Abstract
Introduction
This study characterized the multidose pharmacokinetic (PK) characteristics of posaconazole tablets used as prophylactic antifungal therapy in Chinese patients with acute myelogenous leukemia (AML) at risk for invasive fungal infection (IFI).
Methods
Participants in this open-label, single-arm, phase 1b study received posaconazole 300 mg twice daily on day 1 and then once daily for up to 28 days. In the intensive PK sampling subgroup, posaconazole was administered under fasting conditions on days 1 and 8, and blood samples were regularly collected over 24 h. Trough PK sampling was conducted in all participants on days 1, 2, 3, 8, 14, 21, and 28 without regard for food intake. Population PK characteristics were predicted using PK modeling. Primary endpoints were steady-state average concentration (Cavg) and percentage of participants with steady-state Cavg (predicted and observed) > 500 ng/ml. Treatment safety and efficacy were secondary endpoints.
Results
Sixty-five adult Chinese participants were enrolled. On day 8, steady-state arithmetic mean Cavg was 1610 ng/ml (% coefficient of variation [%CV] 42.8%) in the intensive PK subgroup (n = 20). All participants achieved a steady-state Cavg > 500 ng/ml. Predicted Cavg (pCavg) was 1770 ng/ml (%CV 33.7%) in the total population (n = 64); 92.2% of participants had pCavg values ≥ 500 ng/ml (n = 59). The posaconazole tablet safety profile was consistent with that of the oral formulation, and the IFI rate was 3%.
Conclusion
In Chinese AML patients, the posaconazole 300-mg tablet provided PK data comparable with those of previous studies and was generally well tolerated and efficacious.
Clinical Trial Registration
ClinicalTrials.gov, NCT02387983.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Data are lacking regarding the pharmacokinetic and safety outcomes of posaconazole tablets when used as antifungal prophylaxis in Chinese patients at high risk for invasive fungal infection. |
This study characterized the multidose pharmacokinetic (PK) characteristics of posaconazole tablets used as prophylactic antifungal therapy in Chinese patients with acute myelogenous leukemia (AML) at risk for invasive fungal infections (IFI). |
What was learned from the study? |
In this analysis of the posaconazole tablet formulation in Chinese AML patients at high risk for IFIs, the PK characteristics were consistent irrespective of the sampling schedule and fed/fasting status; all evaluable study participants had Cavg > 500 ng/ml. |
In this analysis, posaconazole tablets achieved exposure levels in Chinese study participants that were consistent with levels previously shown to be efficacious and safe. |
Introduction
In recent decades, a rising number of immunocompromised hosts in China has resulted in an increased prevalence of invasive fungal infection (IFI), primarily caused by Candida, Aspergillus, Cryptococcus, Mucorales, and Pneumocystis species [1, 2]. These opportunistic infections most commonly affect the lungs, systemic circulation, and sinuses [2].
Chinese patients with acute myelogenous leukemia (AML) have a relatively high incidence of invasive fungal infection (IFI), 3.8%, and prominent risk factors including a history of IFI, prolonged neutropenia, parenteral nutrition, and smoking [2,3,4]. However, antifungal prophylaxis continues to be underused in China, despite the fact that it reduces IFI-related death and increases IFI-free survival [3, 4].
Posaconazole is a broad-spectrum triazole antifungal with potent antifungal activity against a variety of yeasts and molds, including strains resistant to amphotericin B, fluconazole, voriconazole, and itraconazole [1]. It is recommended that posaconazole, initially developed as an oral suspension, be administered with food to enhance absorption [5]. Time to reach maximum plasma concentration is 3–6 h, apparent clearance (CL) is 0.2–0.5 l/h/kg, and half-life (t½) is 15–35 h [5]. Its bioavailability is dependent on dosage regimen and food intake— > 98% protein bound—with an apparent volume of distribution after oral administration of 7–25 l/kg [5]. Posaconazole is metabolized primarily in the liver through glucuronidation to inactive metabolites, and 66% is excreted unchanged in feces [5].
Although limited data are available for Asian patients, prophylactic posaconazole therapy has been more effective at reducing the incidence of IFI than fluconazole (9% vs. 22%) in Chinese patients with AML or myelodysplastic syndrome (MDS) and persistent chemotherapy-induced neutropenia [6]. Following approval in 2013 by the China Food and Drug Administration of the oral suspension of posaconazole for prophylaxis of Aspergillus and Candida infections, several additional studies also confirmed that prophylactic posaconazole, compared with other antifungals, provides superior protection against IFI in Chinese patients with AML, acute lymphocytic leukemia, or MDS [3, 7, 8].
A drawback of the posaconazole oral suspension formulation is the requirement for dosing 3 ×/day and for the drug to be administered with a high-fat meal to improve bioavailability [9, 10]. The high-frequency dosing regimen and the food requirement are potential barriers to achieving and maintaining plasma posaconazole concentrations required for effective prophylaxis, especially when patients with hematologic malignancies are at risk for chemotherapy-related mucositis or neutropenic enterocolitis, which can reduce oral drug absorption, or when they are unable to eat because of nausea [11,12,13].
To overcome these challenges to effective antifungal prophylaxis, an extended-release tablet formulation of posaconazole was developed, and a bridging strategy was used in clinical trials to demonstrate comparable exposure and safety between the tablet and the suspension formulations. Combining posaconazole with a pH-dependent polymer prevents dissolution in the low pH of the stomach, delaying release in the small intestine and improving exposure with less variability than the oral suspension in fasting patients [14,15,16]. Specifically, posaconazole exposure was 3.6-fold higher with the tablet formulation than with the oral suspension (mean area under the curve [AUC], 11,700 h·ng/ml vs. 3240 h·ng/ml), whereas peak plasma concentrations (Cmax) were also 4.5-fold higher (385 ng/ml vs. 84 ng/ml) [15]. A subsequent phase 1b/3 study conducted in the USA and Europe showed that the new posaconazole 300-mg tablet was well tolerated and safe, rapidly producing steady-state average plasma concentrations (Cavg) ≥ 500 ng/ml in 97%–99% of patients at risk for IFI and trough plasma concentrations (Cmin) ≥ 500 ng/ml in 95% of patients [17, 18]. Indeed, a lower incidence of breakthrough IFI (0.5%) was reported for the tablet formulation of posaconazole than the oral solution (2–5%) [18,19,20].
To support registration of the tablet formulation in China, we characterized the pharmacokinetic (PK) profile of multiple-dose posaconazole 300-mg extended-release tablets in Chinese patients with AML at risk for IFI.
Methods
Study Design and Population
An open-label, single-arm, phase 1b study was performed at four study sites in China (Peking University People’s Hospital, The First Affiliated Hospital of Soochow University, The 307th Hospital of Chinese People’s Liberation Army, and Shanghai Ruijin Hospital) (Merck protocol 5592-117; ClinicalTrials.gov, NCT02387983). The study was conducted in accordance with the principles of Good Clinical Practice, consistent with the principles enunciated in the Declaration of Helsinki, and written informed consent was obtained from each participant. The protocol was reviewed and approved by independent ethics committees at all participating study centers.
Participants were Chinese men and women aged ≥ 18 and ≤ 70 years who had AML and were considered at high risk for serious IFI. Although subjects with MDS were also eligible for study participation, none were enrolled. Before the study, eligible participants had a body mass index of 15–30 kg/m2 and a baseline weight of 45–80 kg and were anticipated to develop (within 3–5 days) or had developed severe prolonged neutropenia (absolute neutrophil count < 500 cells/mm3) after receiving intensive induction or re-induction chemotherapy for the treatment of a new diagnosis or a first relapse of AML. Participants were excluded if they had received nonprophylactic oral, intravenous, or inhaled antifungal therapy within 30 days of entering the study, had a known or suspected IFI, or had received posaconazole within 10 days before study entry. Other exclusion criteria included history of type 1 hypersensitivity or idiosyncratic reactions to azoles, moderate or severe liver dysfunction (defined as aspartate aminotransferase [AST] or alanine aminotransferase [ALT] levels > 3 × upper limit of normal [ULN] and total bilirubin levels > 2 × ULN), prolonged QTc interval (> 500 ms) on baseline electrocardiography (ECG), recent surgery or blood donation, pregnancy, and breastfeeding. Women of childbearing potential were required to use a medically accepted form of contraception throughout the study treatment period.
Treatment
Participants were allocated to “intensive” (first 20 participants enrolled) and then to “sparse” (subsequent participants, n = 45) PK sampling subgroups. Each participant received two doses of posaconazole 300-mg tablet on day 1 (12 h apart), followed by once-daily dosing until at least day 8, for a maximum of 28 days. On days 1 and 8, participants in the intensive PK subgroup were administered posaconazole under fasting conditions (4 h before and 4 h after study drug administration). All other dosing in this group and all dosing in the sparse PK subgroup were administered irrespective of fasting/fed state (though food intake 2 h before and 1 h after posaconazole administration was recorded).
PK Sampling and Analysis
Blood samples for the intensive PK subgroup were collected on days 1 and 8 predose and at 2, 4, 6, 8, 12, and 24 h postdose. For the intensive and sparse PK sampling subgroups, trough blood samples were collected on days 1, 2, 3, 8, 14, 21, and 28 (or the end of treatment) immediately before daily dosing.
Primary PK parameters in the intensive PK sampling subgroup were Cavg and proportion of participants with Cavg > 500 ng/ml at day 8. Cavg was chosen as the main PK variable based on positive association between posaconazole Cavg ≥ 500 ng/ml and response in a nonrandomized invasive aspergillosis study [21] and a phase 3 PK study of the tablet formulation of posaconazole in patients at high risk for IFI [18]. Additional PK parameters calculated for the intensive subgroup included AUC from time 0 to 24 h, Cmax, Cmin, observed maximum plasma concentration at 12 h (C12), time to Cmax, apparent total body clearance, and Cmax accumulation ratio. Cmin was the only PK parameter observed for the sparse PK sampling subgroup.
Posaconazole levels were analyzed by WuXi AppTec, Inc. (Shanghai, China) using a validated liquid chromatography-tandem mass spectrometry assay with a nominal quantification range of 5–5000 ng/ml [22].
Safety
Safety assessments included adverse events (AE) and vital sign (heart rate, blood pressure, respiratory rate, body temperature) monitoring on days 1, 2, 3, 8, 14, 21, and 28 and follow-up 14 days after the last posaconazole dose. Additional safety assessments included clinical laboratory (hematology, chemistry, urinalysis [days 1, 8, 14, 21, 28]), ECG (days 1, 3, 8, 28), and radiologic (computed tomography) tests (days 8, 28).
An AE was defined as any unfavorable and unintended medical occurrence, which could be a symptom, an abnormal test finding, a disease, or a worsening preexisting condition, that occurred during treatment or follow-up. A serious AE was any event that resulted in death or was life threatening, resulted in persistent or significant disability or incapacity, prolonged inpatient hospitalization, or caused another important medical event. AEs were evaluated by a qualified physician and described in terms of intensity (mild, moderate, or severe), seriousness (e.g., life threatening), duration, relationship to study drug, and outcome. Participants could withdraw from the study at any time for any reason or be withdrawn from the study by the investigator because of an AE, a safety concern, or a protocol violation.
Efficacy
Although the study was not designed primarily as an efficacy study, clinical signs and symptoms of IFI were monitored throughout. Investigators used a descriptive assessment of the clinical signs and symptoms for evaluation of possible, probable, or proven IFI according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG version 2008) criteria [23]. Participants were discontinued from the study on confirmation of a possible, probable, or proven IFI.
Statistical Analysis
Descriptive statistics were generated for population demographics, PK parameters, safety, and efficacy using Phoenix® WinNonlin® Professional (version 6.3; Certara USA, Inc., Princeton, NJ, USA). The safety and efficacy population included all participants who received ≥ 1 dose of the posaconazole tablet formulation. The PK-evaluable population included participants who complied with the study protocol, including documented adherence to dosing and PK regimens through day 8. Pharmacokinetic parameters were calculated by noncompartmental analyses using the Phoenix® WinNonlin® Professional (version 6.3) software. AUC was calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations (linear-up/log-down). Cavg was calculated by taking the AUC from time 0 to 24 h and dividing by 24 h to yield the average concentration across the dosing interval.
The predicted Cavg (pCavg) and the percentage of the population with exposures > 500 ng/ml were estimated using a population PK model developed with data from global studies that enrolled healthy volunteers and participants who received posaconazole tablets as prophylactic antifungal therapy [24]. Data from the current study were added to the population PK data set.
Results
After screening 67 Chinese AML patients for study eligibility, this study was fully enrolled with 65 recruited participants; the first 20 participants were assigned to the intensive PK sampling subgroup and the remaining 45 to the sparse PK sampling subgroup. Mean age was 42.7 years (range, 18–67), most participants (35; 53.8%) were men, mean weight was 63.3 kg (range, 45.1–80.0), and mean body mass index was 23.0 kg/m2 (range, 17–30) (Table 1). All participants had a primary diagnosis of AML and received ≥ 1 dose of study drug.
Of the 65 participants, 58 (89.2%) completed the full 28-day study: 18 in the intensive PK sampling subgroup, 40 in the sparse PK subgroup. Seven participants (10.8%) prematurely discontinued the study (six during the treatment period and one during follow-up) because of AEs (n = 4; one and three from the intensive and sparse PK subgroups, respectively), protocol violation (ECG QTc interval > 500 ms, n = 1, from the sparse PK subgroup), and participant withdrawal (n = 2; one from each sampling subgroup). The participant with a prolonged QTc interval received a single dose of posaconazole before being withdrawn and was excluded from the PK analysis population. Another five discontinued participants (two from the intensive PK sampling subgroup, three from the sparse subgroup) did not complete day 8 study medication and were also excluded from the PK analysis population; the subject in the sparse sampling subgroup who discontinued from the study during follow-up had completed day 8 medication and was included in the PK analysis population.
During the study, two participants used ebastine (H1 histamine receptor antagonist, a prohibited medication) to manage rash (n = 1) and erythema (n = 1). Given that ebastine was administered after posaconazole dosing on day 8 and posaconazole concentrations were unaffected, data from these participants were included in the PK analysis population.
PK Analysis
PK Sampling Analysis
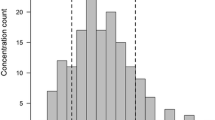
The PK-evaluable population comprised 59 participants (18 from the intensive subgroup, 41 from the sparse subgroup) who adhered to dosing and PK sampling through day 8. All participants in the intensive PK sampling subgroup (n = 18) achieved target steady-state Cavg > 500 ng/ml on day 8, with 16 participants (88.9%) achieving steady-state Cavg exposures between 500 and 2500 ng/ml and two participants (11.1%) reaching Cavg between 2500 ng/ml and 3750 ng/ml (Fig. 1). PK parameters for participants in the intense PK subgroup are summarized in Table 2.
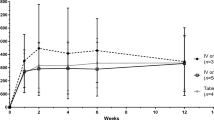
Posaconazole was readily absorbed; an average Cmax of 730 ng/ml was reached at a median of 4 h on day 1, indicating that therapeutic plasma drug concentrations (> 500 ng/ml) were reached after just one 300-mg dose. After 24 h (i.e., after completion of the 2 × 300-mg loading dose), mean Cmin was 997 ng/ml. Posaconazole plasma concentrations reached steady state by day 8 (Fig. 2), with a mean Cmin of 1310 ng/ml and a mean Cavg of 1610 ng/ml (range, 683–3100 ng/ml). A similar day 8 mean Cmin of 1410 ng/ml was observed for the sparse sampling subgroup.
Population PK Analysis
Population PK analysis was used to integrate PK data from participants with ≥ 1 PK data point (all data from the intensive sampling subgroup [n = 20] and Cmin data from the sparse sampling subgroup [n = 44]), with PK data from a global population of participants administered posaconazole 300-mg tablets, to model and calculate predicted PK parameters (i.e., Cavg) across this entire Chinese cohort (n = 64). The predicted steady-state Cavg for the intensive sampling, sparse sampling, and total populations are shown in Table 3. The predicted mean (% coefficient of variation) steady-state Cavg (pCavg) on day 8 was 1770 ng/ml (33.7%) for the total population, consistent with that observed in the intensive PK sampling population. Specifically, all participants had a pCavg > 500 ng/ml; 59 (92.2%) had a steady-state pCavg between 500 and 2500 ng/ml, four (6.3%) reached exposures between 2500 and 3750 ng/ml, and one (1.6%) achieved a pCavg > 3750 ng/ml (Table 3).
Safety
The safety analysis population included all participants who received ≥ 1 dose of posaconazole (n = 65). Posaconazole was generally well tolerated. Almost all participants (98.5%, n = 64) reported ≥ 1 AE, with 23 participants (35.4%) experiencing a study drug-related AE. Of the five (7.7%) participants who discontinued because of an AE, three (4.6%) did so as a result of a drug-related AE. Drug-related AEs leading to study discontinuation included maculopapular rash (n = 1), renal failure (n = 1), and increased ALT and AST (n = 1).
The AE profile was similar to that reported previously for posaconazole oral suspension. The most common AEs overall in this study (occurring in ≥ 20% of participants) were pyrexia, hypokalemia, diarrhea, cough, hypocalcemia, blood bilirubin increased, and rash (Table 4). The most common drug-related AEs (> 5% incidence) were ALT increased, blood bilirubin increased, AST increased, and rash. Most adverse events were mild to moderate and resolved spontaneously. No participant (except for the single patient enrolled in error and discontinued for safety reasons) had a QTc interval measurement ≥ 500 ms during the treatment phase. No clinically meaningful relationships were observed for differences in vital signs and physical examinations as a function of treatment.
Four participants (6.2%) reported serious AEs that were considered by the investigators to be unrelated to the study drug. Similarly, two participants died during the follow-up period (within 14 days of the last dose of posaconazole), but the causes of death (respiratory failure and intracranial hemorrhage) were judged unlikely to be related to the study drug.
Efficacy
The overall incidence of IFI in this study was low, and no proven IFI was reported. However, probable IFI was diagnosed in two participants (3%).
A 50-year-old woman, treated with induction chemotherapy for acute AML, received a diagnosis of probable IFI of the lung following an abnormal radiography result on day 8, and blood culture was positive for an unspecified fungal pathogen on day 15. She was withdrawn from the study on day 16. The participant’s posaconazole Cmin values at days 8, 14, and 15 were 916, 648, and 796 ng/ml, respectively.
A second participant, a 36-year-old man, also treated with chemotherapy for acute AML, received a diagnosis of probable IFI of the lung following an abnormal radiography result on day 20. He was withdrawn from the study on day 21. His posaconazole Cmin values at days 8, 15, and 20 were 1990, 1670, and 796 ng/ml, respectively.
Five additional participants were considered to have possible IFI, though no mycologic, serologic, or specified radiologic evidence of infection was found on further investigation.
Discussion
In this analysis of the posaconazole tablet formulation in Chinese AML patients at high risk for IFI, PK characteristics were consistent irrespective of the sampling schedule and the fed/fasting status; all evaluable study participants had Cavg > 500 ng/ml. The PK profile of posaconazole predicted by the population PK model in Chinese participants was also consistent with that observed in an earlier study of the tablet formulation in a Western population [24]. Approximately twofold increases occurred in posaconazole exposures when the tablet formulation was taken with a high-fat meal in healthy Chinese subjects in a single-dose tablet evaluation [25]. Despite the food effect for the tablet—much smaller than for the oral suspension—the tablet, unlike the oral suspension, does not have to be taken with food to produce adequate exposure. The safety profile for the tablet formulation was similar to that observed with the suspension formulation [6]. Based on the EORTC/MSG criteria for the classification of IFIs, two participants in the current study experienced probable IFIs, even though both participants had steady-state Cmin values > 500 ng/ml at the time of diagnosis. This IFI rate of 3% was in line with that found in previous studies, in which posaconazole prophylaxis was associated with IFI rates of 2–2.4% [19, 20].
The posaconazole tablet formulation was developed to address limitations of the posaconazole oral suspension, primarily the variability of exposure to the oral suspension and the need to consume the oral suspension in combination with a meal, preferably a high-fat meal, to achieve adequate bioavailability [17, 18], and it fulfills a key unmet medical need for participants who require antifungal prophylaxis. Of note, compared with oral suspension, the tablet formulation of posaconazole is associated with higher proportions of subjects achieving target serum and plasma posaconazole concentrations and higher Cavg, all attributed to improved absorption and bioavailability [26,27,28,29,30,31,32,33]. Higher plasma concentrations with the tablet versus the oral suspension formulation have also been observed in Korean patients [34]. Furthermore, no difference in prophylactic efficacy has been observed with the tablet versus the oral formulation of posaconazole [28, 30, 32, 35].
However, real-world studies have highlighted that the variability of plasma posaconazole concentrations following tablet administration remains high, albeit lower than with oral suspension [36,37,38]. In particular, plasma posaconazole concentrations above and below therapeutic ranges continue to be observed in patients administered the tablet formulation [36,37,38]. Concentrations above the therapeutic range may increase the risk for liver function abnormalities, raising the prospect that therapeutic drug monitoring (TDM) may still be required for the tablet formulation, as it is for the oral suspension. However, rather than using TDM to monitor for subtherapeutic plasma posaconazole concentrations with the tablet formulation, TDM may be required only once after treatment initiation—for instance, on day 8 once Cavg has been reached—to identify patients for whom a lower dose may be appropriate to reduce the risk for AEs and to reach treatment discontinuation [33, 36, 39,40,41].
Although the tablet formulation has been associated with an increased prevalence of elevated liver enzyme levels in some patients, no differences in treatment-limiting hepatotoxicity have been reported [26,27,28, 37, 42]. Evidence suggests that a 200-mg dose may achieve comparable serum and plasma posaconazole levels with improved tolerability in some patients, but coadministered medications in patients with hematologic malignancies potentially confound assessments of AEs, especially hepatotoxicity [38, 39, 42]. The prevalence and impact of oral and intestinal mucositis on administering prophylactic antifungal treatment with posaconazole must also be considered. Investigations have indicated that the presence of mucositis in patients is associated with lower plasma posaconazole concentrations when administered as a tablet; however, given that target serum and plasma concentrations were still reached, the clinical relevance of any mucositis-related reduction in tablet plasma posaconazole concentrations appears to be limited [28, 31].
A number of other confounding factors could also influence plasma posaconazole concentrations. For example, consuming a high-fat meal with the tablet formulation modestly increases posaconazole exposure by 50% compared with increases of 160% and 300%, respectively, when the oral suspension is administered in combination with a nonfat or a high-fat meal [43]. However, results from the current study confirmed that Chinese patients do not have to consume posaconazole tablets with a high-fat meal to achieve therapeutic concentrations, an option likely to be preferred by patients [44].
Proton pump inhibitors and intermediate- or high-dose steroids (including steroids for asthma) have also been linked to an increased risk for subtherapeutic plasma posaconazole levels [38, 45]. Other studies have suggested no interaction between drugs that alter gastric pH and gastric motility and the PK characteristics of posaconazole in the tablet formulation after administration of a single higher (400-mg) dose of posaconazole [14, 31]. Similarly, reports of an association between sex and lower plasma posaconazole levels are conflicting [38, 40, 45].
Safety and tolerability assessments for the posaconazole tablet in this study were similar to those in previous studies, and no new safety signals were identified in Chinese participants [18]. The prevalence of treatment-related AEs, including increased liver enzyme levels, rash, and ECG abnormalities, in Chinese participants was consistent with that in earlier studies of the posaconazole oral suspension formulation [6]. Nevertheless, the AE profile of posaconazole in Chinese participants presents some differences from that observed in Western populations. In particular, the incidence of nausea and diarrhea observed in Western populations has not been seen in Chinese participants, though the prevalence of liver function abnormalities appears to be higher in Chinese participants [18]. The overall safety profile of the posaconazole tablet formulation was more favorable than that of voriconazole in patients with AML or MDS and maintained comparable efficacy [46].
A once-daily tablet formulation can also help improve patient adherence. A once-daily treatment option is preferred by patients and has been associated with improved adherence in patients with chronic diseases [44, 47]. Adherence among patients prescribed a 28-day prophylactic posaconazole tablet regimen has been reported as only 76% [40]. Given the challenge of maintaining adherence in this patient population, the extended-release tablet formulation combined with Cavg plasma posaconazole concentrations well above the minimum therapeutic target may also help maintain effective prophylaxis.
One limitation of this study is that the target exposure level (500 ng/ml) was set on the basis of a similar target specified in a multicenter global study of posaconazole tablet when used as antifungal prophylaxis for preventing invasive fungal infections in patients who have AML or MDS or who are undergoing hematopoietic stem cell transplantation [18]. Many other studies have set minimum target plasma posaconazole levels at 700 ng/ml, which is consistent with US Food and Drug Administration recommendations for achieving effective prophylaxis with posaconazole; even then, this threshold has been suggested as too low to achieve adequate efficacy [30]. No statistically significant difference in efficacy was observed between a minimum exposure target of Cavg 500 versus 700 ng/ml in a meta-analysis [48]. Additional limitations of this study include the presence of confounding factors and the small sample size. Accordingly, the characteristics of the population PK model for the posaconazole tablet described in this study must be confirmed prospectively in Chinese patients.
Conclusion
In conclusion, the PK characteristics of the posaconazole tablet formulation in Chinese patients with hematologic malignancies are consistent with those reported in earlier studies in Western populations, providing an efficacious and well-tolerated prophylactic antifungal treatment option. The posaconazole tablet formulation provides a needed treatment option for Chinese patients at risk for IFI.
References
Keating GM. Posaconazole. Drugs. 2005;65(11):1553–677.
Lien MY, Chou CH, Lin CC, et al. Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: a retrospective cohort study. PLoS One. 2018;13(6):e0197851.
Xu XH, Zhang L, Cao XX, et al. Evaluation of the implementation rate of primary antifungal prophylaxis and the prognosis of invasive fungal disease in acute leukemia patients in China. J Infect Chemother. 2017;23(6):360–7.
Sun Y, Huang H, Chen J, et al. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: a multicenter, prospective, observational study in China. Tumour Biol. 2015;36(2):757–67.
Li Y, Theuretzbacher U, Clancy CJ, Nguyen MH, Derendorf H. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin Pharmacokinet. 2010;49(6):379–96.
Shen Y, Huang XJ, Wang JX, et al. Posaconazole vs. fluconazole as invasive fungal infection prophylaxis in China: a multicenter, randomized, open-label study. Int J Clin Pharmacol Ther. 2013;51(9):738–45.
Tang L, Yang XF, Qiao M, et al. Posaconazole vs. voriconazole in the prevention of invasive fungal diseases in patients with haematological malignancies: a retrospective study. J Mycol Med. 2018;28(2):379–83.
Wang Y, Xing Y, Chen L, et al. Fluconazole versus mould-active triazoles for primary antifungal prophylaxis in adult patients with acute lymphoblastic leukemia: clinical outcome and cost-effectiveness analysis. Int J Hematol. 2018;107(2):235–43.
Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol. 2004;57(2):218–22.
Krishna G, Moton A, Ma L, Medlock MM, McLeod J. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53(3):958–66.
Pille S, Bohmer D. Options for artificial nutrition of cancer patients. Strahlenther Onkol. 1998;174:52–5.
Vehreschild MJT, Meissner AMK, Cornely OA, et al. Clinically defined chemotherapy-associated bowel syndrome predicts severe complications and death in cancer patients. Haematologica. 2011;96(12):1855–60.
Sansone-Parsons A, Krishna G, Calzetta A, et al. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob Agents Chemother. 2006;50(5):1881–3.
Kraft WK, Chang PS, van Iersel MLPS, Waskin H, Krishna G, Kersemaekers WM. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother. 2014;58(7):4020–5.
Krishna G, Ma L, Martinho M, O'Mara E. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole new tablet and capsule formulations relative to oral suspension. Antimicrob Agents Chemother. 2012;56(8):4196–201.
Krishna G, Ma L, Martinho M, Preston RA, O'Mara E. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother. 2012;67(11):2725–30.
Duarte RF, López-Jiménez J, Cornely OA, et al. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother. 2014;58(10):5758–65.
Cornely OA, Duarte RF, Haider S, et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother. 2016;71(3):718–26.
Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–59.
Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–47.
Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44(1):2–12.
Shen JX, Krishna G, Hayes RN. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J Pharmaceut Biomed Anal. 2007;43(11):228–36.
de Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21.
van Iersel MLPS, Rossenu S, de Greef R, Waskin H. A population pharmacokinetic model for a solid oral tablet formulation of posaconazole. Antimicrob Agents Chemother. 2018;62(7):e02465–e2517.
Li H, Wei Y, Zhang S, et al. Pharmacokinetics and safety of posaconazole administered by intravenous solution and oral tablet in healthy Chinese subjects and effect of food on tablet bioavailability. Clin Drug Investig. 2019;39(11):1109–16.
Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother. 2014;58(11):6993–5.
Cumpston A, Caddell R, Shillingburg A, et al. Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies. Antimicrob Agents Chemother. 2015;59(8):4424–8.
Belling M, Kanate AS, Shillingburg A, et al. Evaluation of serum posaconazole concentrations in patients with hematological malignancies receiving posaconazole suspension compared to the delayed-release tablet formulation. Leuk Res Treatment. 2017;2017:3460892.
Durani U, Tosh PK, Barreto JN, et al. Retrospective comparison of posaconazole levels in patients taking the delayed-release tablet versus the oral suspension. Antimicrob Agents Chemother. 2015;59(8):4914–8.
Leclerc E, Combarel D, Uzunov M, et al. Prevention of invasive Aspergillus fungal infections with the suspension and delayed-release tablet formulations of posaconazole in patients with haematologic malignancies. Sci Rep. 2018;8(1):1681.
Pham AN, Bubalo JS, Lewis JS 2nd. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses. 2016;59(4):226–33.
Liebenstein TK, Widmer KM, Fallon MJ. Retrospective analysis of goal drug level attainment of posaconazole for invasive fungal infection prophylaxis in patients with acute myeloid leukemia pre- and post-switch to tablet formulation. J Oncol Pharm Pract. 2018;24(8):599–603.
Lenczuk D, Zinke-Cerwenka W, Greinix H, et al. Antifungal prophylaxis with posaconazole delayed-release tablet and oral suspension in a real-life setting: plasma levels, efficacy, and tolerability. Antimicrob Agents Chemother. 2018;62:6.
Suh HJ, Kim I, Cho JY, et al. Comparison of plasma concentrations of posaconazole with the oral suspension and tablet in Korean patients with hematologic malignancies. Infect Chemother. 2017;49(2):135–9.
Furuno JP, Tallman GB, Noble BN, et al. Clinical outcomes of oral suspension versus delayed-release tablet formulations of posaconazole for prophylaxis of invasive fungal infections. Antimicrob Agents Chemother. 2018;62:10.
Boglione-Kerrien C, Picard S, Tron C, et al. Safety study and therapeutic drug monitoring of the oral tablet formulation of posaconazole in patients with haematological malignancies. J Cancer Res Clin Oncol. 2018;144(1):127–34.
Martson AG, Veringa A, van den Heuvel ER, et al. Posaconazole therapeutic drug monitoring in clinical practice and longitudinal analysis of the effect of routine laboratory measurements on posaconazole concentrations. Mycoses. 2019;62(8):698–705.
Kosmidis C, Rodriguez-Goncer I, Rautemaa-Richardson R, et al. Therapeutic drug monitoring and adverse events of delayed-release posaconazole tablets in patients with chronic pulmonary aspergillosis. J Antimicrob Chemother. 2019;74(4):1056–61.
Petitcollin A, Boglione-Kerrien C, Tron C, et al. Population pharmacokinetics of posaconazole tablets and Monte Carlo simulations to determine whether all patients should receive the same dose. Antimicrob Agents Chemother. 2017;61:11.
Peterlin P, Chauvin C, Le Gouill S, et al. Fungal prophylaxis with a gastro-resistant posaconazole tablet for patients with hematological malignancies in the POSANANTES study. Antimicrob Agents Chemother. 2018;62:2.
Lewis RE, Kontoyiannis DP, Viale P, Sarpong EM. Using state transition models to explore how the prevalence of subtherapeutic posaconazole exposures impacts the clinical utility of therapeutic drug monitoring for posaconazole tablets and oral suspension. Antimicrob Agents Chemother. 2019;63:12.
Pettit NN, Miceli MH, Rivera CG, et al. Multicentre study of posaconazole delayed-release tablet serum level and association with hepatotoxicity and QTc prolongation. J Antimicrob Chemother. 2017;72(8):2355–8.
Kersemaekers WM, Dogterom P, Xu J, et al. Effect of a high-fat meal on the pharmacokinetics of 300-milligram posaconazole in a solid oral tablet formulation. Antimicrob Agents Chemother. 2015;59(6):3385–9.
Witticke D, Seidling HM, Klimm HD, Haefeli WE. Do we prescribe what patients prefer? Pilot study to assess patient preferences for medication regimen characteristics. Patient Prefer Adherence. 2012;6:679–84.
Cojutti PG, Candoni A, Lazzarotto D, et al. Co-administration of proton pump inhibitors and/or of steroids may be a risk factor for low trough concentrations of posaconazole delayed-released tablets in adult patients with haematological malignancies. Br J Clin Pharmacol. 2018;84(11):2544–50.
Phillips K, Cirrone F, Ahuja T, Siegfried J, Papadopoulos J. Posaconazole versus voriconazole as antifungal prophylaxis during induction therapy for acute myelogenous leukemia or myelodysplastic syndrome. J Oncol Pharm Pract. 2019;25(2):398–403.
Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18(7):527–39.
Chen L, Wang Y, Zhang T, et al. Utility of posaconazole therapeutic drug monitoring and assessment of plasma concentration threshold for effective prophylaxis of invasive fungal infections: a meta-analysis with trial sequential analysis. BMC Infect Dis. 2018;18(1):155.
Acknowledgements
The authors thank the participants of the study.
Funding
The design, study conduct, and financial support for this research were provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. They have also funded the journals Rapid Service fee associated with manuscript publication.
Medical Writing and Editorial Assistance
Medical writing assistance, supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, was provided by Jennifer M. Kulak, PhD, of ApotheCom (Yardley, PA) in the preparation of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
KL, DW, JL, HC, TZ, HD, LC, and XMZ contributed to acquisition of the data and reviewing/revising the manuscript. HN contributed to acquisition of the data, interpretation of the results, and reviewing/revising the manuscript. EM contributed to analysis of the data and reviewing/revising the manuscript. GAW and HW contributed to study design, analysis of data, interpretation of results, and reviewing/revising the manuscript. JJ contributed to interpretation of results and reviewing/revising the manuscript. YQ contributed to analysis of the data. All authors reviewed the final version of the manuscript and agreed with its submission.
Disclosures
Kaiyan Liu, Depei Wu, Junmin Li, Hu Chen, Hongmei Ning, Ting Zhao, Haiping Dai, and Li Chen have no conflicts to disclose. Eric Mangin, Hetty Waskin, and Xu Min Zhao are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). Gregory A. Winchell is a subcontractor to Certara providing paid services to MSD. Jun Jiang and Yanping Qiu were employees of MSD at the time of the study. Hetty Waskin holds stock in Merck & Co., Inc., Kenilworth, NJ, USA, and is an author on a submitted patent application. Xumin Zhao has changed affiliation from Merck & Co., Inc., Kenilworth, NJ, USA, to Bayer Healthcare Company Limited.
Compliance with Ethics Guidelines
The study was conducted in accordance with the principles of Good Clinical Practice, and written informed consent was obtained from each participant. The protocol was reviewed and approved by independent ethics committees at all participating study centers (Peking University People’s Hospital, The First Affiliated Hospital of Soochow University, The 307th Hospital of Chinese People’s Liberation Army, and Shanghai Ruijin Hospital). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all participants included in the study.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available but are available from http://engagezone.merck.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12086820.
Rights and permissions
About this article
Cite this article
Liu, K., Wu, D., Li, J. et al. Pharmacokinetics and Safety of Posaconazole Tablet Formulation in Chinese Participants at High Risk for Invasive Fungal Infection. Adv Ther 37, 2493–2506 (2020). https://doi.org/10.1007/s12325-020-01341-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01341-x