Abstract
Introduction
Hemifacial spasm (HFS) is a condition causing poor quality of life. Treatment with botulinum toxin A (BTX) injection is effective. Only one randomized controlled trial with a single-blind fashion has evaluated if oral injection is needed in HFS. The present study aimed to evaluate the necessity of oral BTX injection in HFS by a randomized, double-blind, placebo-controlled method.
Methods
We conducted a double-blind, placebo-controlled trial in patients with HFS who never received BTX treatment. Eligible patients randomly received either 15 units of BTX around the eye and normal saline around the mouth (group A) or 15 units of BTX around both the eye and the mouth (group B). The primary outcomes were self-reported symptoms and observed frequency of spasms, while the secondary outcome was the duration of improvement or the time between the injection and the recurrence of symptoms to the same condition as before treatment. Student t test and survival analyses were used to compare the duration of symptoms between both groups. The mean changes were compared to secondary outcomes between the two groups.
Results
There were 60 patients enrolled, half in each group. Baseline characteristics between both groups were similar. The mean (SD) of the duration of improvement in group A and B was 22.97 (18.85) and 17.53 (14.90) weeks, respectively (p = 0.220). There was no difference between both groups by survival analysis. Group B had a higher percentage of mouth improvement but there was no difference in the percentage of eye improvement, visual analog scale of eye and mouth spasm, or frequency of eye and mouth spasm. Group B had a higher incidence of side effects particularly mouth drooping (30% vs 10%) than group A (p = 0.053).
Conclusion
The mouth injection of BTX may not be necessary for HFS. It may be beneficial to reduce mouth symptoms with a higher rate of mouth drooping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hemifacial spasm (HFS) can be treated with botulinum toxin (BTX) injection. Only one study compared ocular plus oral injection versus ocular injection alone by a single-blind study. |
This is a randomized, double-blind, placebo-controlled study to evaluate the necessity of oral BTX injection in HFS. |
There was no significant difference of duration of improvement between both injections. |
The mouth injection of BTX may not be necessary for HFS. |
Introduction
Hemifacial spasm (HFS) is characterised by unilateral intermittent clonic and/or tonic contractions of muscles of facial expression supplied by the facial nerve [1]. The most likely cause is due to microvascular compression at the root exit zone. Although it is not a life-threatening condition, HFS is a chronic and usually progressive disease. It may cause patients unremitting social handicap or poor quality of life [1].
Botulinum toxin type A (BTX) is a neurotoxin produced by Clostridium botulinum bacteria. The action is to paralyse the injected muscles by irreversible blockage of the cholinergic transmission at the presynaptic nerve terminals [2]. BTX is a lyophilised formulation, purified from the culture solution by a series of acid preparations to a crystalline complex.
BTX was reported to be very effective, safe, and cost-effective in the treatment of HFS [3]. This recent review showed that several randomized controlled trials are comparing BTX treatment in HFS in terms of site of injection, dosage of injection, or source of BTX [3]. However, there is only one randomized trial that compared periocular and perioral injection versus periocular injection alone [4]. The periocular plus perioral injection had a better outcome in perioral muscle than periocular injection alone with significantly different severity scores: the visual analog scales were 5.0 vs 3.45. As the previous study was the only study with a single-blind fashion, the present study aimed to evaluate the necessity of perioral BTX injection in HFS by a randomized, double-blind, placebo-controlled method.
Methods
Study Design
This study was a randomized, double-blind, placebo-controlled study comparing periocular versus periocular plus perioral injection of BTX in patients with HFS. We conducted the study at HFS clinic, Srinagarind Hospital, Khon Kaen University, Thailand. The research protocol was approved by the Human Ethics Committee of Khon Kaen University (No. HE531456) and based on the Declaration of Helsinki. An informed consent was obtained from all participants prior to study participation.
Study Population
The inclusion criteria were patients aged more than 15 years old, did not respond to oral medication, were never treated with BTX, and had both periocular and perioral spasm. The exclusion criteria included patients who previously had surgical treatment for HFS, were pregnant, were allergic to BTX, or have myasthenia gravis.
We recorded all eligible patients’ baseline characteristics, duration of symptoms, the severity of eye spasms, and frequency of eye and mouth spasms in 5 min. The severity of eye spasm was recorded and graded as follows: grade 1, spasm at orbicularis oculi only; grade 2, eye closed less than 50%; grade 3, eye closed between 50% and 100%; and grade 4, eye closed 100%. The frequency of eye and mouth spasms was an objective measurement. We counted the frequency of spasms at the eye and the mouth separately in 5 mins.
Study Intervention
Patients were randomized into two groups (A and B). Both groups received two injections. Group A received the first injection with 15 units of BTX around the eye and normal saline (placebo) around the mouth (a total of 15 units). Group B received 15 units around the eye and 15 units around the mouth (a total of 30 units). The 15-unit injection was representative of the eye injection; on the other hand, the 30-unit injection was representative of the eye and the mouth injection.
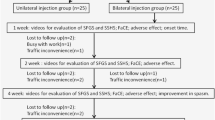
Reinjection was performed when the spasm resumed, and the spasm was as severe as before treatment. Patients were seen at 6 weeks after injections and when the spasm resumed. Each patient was followed up for five visits and at each visit patients were assessed for the treatment outcomes and side effects; thereafter, the patients were followed up clinically for 18 months. The BTX used in this study was onabotulinumtoxinA (BoNTA; BOTOX®; Allergan, Inc., Irvine, CA, USA).
Method of Injection
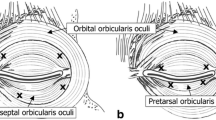
BTX was reconstituted with 2 ml of sterile 0.9% normal saline which made 5 units of BTX per 0.1 ml. We used an equal volume of normal saline as a placebo. All patients were injected subcutaneously with a 27-gauge insulin syringe into the medial, lateral part of the lower eyelid and the lateral part of orbital orbicularis oculi, just above the lateral eyebrow. We also injected into the upper part and lower part of the orbicularis oris. In the 15-unit treatment, we injected 15 units around the eye and normal saline around the mouth. In the 30-unit treatment, we injected 15 units around the eye and 15 units around the mouth (Fig. 1). Note that there was no pretarsal injection in this study.
Randomization
Eligible patients were randomized in blocks of six into two groups by a research coordinator. A research nurse prepared both BTX and placebo for each patient according to the study code. Both BTX and placebo had identical appearance in terms of colour and volume. Injector, research coordinator, research assistant, and patients were blinded to treatment allocation. Study codes were declared at the end of study.
Study Outcomes
The primary outcomes of the study were self-reported symptoms and observed frequency of spasms at week 6 after BTX injection. Each patient reported their symptoms at the eye and the mouth separately by using the visual analog scale (VAS). The scale ranged from 0 to 10; 10 represented no spasm and 10 represented the most severe of spasm as before treatment [5]. The initial VAS was set at 10 in all patients. Patients scored the VAS weekly after treatment and if the severity decreased by half, patients were advised to score as 5.
In addition, patients were asked to assess the percentage improvement at the eye and the mouth from 0% to 100%. The frequency of spasms was observed and counted for 5 min at each follow-up visit by a rater who was a research assistant and blinded to treatment [6].
We also recorded any adverse events occurring during the study period both reported by patients and detected at the follow-up.
The secondary outcome was the duration of improvement or the time between the injection and the recurrence of symptoms to the same condition as before treatment.
Sample Size Calculation
According to the previous study [4], the VAS of perioral muscles by BTX and placebo were 3.5 and 2.1, respectively. With a power of 80% and confidence of 95%, the required sample size was 29 in each arm for two-sided and two-group comparison with equal sample size of each group. We rounded up to 30 patients per group.
Statistical Methods
For each variable of the primary outcome measures and side effects were calculated within the subject. The mean changes were compared between the two groups. The differences of the mean changes and the 95% confidence intervals (CI) were estimated.
For the secondary outcome measure (duration of improvement), survival analysis was performed as there were censoring observations. Patients who did not respond to BTX, withdrawals, or lost to follow-up were considered as censored observations. Kaplan–Meier survival curve, log-rank test, and Cox proportional hazard model were applied to show the improvement and relapse of symptoms between groups. Relapse was defined when patients needed additional injections or symptoms worsened.
All analyses were two-sided tests with a level of significance of 0.05 and done by using STATA statistical package.
Results
Baseline Data
A total of 60 patients with HFS were recruited and randomized in the trial, half in each group (Fig. 2). Demographic data and baseline characteristics are summarized in Table 1. All variables were comparable between both groups. About two-thirds of each group were female. Mean age and mean duration of symptoms (SD) before participating in the study in group A and B were 49.7 and 51.3 years, and 5.0 (5.7) and 3.1 (3.1) years, respectively. More than half of the subjects had their symptoms at grade 4 (very severe). The frequency of spasm and frequency of tonic spasm before the BTX injection at the eye and mouth were similar in both groups.
Outcomes
Primary outcomes
The primary outcomes are summarized in Table 2.
The VAS of the eye and mouth spasm The VAS score fell to about half within the first week after injection for both groups. At the end of the study, the difference of VAS of the eye and mouth spasm between the two groups was 0 (95% CI − 0.9 to 0.9, p = 0.941) and − 0.6 (95% CI − 1.7 to 0.5, p = 0.249), respectively.
Percentage of eye improvement The difference in the mean changes between the two groups was 0.3% (95% CI − 8.4 to 9.0 %, p = 0.940).
Percentage of mouth improvement The difference in the mean changes between the two groups was − 15.4% (95% CI − 26.2% to − 4.5%, p = 0.006).
Frequency of eye spasm The difference of the mean changes between the two groups was 39.0 (95% CI − 129.0 to 50.9, p = 0.389).
Frequency of mouth spasm The difference of the mean changes between the two groups was − 6.7 (95% CI − 111.2 to 79.8, p = 0.630).
Secondary Outcome
The duration of improvement indicated the efficacy of BTX. The mean (SD) of the duration of improvement in group A and B was 22.97 (18.85) and 17.53 (14.90) weeks, respectively (p = 0.220).
The Kaplan–Meier survival curve (Fig. 3) showed that the probability of being symptom-free between the two groups was similar during the first 20-week period. After 20 weeks, group A (15 units) had slightly higher proportions than group B (30 units). The difference of median survival between the two groups was 2 weeks (log-rank test, p = 0.18).
Patients who received 15 units are 30% less likely to relapse at any time than those who received 30 units. The hazard ratio of being relapsed among those who received 15 units compared to those who received 30 units was 0.70 (95% CI 0.41–1.21, p = 0.20). The effects of duration of illness before the treatment, severity of eye spasm, frequency of spasm at the eye, and frequency of spasm at the mouth were evaluated between both groups by multivariable analysis. The effect of 15 units compared to 30 units was similar without any adjustment. The hazard ratio was 0.73 (95% CI 0.38–1.40, p = 0.20). The test for proportional hazard assumption indicated that the assumption was not violated (p = 0.81).
Adverse Events
Two patients in group B (30 units BTX) discontinued the study because of side effects. Adverse events monitored both by spontaneous report and formal ascertainment showed that at 6 weeks after the first injection the incidence of ptosis and double vision was comparable. By contrast, the symptoms of water getting into the eyes, dry eyes, mouth drooping, and dynamic facial asymmetry were more frequent in group B (30 units) than group A (15 units) without statistical significance (Table 3).
Discussion
Our randomized, placebo-controlled design indicated that the mouth injection for HFS was not necessary. Both primary and secondary outcomes were almost all comparable. Although BTX was not injected into the mouth area of patients in group A, the frequency and the visual analog scale of severity of mouth spasm were improved similarly to group B (Table 2). Only the percentage of mouth improvement after injection was statistically different between both groups. However, this secondary outcome was subjectively rated by the patients.
In most patients with HFS, facial contractions start around the eye and spread down to the mouth. We believe that spasm originates in the muscle around the eye and spreads to the mouth area [7]. Ogawara et al. also showed that injection of BTX only into the orbicularis oculi can reduce the excitability of the facial motor neuron. As a result, the mouth spasm is reduced [8]. However, mouth injection may have a direct benefit on mouth improvement as previously reported and shown in Table 2 [4]. The present study also found nearly significant differences in mouth drooping (10% vs 30%) between group A and B (Table 3) which was similar to the previous study [4]. Note that mouth injection more laterally in this study may reduce occurrence of mouth weakness. The ocular and oral injection had lower facial paresis of 47.7% for a mild and moderate degree, while the ocular injection had 13% for only mild degree facial paresis [4].
There are some differences between this and the previous study [4]. First, BTX doses were different. The previous study used BTX of 11–30 units for ocular injection with 3–11 units for oral injection. This study used a fixed dose of 15 units in each site. Second, the mean duration of spasms was longer in the previous study (9.26 vs 4 years). Third, the outcomes were evaluated after 3 months of BTX injection in the previous study, while this study evaluated the duration of improvement. Finally, this study was a double-blind, placebo-controlled trial, while the previous study was a single-blind study.
There is some strength in this study. The size of the study population was relatively large for HFS. Second, the study design was a double-blind, placebo-controlled trial. Finally, only de novo patients were included which eliminates the bias of previous injections. However, some limitations exist. First, the dose of mouth injection may be high as this was a part of the blinding process. We selected 15 units as the previous report found that the highest dose of 11 units was not effective [4]. The 15-unit injection was similar to the ocular injection. Second, the mouth injection site may not be a typical site. Finally, some outcomes were rated subjectively and some factors are not studied such as obstructive sleep apnea [9,10,11,12,13].
Conclusions
The mouth injection of BTX may not be necessary for HFS. It may be beneficial to reduce mouth symptoms with a higher rate of the mouth drooping.
References
Kongsaengdao S, Maneeton N, Maneeton B. The five-year prospective study of quality of life in hemifacial spasm treated with abo-botulinum toxin A. Toxins (Basel). 2021;13(3):215.
Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69(2):200–35.
Anandan C, Jankovic J. Botulinum toxin in movement disorders: an update. Toxins (Basel). 2021;13(1):42.
Colakoglu BD, Cakmur R, Uzunel F. Is it always necessary to apply botulinum toxin into the lower facial muscles in hemifacial spasm? A randomized, single-blind, crossover trial. Eur Neurol. 2011;65(5):286–90.
Jitpimolmard S, Tiamkao S, Laopaiboon M. Long term results of botulinum toxin type A (Dysport) in the treatment of hemifacial spasm: a report of 175 cases. J Neurol Neurosurg Psychiatry. 1998;64(6):751–7.
Yoshimura DM, Aminoff MJ, Tami TA, Scott AB. Treatment of hemifacial spasm with botulinum toxin. Muscle Nerve. 1992;15:1045–9.
Mauriello JA Jr, Coniaris H, Haupt EJ. Use of botulinum toxin in the treatment of one hundred patients with facial dyskinesia. Ophthalmology. 1987;94:96–9.
Ogawara K, Kuwabara S, Kamitsukasa I, et al. Trigeminal afferent input alters the excitability of facial motoneurons in hemifacial spasm. Neurology. 2004;62:1C – 1752.
Kasemsap N, Netwijitpan S, Limpawattana P, et al. CPAP therapy improves intractable hemifacial spasm. Case Rep Neurol Med. 2015;2015:846234.
Khamsai S, Chootrakool A, Limpawattana P, et al. Hypertensive crisis in patients with obstructive sleep apnea-induced hypertension. BMC Cardiovasc Disord. 2021;21:310.
Soontornrungsun B, Khamsai S, Sawunyavisuth B, et al. Obstructive sleep apnea in patients with diabetes less than 40 years of age. Diabetes Metab Syndr. 2020;14:1859–63.
Sawunyavisuth B. What are predictors for a continuous positive airway pressure machine purchasing in obstructive sleep apnea patients? Asia-Pac J Sci Technol. 2018;23:APST-23-03-10.
Sawunyavisuth B, Ngamjarus C, Sawanyawisuth K. Any effective intervention to improve CPAP adherence in children with obstructive sleep apnea: a systematic review. Glob Pediatr Health. 2021; https://doi.org/10.1177/2333794X211019884.
Acknowledgements
Supported in part by a grant from the Faculty of Medicine, Neurology Research Fund, Allergan, Inc. for providing botulinum toxin (Botox), and Neuroscience Research and Development Group, Khon Kaen University. We thank Eric and Jeanette Rental for their assistance in proofreading without any fee or funding. We also thank the participants of the study.
Funding
No funding or sponsorship was received for this study. The Rapid Service Fee for this article was funded by Botulinum toxin fund, Faculty of Medicine, Khon Kaen University, Thailand.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, have given their approval for this version to be published and assure it will not be published elsewhere in the same form, in English or in any other language, including electronically.
Author Contributions
Suthipun Jitpimolmard and Somsak Tiamkao contributed to study conception and design, collection and assembly of data, and data interpretation. Bandit Thinkhamrop contributed to data analysis. Suwanna Arunpongpaisal, Preeda Arayavichanon, Weerachai Kosuwan, and Siriya Jitpimolmard contributed to data interpretation. Kittisak Sawanyawisuth contributed to data interpretation and wrote the manuscript. All authors reviewed and approved the final content of this manuscript.
Disclosures
Suthipun Jitpimolmard, Bandit Thinkhamrop, Somsak Tiamkao, Suwanna Arunpongpaisal, Preeda Arayavichanon, Weerachai Kosuwan, Siriya Jitpimolmard, Kittisak Sawanyawisuth have nothing to disclose in relation to this article.
Compliance with Ethical Guidelines
The research protocol was approved by the Human Ethics Committee of Khon Kaen University (No. HE531456). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Eligible patients gave an informed consent prior to study participation.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jitpimolmard, S., Thinkhamrop, B., Tiamkao, S. et al. A Double-Blind, Placebo-Controlled Study of Appropriate Site of Botulinum Toxin Therapy in Hemifacial Spasm. Adv Ther 39, 2025–2034 (2022). https://doi.org/10.1007/s12325-022-02077-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02077-6