was consistently a risk factor in breast and lung cancer across all analyses. In prostate cancer, there was no apparent increased risk associated with G-CSF, but the numbers of patients and MDS-AML events were both very small. The small number of events among patients with prostate cancer may be because most of the MDS-AML events in these patients occurred between diagnosis and the completion of systemic therapy and these patients were therefore excluded from our study. This suggests that radiation therapy, which is commonly used earlier in the course of prostate cancer, was potentially the causative agent and resulted in the removal of many susceptible patients from the risk pool.
In analyses of both breast and lung cancer, both filgrastim and pegfilgrastim were associated with MDS-AML in a dose-dependent way when using cumulative administrations as the exposure. The risk associated with pegfilgrastim, per administration, was about 5.5 times higher than the risk associated with filgrastim per administration, and varied depending on cancer and the specific analysis. This implies that the risk associated with a single pegfilgrastim administration is about half of the risk of 11 daily doses of filgrastim. Since pegfilgrastim is administered once per cycle, and filgrastim is dosed daily for up to 2 weeks or until the absolute neutrophil count has reached 10,000/mm3, it is not surprising that the risks are different on a per administration basis. However, it should be noted that in routine clinical practice, the mean number of administrations of filgrastim per systemic therapy cycle is less than 5 [38, 39].
In contrast to our findings, Calip et al. showed a higher risk associated with any filgrastim use (hazard ratio 2.11) compared to pegfilgrastim use (hazard ratio 1.21). This may be related to the way their model was parameterized. The authors included dichotomous variables for both filgrastim and pegfilgrastim but did not include an interaction term [2]. This can create a statistical issue for estimating risk in patients who used both agents, something that occurred in 12% our breast and lung cancer G-CSF cohorts. Without an interaction term, the risk in patients who received both agents would be estimated by combining the risk estimates for both products. However, in the absence of a synergistic effect, such a model biases the risk estimates for each individual agent downward to balance overestimation of risk in those who received both formulations. The strength of this bias is a function of the number of people who received both agents and type of overlapping use. Our study adds a more precise assessment of filgrastim and pegfilgrastim exposure based on dose per day, which allows for a fully independent exposure assessment for each formulation.
We conducted several sensitivity analyses to explore the effects of some of our analysis decisions. In breast cancer, limiting follow-up to 3 years increased the relative risks seen in the primary analyses. This suggests that, in breast cancer, the highest risk periods are in the 2–3 years after exposure. This was not observed with lung cancer. This difference may be related to the shorter follow-up in the lung cancer cohort, which is a consequence of the higher lung cancer mortality rate. In our sensitivity analysis using time-dependent covariates for G-CSF, radiation, and myelosuppressive chemotherapy, the risk estimates per administration were similar for pegfilgrastim, and the risk estimates per administration were lower for filgrastim. However, because these models did not address time-dependent confounding factors, their interpretation may be limited.
As with any analyses of observational data, there are limitations to the analyses. In particular, estimating the cumulative dose of systemic therapy or radiotherapy is very difficult with claims data. This is an important potential confounding factor because the use of G-CSF is likely to be associated with higher doses of systemic therapy and radiotherapy, making it difficult disentangle their separate contributions to risk. The difficulty with systemic therapy arises because infused medications are reimbursed on the basis of quantities that may not correspond to the actual dose used (e.g., wastage from partial use of vials is not consistently reported). We used cumulative administrations as a proxy for cumulative dose, but this may not be accurate because of our inability to assess dose reductions or delays, and we do not have measures like height, weight, and body surface area that would allow us to calculate dosing more accurately. However, in breast cancer, the higher MDS-AML risk for patients with node positive disease (HR 1.62, 95% CI 1.12–2.34) may be a marker for higher dose intensity since this subgroup of patients is likely to receive more aggressive treatment (i.e., higher dose or dose dense chemotherapy).
The effects of other exposures deserve comment, noting that the causal interpretation of other covariates from a model may be confounded even if the primary exposure of interest is not [40]. We observed that taxane use was associated with a lower risk of MDS-AML in several analyses. Whether this is a causal effect or simply correlated with some other treatment pattern (e.g., less aggressive treatment) is beyond the scope of these analyses. Also, in lung cancer, the effect of calendar year may be a marker of different treatment patterns over time (e.g., more aggressive radiation or chemotherapy).
The identification of MDS-AML is challenging for several reasons. MDS was first reported in the SEER program starting in 2001 and this study may have missed MDS cases as reporting improved over time. Also, leukemias are generally reported more slowly than solid tumors, so it is possible that MDS and AML are underreported by SEER for the most recent years of follow-up. Because of this, we used claims-based algorithms with high sensitivity and specificity to improve our capture. The benefit of this approach is that more events can be captured, improving the power of our study to identify associations. However, as with any study using claims data, there is a risk that false positive cases may be included, potentially introducing bias.
The death rate was substantially higher than the MDS-AML rate in the breast, lung, and prostate cancer cohorts (i.e., greater than 27-fold for breast cancer and even higher for prostate and lung cancer) and was also higher among non-G-CSF patients than G-CSF patients. Because of this, the competing risk of death deserves special mention since death preempts MDS-AML events from occurring. The rationale in considering this as a potential bias is that it assumes that some MDS-AML in the G-CSF group could be caused indirectly by improved survival, which would allow more time for MDS-AML events to accumulate. While the meta-analysis of Lyman et al. showed a protective effect of G-CSF on mortality in breast cancer [1], the additional survival time would lead to a very small number of additional MDS-AML cases because the MDS-AML rate is so low, meaning that only a tiny portion of the patients who survive longer would develop MDS-AML.
In terms of the interpretation of the hazard ratios, we note that the hazard ratio for G-CSF represents the risk for the “average” exposure across filgrastim and pegfilgrastim over time, which can vary depending on whether the use is for treatment or prophylaxis of neutropenia. The number of cycles, dose, and dose density of systemic therapy very likely differ between the treatment and prophylaxis settings. We also included variables for the administrations of systemic therapy, and for breast cancer, we included a variable for node positivity to account for the generally higher doses and/or dose density. However, there is still likely to be residual confounding. If there were more MDS-AML events, we could have conducted stratified analyses or considered more sophisticated analyses (e.g., interaction terms); however, the small number of events made this difficult.
Finally, the G-CSF and no G-CSF groups may have varied over time as a result of changes in practice patterns, including systemic therapy doses, systemic therapy dose frequency, and choice of systemic agents. While we included year as a covariate to try to capture some of this effect, this approach is limited because the changes may not be smooth over time.
Conclusions
The use of G-CSF in patients diagnosed with breast and lung cancer is associated with an increased risk of MDS-AML. However, the incidence of MDS-AML is very low, regardless of whether patients received G-CSF or not, and the risk difference is less than 0.5% in the breast and lung cancer cohorts.
References
Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol. 2010;28(17):2914–24. https://doi.org/10.1200/JCO.2009.25.8723.
Calip GS, Malmgren JA, Lee WJ, Schwartz SM, Kaplan HG. Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer. Breast Cancer Res Treat. 2015;154(1):133–43. https://doi.org/10.1007/s10549-015-3590-1.
Castro GA, Church A, Pechet L, Snyder LM. Leukemia after chemotherapy of Hodgkin’s disease. N Engl J Med. 1973;289(2):103–4. https://doi.org/10.1056/nejm197307122890215.
Preisler HD, Lyman GH. Acute myelogenous leukemia subsequent to therapy for a different neoplasm: clinical features and response to therapy. Am J Hematol. 1977;3(3):209–18. https://doi.org/10.1002/ajh.2830030301.
Kaplan HG, Malmgren JA, Atwood MK. Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990–2005. BMC Cancer. 2011;11:260. https://doi.org/10.1186/1471-2407-11-260.
Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol. 2007;20(1):29–37. https://doi.org/10.1016/j.beha.2006.10.006.
Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35(4):418–29. https://doi.org/10.1053/j.seminoncol.2008.04.012.
Praga C, Bergh J, Bliss J, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol. 2005;23(18):4179–91. https://doi.org/10.1200/JCO.2005.05.029.
Smith RE, Bryant J, DeCillis A, Anderson S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the national surgical adjuvant breast and bowel project experience. J Clin Oncol. 2003;21(7):1195–204. https://doi.org/10.1200/JCO.2003.03.114.
Patt DA, Duan Z, Fang S, Hortobagyi GN, Giordano SH. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J Clin Oncol. 2007;25(25):3871–6. https://doi.org/10.1200/JCO.2007.12.0832.
Weycker D, Bensink M, Lonshteyn A, Doroff R, Chandler D. Risk of chemotherapy-induced febrile neutropenia by day of pegfilgrastim prophylaxis in US clinical practice from 2010 to 2015. Curr Med Res Opin. 2017;33(12):2107–13. https://doi.org/10.1080/03007995.2017.1386858.
Touw IP, Bontenbal M. Granulocyte colony-stimulating factor: key (f)actor or innocent bystander in the development of secondary myeloid malignancy? J Natl Cancer Inst. 2007;99(3):183–6. https://doi.org/10.1093/jnci/djk057.
Dong F, Brynes RK, Tidow N, Welte K, Löwenberg B, Touw IP. Mutations in the gene for the granulocyte colony-stimulating–factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N Engl J Med. 1995;333(8):487–93. https://doi.org/10.1056/NEJM199508243330804.
Voso MT, Falconi G, Fabiani E. What’s new in the pathogenesis and treatment of therapy-related myeloid neoplasms. Blood. 2021;138(9):749–57. https://doi.org/10.1182/blood.2021010764.
Pich O, Cortes-Bullich A, Muiños F, Pratcorona M, Gonzalez-Perez A, Lopez-Bigas N. The evolution of hematopoietic cells under cancer therapy. Nat Commun. 2021. https://doi.org/10.1038/s41467-021-24858-3.
Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99(3):196–205. https://doi.org/10.1093/jnci/djk028.
Food and Drug Administration. Filgrastim [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5183lbl.pdf. Accessed 13 June 2018.
Food and Drug Administration. Pegfilgrastim [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125031s180lbl.pdf. Accessed 13 June 2018.
Molineux G, Foote MA, Arvedson T. Twenty years of G-CSF: clinical and nonclinical discoveries. Basel: Springer; 2012.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. https://doi.org/10.3322/caac.21332.
Wang L, Barron R, Baser O, Langeberg WJ, Dale DC. Cancer chemotherapy treatment patterns and febrile neutropenia in the US Veterans Health Administration. Value Health. 2014;17(6):739–43. https://doi.org/10.1016/j.jval.2014.06.009.
Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87. https://doi.org/10.1016/j.ejca.2018.07.005.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med care. 2002;40(8 Suppl):IV-3–18. https://doi.org/10.1097/01.MLR.0000020942.47004.03.
Enewold L, Parsons H, Zhao L, et al. Updated overview of the SEER-medicare data: enhanced content and applications. J Natl Cancer Inst Monogr. 2020;2020(55):3–13. https://doi.org/10.1093/jncimonographs/lgz029.
Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167(4):492–9. https://doi.org/10.1093/aje/kwm324.
Sargramostim Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103362s5240lbl.pdf. Accessed 15 Feb 2021
National Cancer Institute. Observational Research in Oncology Toolbox. https://seer.cancer.gov/oncologytoolbox/. Accessed 17 Oct 2019.
Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with medicare claims. Med Care. 2016;54(9):e55-64. https://doi.org/10.1097/MLR.0000000000000073.
Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among medicare patients with cancer. J Oncol Pract. 2015;11(5):403–9. https://doi.org/10.1200/jop.2015.005694.
Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–9. https://doi.org/10.1001/jamaoncol.2015.4508.
Beebe-Dimmer JL, Ruterbusch JJ, Cooney KA, et al. Racial differences in patterns of treatment among men diagnosed with de novo advanced prostate cancer: a SEER-Medicare investigation. Cancer Med. 2019;8(6):3325–35. https://doi.org/10.1002/cam4.2092.
Nadpara PA, Madhavan SS, Tworek C. Disparities in lung cancer care and outcomes among elderly in a medically underserved state population—a cancer registry-linked database study. Popul Health Manag. 2016;19(2):109–19. https://doi.org/10.1089/pop.2015.0027.
Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–90. https://doi.org/10.1016/j.annepidem.2007.03.011.
Cogle CR, Craig BM, Rollison DE, List AF. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: high number of uncaptured cases by cancer registries. Blood. 2011;117(26):7121–5. https://doi.org/10.1182/blood-2011-02-337964.
Craig BM, Rollison DE, List AF, Cogle CR. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomark Prev. 2012;21(3):474–81. https://doi.org/10.1158/1055-9965.EPI-11-1087.
Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–56. https://doi.org/10.1093/aje/kwp107.
Comprehensive R Archive Network. Vienna: R Foundation for Statistical Computing.
Naeim A, Henk HJ, Becker L, et al. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013;13:11. https://doi.org/10.1186/1471-2407-13-11.
Gawade PL, Li S, Henry D, et al. Patterns of granulocyte colony-stimulating factor prophylaxis in patients with cancer receiving myelosuppressive chemotherapy. Supportive Care Cancer. 2020. https://doi.org/10.1007/s00520-020-05295-2.
Westreich D, Greenland S. The Table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–8. https://doi.org/10.1093/aje/kws412.
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Funding
Outcomes Insights, Inc was funded by Amgen to conduct this study. Amgen also paid the journal’s Rapid Service fees. Prior to conducting the research, Outcomes Insights, Inc. was permitted to publish the results of this study by Amgen, Inc., which is a requirement in the data use agreement with the National Cancer Institute.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conception, study design, and protocol development. Mark Danese conducted the data analyses. Mark Danese and Jennifer Schenfeld were responsible for creating the initial draft. All authors were involved in revising the initial draft and approving the final version. All authors agree to be accountable for the integrity of the research.
Disclosures
At the time this research was conducted Jennifer Schenfeld, Jaime Shaw, Prasad Gawade, Akhila Balasubramanian, Michael Kelsh, and Rohini K. Hernandez were Amgen employees and Amgen stockholders. Gary Lyman served as a Principal Investigator on an institutional grant from Amgen, Inc. and received speaking or consulting fees from G1 Therapeutics, Partners Healthcare, BeyondSpring, Squibb (institutional), Sandoz, Merck, Jazz, Kallyope, and TEVA. Mark Danese was an owner of Outcomes Insights, Inc. Outcomes Insights, Inc. received research funding to conduct this study, including funding for developing the manuscript. Outcomes Insights, Inc. has also received consulting fees from Amgen related to methods for observational research. Outcomes Insights, Inc. provided research and consulting services in oncology to Bristol Myers Squibb, Seattle Genetics, Boston Scientific, Mirati, EMD Serono, and Taiho.
Compliance with Ethics Guidelines
The study protocol was reviewed by Advarra and received an exemption determination on 24 May 2019. The authors received permission to access and use the USRDS data from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Data Availability
The data sets generated for the current study are not publicly available to ensure patient privacy, as required by the data use agreement with the National Cancer Institute
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Danese, M.D., Schenfeld, J., Shaw, J. et al. Association Between Granulocyte Colony-Stimulating Factor (G-CSF) Use and Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukemia (AML) Among Elderly Patients with Breast, Lung, or Prostate Cancer. Adv Ther 39, 2778–2795 (2022). https://doi.org/10.1007/s12325-022-02141-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02141-1