Abstract
Introduction
Synthesis of evidence on the long-term use of first-line biologic therapy in patients with early rheumatoid arthritis (RA) is required. We compared the efficacy of up to 5 years’ treatment with first-line tumor necrosis factor inhibitors (TNFis) versus other treatment strategies in this population.
Methods
Previous systematic reviews, PubMed and the Cochrane Central Register of Controlled Trials were searched for randomized controlled trials (RCTs) involving treatment of methotrexate-naïve RA patients with first-line TNFis. Literature was synthesized qualitatively, and a meta-analysis conducted to evaluate American College of Rheumatology (ACR) responses, clinical remission defined by any standard measure, and Health Assessment Questionnaire Disability Index (HAQ) at Years 2 and/or 5.
Results
Ten RCTs involving 4306 patients [first-line TNFi, n = 2234; other treatment strategies (control), n = 2072] were included in the meta-analysis. Three studies were double-blind for the first 2 years, while seven were partly/completely open label during this period. Five studies reported data at Year 5; all were open label at this time point. At Year 2, ACR50 response, ACR70 response and remission rates were significantly improved with first-line TNFi versus control in double-blind RCTs [log-odds ratio (OR) 0.32 [95% confidence interval (CI) 0.02, 0.62; p = 0.035], log-OR 0.48 (95% CI 0.20, 0.77; p = 0.001), and log-OR 0.44 (95% CI 0.13, 0.74; p = 0.005), respectively], but not in open-label studies. No significant between-group differences were observed in mean HAQ at Year 2 in double-blind or open-label RCTs or in ACR response or remission outcomes at Year 5.
Conclusion
In double-blind studies, 2-year efficacy outcomes were significantly improved with first-line TNFi versus other treatment strategies in patients with MTX-naïve RA. No significant differences in these outcomes were observed when data from open-label RCTs were considered on their own. Further data on the efficacy of TNFi therapy over ≥ 2 years in patients with methotrexate-naïve RA are required.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain Language Summary
Rheumatoid arthritis (RA) is a disease of the joints and surrounding tissue, which often becomes worse over time. It causes inflammation, pain, stiffness and swelling that can destroy the joints and lead to disability. Biologics are a type of disease-modifying antirheumatic drug (DMARD) that suppress the immune system and reduce inflammation in the joints, thereby preventing joint damage.
Messenger proteins such as tumor necrosis factor (TNF) play an important role in inflammation. Tumor necrosis factor inhibitors (TNFis) are biologic drugs that block TNF and can reduce or stop inflammation in patients with RA. Currently, TNFis are not often used as the first treatment for patients with RA. Patients usually receive other drugs (conventional synthetic DMARDs) first.
Doctors face the challenge of identifying which type of DMARD to use first in patients with RA. Although previous studies have demonstrated the short-term benefits of using biologics as the first treatment, the longer-term effects of doing this have yet to be proven.
We conducted this analysis to find out if patients with RA who were first treated with TNFis in clinical trials had long-term improvements in their disease compared with patients receiving other treatments. We conducted a thorough review of the literature and used a meta-analysis approach to combine data from relevant randomized controlled trials. We found that, in certain types of tightly controlled trials, using TNFis as the first treatment can improve disease in the long term (up to 2 years at least). However, further studies are required to confirm this finding.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting 0.5–1% of the general population [1]. The disease is characterized by persistent joint inflammation, pain, stiffness and swelling that can lead to irreversible damage and disability if not adequately treated [2]. Twenty years ago, authorization of the first biologic drugs for use in RA, which act by inhibiting the activity of tumor necrosis factor (TNF), initiated a new age of treatment [2, 3]. Since then, biologic drugs possessing different mechanisms of action (e.g., B-lymphocyte depletion, T-cell co-stimulation modulation and interleukin receptor antagonism) have also become part of the therapeutic landscape [2]. Today, RA is undoubtedly one of the most studied diseases in rheumatology, for which the highest number of biologic drugs are approved, with TNF inhibitors (TNFis) the largest group.
Since their introduction into clinical practice, biologic drugs have proven to be effective in patients with RA who have had inadequate response to previous conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as methotrexate (MTX). Initially, biologics were used to treat patients with long-standing disease who had failed multiple prior csDMARDs. Data from the British Society for Rheumatology Biologics Register published in 2005 showed that mean disease duration at first initiation of TNFi therapy was 14 years. Patients beginning on TNFis had previously received a mean of four different DMARDs [4]. However, with the emergence of new diagnostic tools (e.g., immunological markers and imaging techniques) and new therapeutic goals (e.g., reaching clinical and radiological remission, and maintaining productivity and quality of life), the global understanding of RA has begun to shift. In particular, recognition of the importance of preventing radiological progression and joint damage has led to prioritization of early diagnosis and treatment [5]. As a consequence, the time between RA diagnosis and administration of first biologic treatment has gradually decreased [6]. In line with this trend, randomized controlled trials (RCTs) have been conducted to formally assess the efficacy of biologic drugs in the first-line treatment of RA [7,8,9].
Today, of the ten available biologic DMARDs (bDMARDs) approved by the European Medicines Agency for use in RA, seven (abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, and tocilizumab) are indicated for the treatment of adult patients with severe, active, and progressive disease not previously treated with MTX or any other csDMARD. However, due to both economic constraints associated with the use of biologic therapy and the concerns of some expert healthcare professionals, the use of biologic drugs in MTX-naïve RA has not yet become a widespread component of everyday practice [10, 11]. European League Against Rheumatism (EULAR) recommendations for the management of RA with synthetic and biological disease-modifying drugs (2016) state that “Therapy with DMARDs should be started as soon as the diagnosis of RA is made” and that “MTX should be part of the first treatment strategy” [12]. Use of early bDMARD treatment, including an induction regimen with subsequent withdrawal of bDMARDs, was debated during the development of the recommendations but no consensus was achieved. Members of the recommendations task force noted that there was a “lack of evidence for superiority of such therapy compared with the use of MTX plus glucocorticoid” and “when placed in the context of a treat-to-target strategy, [initial use of csDMARDs] yields equal results in the long-term”. The recommendations also state that “the cost-effectiveness of first-line bDMARD therapy, especially in light of the reasons just mentioned, is very poor” [12]. Thus, while there is great interest in the long-term benefits of using biologics in MTX-naïve RA, there is a discrepancy between already available (and approved) treatment options and current professional recommendations.
The high price of biologic drugs has been a barrier to their widespread use in many countries [13, 14]. However, the introduction of biosimilar versions of RA-approved drugs from 2013 onwards has enabled biologic therapy to be offered at considerably reduced cost [15, 16]. Currently, biosimilars of three of the five ‘originator’ TNFis (adalimumab, etanercept, infliximab) are approved for use in RA in Europe. The availability of biosimilars has also led to a reduction in the price of originator biologics [17]. Currently, there is a shortage of up-to-date economic evaluations of biologics (originators and/or biosimilars) in MTX-naïve RA that incorporate these recent reductions in costs. Before such cost-effectiveness analyses can be properly performed, good-quality input data on the relative long-term effectiveness of biologics and csDMARDs, based on the meta-analysis of RCTs, are needed.
The benefits of biologic therapy given first line over delayed therapy (i.e., biologics given later in the treatment line after MTX failure/intolerance) have been demonstrated for up to 1 year in recent systematic reviews/meta-analyses [7,8,9]; however, longer-term comparisons have not been performed. The aims of our study were, therefore, to systematically review the literature then qualitatively review and quantitatively synthesize available evidence on the long-term efficacy of first-line treatment with TNFi (adalimumab, certolizumab pegol, etanercept, golimumab, or infliximab) versus other treatment strategies in MTX-naïve RA.
Methods
Systematic Literature Search
To identify evidence in the literature published before January 2015, we used two systematic reviews and meta-analyses on the first-line biologic treatment of RA: the Cochrane review of Singh et al. published in 2017 [7] and the 2018 work of Cai et al. [8]. Both included and excluded studies reported by Singh et al. were reviewed, as were lists of ongoing RCTs from that publication. A detailed list of excluded studies was not reported by Cai et al.
For the period from January 2015 to July 2018, we conducted a systematic search in PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL). Our search included the Medical Subject Headings term for RA, the Cochrane filter for RCTs, and the international non-proprietary name of any biologic drug indicated for the treatment of RA (abatacept, adalimumab, anakinra, certolizumab, etanercept, golimumab, infliximab, rituximab, sarilumab and tocilizumab). As we focus on bDMARDs in this review, targeted synthetic DMARDs, such as baricitinib and tofacitinib, were not included. We added a selection of keywords to narrow our search to studies involving treatment-naïve patients with RA. The keywords most frequently used to identify treatment-naïve patients with RA in the RCT publications retrieved by Singh et al. [7] and Cai et al. [8] were ‘naïve’ and ‘early’; therefore, we used these two keywords in our search, alongside some less commonly used ones (e.g., ‘untreated’). In addition, the acronyms of RCTs identified by Singh et al. and Cai et al. were included in our search strategy so that publications reporting long-term extension studies of these RCTs were identified. Full details of our PubMed and CENTRAL search strategies are shown in Supplementary Tables S1 and S2, respectively. Additional searches using trial identifiers and RCT acronyms in PubMed and Google Scholar [18] were conducted.
Selection of Studies
We considered studies as the unit of our analysis; therefore, multiple publications of the same study or its extensions were only retained if these reported new data on outcomes of interest from the initially randomized patient populations. Peer-reviewed journals were included without language restrictions. Review articles, pooled analyses, case studies and conference abstracts were excluded.
Eligible studies involved patients with a diagnosis of RA who were naïve to MTX therapy. Patients very recently (< 4 weeks) initiated on MTX were considered MTX-naïve. Treatment interventions could include any pharmacological therapeutic strategy for RA that started with TNFi treatment (adalimumab, certolizumab pegol, etanercept, golimumab or infliximab) with or without concomitant MTX (hereinafter ‘first-line TNFi treatment’; FL-TNFi). No distinction was made between originator and biosimilar TNFis. Control treatments could include any synthetic pharmacological therapeutic strategy for RA (i.e., placebo, csDMARDs and/or steroids), with or without concomitant MTX, that did not involve any biologic therapy at study initiation [although TNFi could be applied per protocol in a later phase of the study (hereinafter ‘delayed TNFi treatment’)]. Outcomes could include responses based on American College of Rheumatology (ACR)20, ACR50 or ACR70 criteria, clinical remission [defined by any standard measure (see “Data extraction”)], or the Health Assessment Questionnaire Disability Index (HAQ-DI, hereinafter ‘HAQ’), measured at Year 2 or Year 5 [19]. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Extraction
A Microsoft Excel spreadsheet was developed to capture the following details of each considered study by treatment arm: study name; reference; start year; treatment and dosing; duration of double-blind, open-label or strategic-treatment phase; funding (with one of two categories: ‘sponsored by pharmaceutical company’ and ‘no pharmaceutical company sponsor’) to be selected based on the information provided); baseline characteristics {number of randomized patients and patient gender, age, disease activity score [DAS, based on erythrocyte sedimentation rate (DAS28ESR) or C-reactive protein (DAS28CRP)], HAQ and duration of disease}; risk of bias assessment; number of patients at Year 2 and Year 5; and ACR20, ACR50, ACR70, HAQ and remission [based on ACR criteria, DAS in 28 or 44 joints (DAS28/DAS44), the Simple Disease Activity Index or Clinical Disease Activity Index] at Years 2 and 5. Missing DAS28 scores were calculated from DAS using the formula DAS28 = 1.072 × DAS + 0.938 [20]. If outcomes on multiple remission criteria were reported, we preferred DAS28ESR if available, otherwise the measure with the highest number of patients achieving remission in the entire study population was selected.
Risk of Bias Assessment
The Cochrane Risk of Bias Tool [21] was applied to all studies. Based on the overall assessment, studies were categorized as having a low, high or unknown risk of bias.
Data Synthesis
All outcomes were included on an intent-to-treat (ITT) basis, using the number of patients initially randomized. If not provided directly, the nearest even number of patients achieving the desired outcome was calculated from the percentages provided in tables or graphs. GraphClick 3.0.3 was used to retrieve data from graphs (Arizona Software, AZ, USA).
In the case of multiple-arm studies, arms were pooled into one of two groups: FL-TNFi or ‘other strategy’ (control group). For complex study designs, one of the two following criteria must have been met for study arms to be included: (1) treatment changes (dose escalations or reductions, switches or additions of drugs) were applied according to predefined criteria based on the clinical status of patients (e.g., in strategic treat-to-target trials), or (2) possible changes (e.g., discontinuation of therapy) were applied to all randomized patients with equal probability. When the placebo (with or without csDMARD) arm of a double-blind study subsequently received open-label biologic therapy, this was considered delayed TNFi treatment and the arm was included in the control group. Multiple TNFi doses, as well as TNFi with or without concomitant MTX, were pooled in the FL-TNFi arm. Data reported for Month 18 were included in Year 2 analyses.
Using ACR70 data, we also investigated how the results of our analyses at Year 2 might have changed if the ASPIRE study, a 1-year double-blind RCT of first-line infliximab versus placebo in combination with MTX [22], had included a long-term extension study. Sensitivity analyses were also performed to determine how the analysis of Year 2 remission rates would change if patients from C-OPERA were excluded and how analyses for ACR70 and Year 2 remission rates would change if OPTIMA and COMET patients were excluded.
Statistical Analysis
All calculations were performed in the Stata14 statistical software package (StataCorp, TX, USA). Baseline characteristics of study populations were compared via one-way analysis of variance (ANOVA) and Chi-squared tests. Predefined outcomes were combined by random-effects meta-analysis according to the method of DerSimonian and Laird [23]. The significance threshold was set at p < 0.05.
Sources of between-study heterogeneity were explored by random-effects meta-regression of the following study-level variables: percentage of total study time used by the double-blind period, risk of bias (low/high or uncertain), funding category (pharmaceutical company/non-pharmaceutical company sponsor), type of TNFi, and percentage of patients who dropped out (treatment and control arms combined).
Results
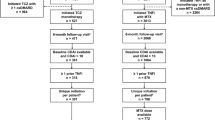
A PRISMA flow diagram for our systematic review is presented in Fig. 1. We identified 18 RCTs (reported in 61 publications) involving TNFi treatment of MTX-naïve patients with RA: Bejarano 2008 [24], BeSt [25,26,27,28,29,30,31,32], C-EARLY [33, 34], C-OPERA [35, 36], COMET [37, 38], Durez 2007 [39], Enbrel ERA [40,41,42], GO-BEFORE [43,44,45,46,47], GUEPARD [48], HIT HARD [49], HOPEFUL [50,51,52], IDEA [53, 54], Marcora 2006 [55], NEO-RACo [56,57,58,59,60,61,62], OPERA [63,64,65,66,67,68,69,70,71,72,73,74], OPTIMA [75,76,77], PREMIER [78,79,80,81,82,83] and Quinn 2005 [84]. None of these 18 RCTs involved biosimilar TNFi treatment.
Twelve of the 18 identified RCTs reported outcomes beyond Year 1. In two of these RCTs (C-EARLY and HOPEFUL), long-term results for the initially randomized populations could not be unambiguously determined. Therefore, we included 10 RCTs (BeSt, C-OPERA, COMET, Enbrel ERA, GO-BEFORE, IDEA, NEO-RACo, OPTIMA, PREMIER and Quinn 2005) in our qualitative review (see Table 1 for study details and citations for the 21 included publications and Supplementary Table S3 for excluded publications and reasons for exclusion).
The total number of patients in the 10 RCTs was 4697 (FL-TNFi: n = 2625; control: n = 2072). The RCTs comprised four studies of infliximab, two of etanercept, two of adalimumab, one of certolizumab pegol and one of golimumab. Patients withdrawn from treatment during the blinded study phases of the OPTIMA and COMET studies were excluded from the meta-analysis. In the withdrawn patient groups, treatment discontinuations were not mirrored in the control arms and treatment withdrawals were neither linked to remission nor performed during the open-label phase; therefore, patients could have received sub-optimal treatment. In OPTIMA, adalimumab was withdrawn after 1:1 re-randomization of responders receiving adalimumab plus MTX treatment. The adalimumab non-responder group was weighted correspondingly by 50.7% in order to preserve response rates in the ITT population. From the randomized population of OPTIMA [75], a total of 254 patients were excluded from the meta-analysis. In COMET, MTX was withdrawn in one of the four randomized study groups after 52 weeks of combination treatment with etanercept plus MTX (n = 137) [38]. After excluding OPTIMA and COMET patients withdrawn from treatment, 4306 patients (FL-TNFi: n = 2234; control: n = 2072) were included in the meta-analysis. Data included in the meta-analysis were from 15 publications on the 10 RCTs [26, 28, 36, 38, 42, 44, 46, 53, 56, 57, 75, 78,79,80, 84].
In addition to ongoing long-term extension studies of previously published RCTs, we identified 14 studies involving treatment of MTX-naïve RA patients with biologic agents (n = 3459) that were listed in clinical trial registries but had yet to be published in peer-reviewed journals [85,86,87,88,89,90,91,92,93,94,95,96,97,98]. Two of these studies [85, 89] started after August 1, 2017 and therefore were not included in the review of Singh et al. [7]. Among the 14 non-fully reported studies, the estimated completion date was not available for 2 [92, 97], 1 was published as a conference poster [93], another was stopped early due to issues with participant recruitment [98], and the results of 2 had not yet been published [87, 88]; the remaining 8 studies were still ongoing. Most of the 14 studies were sponsored by academic or clinical institutions; 2 were funded by industry [86, 95] and 2 by government [97, 98]. Seven of the studies were blinded RCTs [86, 88,89,90, 92, 93, 98], 5 were open-label randomized trials [85, 87, 91, 95, 96] and 2 were non-randomized open-label studies [94, 97]. Ten of the studies investigated the clinical efficacy of biologic agents in various settings [86, 88,89,90,91,92,93, 96,97,98], 2 investigated the pathophysiology of inflammatory processes [85, 95] and 2 evaluated radiological techniques [87, 94]. TNFis were investigated in 9 of the 14 studies (N = 1828) [87,88,89,90,91,92, 96,97,98].
Risk of Bias Analysis
Two RCTs (COMET and PREMIER) were double-blind at the end of Year 2 and therefore their risk of bias at this time point was categorized as low. All other studies except OPTIMA had an open-label arm at Year 2 and were considered high risk. Risk of bias at Year 2 for OPTIMA was categorized as unknown due to the partial unblinding of treatment for study non-responders. At Year 5, all studies were open label and therefore had a high risk of bias.
Methodological Heterogeneity of Studies
The methodology of included studies was diverse. Most were double-blind RCTs that varied in duration between 6 and 24 months, were followed by open-label or strategic-treatment extension phases of varying durations (≤ 10 years) and involved changes in treatment at different time points. One included study employed an open-label, treat-to-target strategy from randomization (BeSt). The longest double-blind period without treatment changes was 24 months (PREMIER). By Year 5, all studies were in open-label phases that featured modifications of originally assigned treatment. The methodological heterogeneity of studies is summarized in Fig. 2.
Characteristics of Patients with RA at Baseline
Baseline study populations were clinically homogenous and comprised MTX-naïve adult patients with RA, most of whom had active early disease. However, previous treatment exposure varied among studies. In IDEA, NEO-RACo and Quinn 2005, patients were naïve to all csDMARDs, whereas in BeSt, COMET, C-OPERA, PREMIER and OPTIMA, previous treatment with some csDMARDs (but not MTX) was permitted. In Enbrel ERA and GO-BEFORE, only previous treatment with MTX led to exclusion. Although comparisons of baseline age, disease duration, HAQ and DAS28 (with ANOVA) and gender distribution (with Chi-squared tests) revealed statistically significant differences between randomized study arms, these baseline characteristics were considered clinically comparable. Mean age was 51.0 years [range 47 (NEO-RACo) to 55 (BeSt) years; F(26, 4279) = 3.17, p < 0.001]. Mean disease duration was 12.4 months [range 3.9 (OPTIMA) to 49.2 (GO-BEFORE) months; F(26, 4279) = 39.27, p < 0.001]. Mean HAQ was 1.47 [range 0.9 (NEO-RACo) to 1.7 (COMET); F(26, 4279) = 10.04, p < 0.001]. Mean DAS28 was 5.85 [range 4.75 (IDEA) to 6.95 (Quinn 2005); F(26, 4279) = 26.10, p < 0.001]. Females comprised 74.7% of study participants [range 63.27% (NEO-RACo) to 100% (Quinn 2005); \(\chi_{{\left( {26} \right)}}^{2}\) = 63.8, p < 0.001]. In four trials that reported anti-citrullinated protein antibody (ACPA) status, 81% of patients were ACPA positive at baseline [range 67% (COMET) to 100% (C-OPERA)].
Results of the Meta-Analysis
For each outcome, and where possible, we performed meta-analysis for the subgroup of RCTs that were double blind for the first 2 years (COMET, OPTIMA and/or PREMIER), for the other subgroup of studies that were partly or completely open label during either time period (seven open-label RCT extensions of double-blind RCTs or open-label strategic-treatment RCTs) and for both types of study overall. Data were not available from all 10 studies for any of the assessed outcomes at either time point. At Year 2, ACR70 and at least one of the predefined remission outcomes were available for nine studies, ACR50 and ACR20 outcomes for eight studies and HAQ results for five studies. At Year 5, remission outcomes were available for four studies, ACR70 for three studies and ACR20 or ACR50 outcomes for two studies.
Year 2
Our analyses showed that for ACR20 at Year 2, FL-TNFi was not significantly different from the control group in either the double-blind RCT or open-label study subgroups, or overall (Supplementary Figure S1). For ACR50, ACR70 and remission outcomes, differences were significant in favor of FL-TNFi in the double-blind RCTs; no significant differences in these outcomes were revealed in analysis of open-label studies (Fig. 3a–c). In overall analyses including both double-blind RCTs and open-label studies, differences between the two groups were significant for ACR70 [log-OR 0.23 (95% CI 0.04, 0.43); p = 0.020; Fig. 3b] and remission outcomes [log-OR 0.23 (95% CI 0.04, 0.41); p = 0.015; Fig. 3c].
Meta-analyses of double-blind and open-label studies of first-line TNFi versus control at Year 2*. a ACR50 response at Year 2. b ACR70 response at Year 2. c Remission at Year 2. d Mean HAQ at Year 2. Weights are from random-effects analysis. *Double-blind RCTs with open-label extension at Year 2 were considered in the open-label subgroup. Data presented for Month 18 in the OPTIMA trial were considered among the double-blind results at Year 2. ACR American College of Rheumatology, CI confidence interval, HAQ Health Assessment Questionnaire Disability Index, OR odds ratio, RCT randomized controlled trial, SMD standardized mean difference, TNFi tumor necrosis factor inhibitor
For HAQ outcomes, standard deviations (SDs) were not reported for the Enbrel ERA and IDEA trials; therefore, the pooled SD of BeSt, GO-BEFORE, NEO-RACo, OPTIMA and PREMIER was used. In the IDEA trial, HAQ change versus baseline was used to calculate mean HAQ score. Meta-analysis revealed no significant difference between the TNFi and control groups in terms of mean HAQ (Fig. 3d).
Following our observation that the benefits of FL-TNFi in terms of response and remission could be demonstrated in the double-blind RCTs but that differences between FL-TNFi and control groups were not apparent in analyses of open-label studies, we investigated how the results might have changed if the ASPIRE study had included a long-term extension study. We projected Year 2 results for ASPIRE based on available Year 2 and Year 1 ACR70 results of studies with at least a 1-year-long double-blind randomized phase (COMET, Enbrel ERA, GO-BEFORE, PREMIER, Quinn 2005). Inclusion of projected ACR70 data for Year 2 of ASPIRE in the overall meta-analysis for this outcome further increased the between-group difference in favor of FL-TNFi (Supplementary Figure S2).
Year 5
At Year 5, five of the ten studies reported data on at least one relevant outcome (BeSt, Enbrel RA, GO-BEFORE, NEO-RACo and PREMIER); however, these trials were no longer double-blind at this time point. Therefore, data available at Year 5 were taken from the open-label extension periods of these studies. Meta-analyses determined that between-group differences were not significant for ACR20, ACR50, ACR70 or remission outcomes (Supplementary Fig. S3–S6). SD values for mean HAQ were not reported by any study at Year 5; therefore, meta-analysis of HAQ data at this time point was not performed.
Meta-Regression of Study-Level Variables
Very high heterogeneity was observed in all models, with I2 ranging from 87.9 to 99.7%. Random-effects meta-regression analysis revealed that low risk of bias was associated with significantly greater effect size in ACR70 (p = 0.046) and remission (p = 0.047) outcomes at Year 2 (Table 2). Other study-related variables (funding source, percentage of double-blind period within the 2-year observation, dropout rate) did not explain the heterogeneity of the effect size for any of the 2-year efficacy outcomes. No significant differences between different TNFi agents for any of the response or remission outcomes at Year 2 were observed.
Sensitivity Analyses
Although the approach to certolizumab pegol discontinuation in C-OPERA was very similar to that in the excluded arm in OPTIMA, patients in C-OPERA were followed up in an open-label phase, potentially allowing physicians to optimize patient response; therefore, C-OPERA patients were not excluded from our main analyses. We did, however, conduct a sensitivity analysis to determine how our results would differ if we had excluded C-OPERA patients. Only Year 2 remission rates were reported in C-OPERA. Although treatment withdrawal in the certolizumab pegol arm would indicate a diminished difference between the FL-TNFi and control groups suggesting a positive effect on the overall effect size after removal of C-OPERA data, the effect size of Year 2 remission rates decreased and became non-significant [log-OR 0.20 (95% CI – 0.002, 0.41); p = 0.067]. We also conducted a sensitivity analysis to establish how the inclusion of all patients from OPTIMA and COMET would influence our results. Following inclusion of these patients, differences between the FL-TNFi and control groups for ACR70 [log-OR: 0.18 (95% CI – 0.003, 0.37); p = 0.064] and remission outcomes [log-OR: 0.19 (95% CI – 0.014, 0.39); p = 0.068] became non-significant.
Discussion
This systematic review and meta-analysis has demonstrated the long-term (2-year) benefits of FL-TNFi treatment versus other treatment strategies in MTX-naïve patients with RA participating in RCTs with a double-blind, randomized, parallel-group design. In contrast, no statistically significant benefits were observed at this time point in open-label studies (randomized strategic-treatment studies or RCT extensions). At Year 2, ACR50, ACR70 and remission outcomes were significantly improved in FL-TNFi versus control groups in double-blind RCTs but not in open-label studies. In terms of mean HAQ, no significant differences between TNFi and control groups were observed at Year 2. At Year 5, data collected during open-label treatment in three studies did not show any significant between-group differences in response and remission outcomes. Meta-regression analysis of Year 2 outcomes did not reveal significant differences between individual TNFi agents. Our sensitivity analyses demonstrated that decisions about the inclusion or exclusion of individual studies could influence conclusions regarding the long-term efficacy advantage of FL-TNFi treatment.
To our knowledge, our study is the first meta-analysis focusing on the long-term efficacy outcomes of FL-TNFi treatment in MTX-naïve RA. Other meta-analyses have established the short-term efficacy benefits of using first-line biologic therapy in MTX/csDMARD-naïve RA [7, 8, 99]. In a small, indirect, pairwise meta-analysis of six RCTs for up to 1 year, all included biologic regimens demonstrated a significantly higher likelihood of achieving an ACR20 response than MTX alone. Furthermore, all but one biologic drug showed significant differences in ACR50 and ACR70 responses versus MTX monotherapy [99]. In a systematic review and network meta-analysis including 19 trials, Singh et al. demonstrated that biologic agents in combination with MTX were associated with clinically meaningful benefits in terms of ACR50, remission and HAQ outcomes versus MTX alone for up to 1 year [7]. In a network meta-analysis including 20 trials, Cai et al. showed that biologics used in combination with MTX were associated with significant improvements in ACR20, ACR50, ACR70 and remission outcomes compared with csDMARDs alone [8]. In that analysis, the majority of included studies had follow-up periods of less than 1 year, with the exception of COMET and PREMIER, which lasted 104 weeks. More recently, a comprehensive network meta-analysis by Donahue et al. demonstrated the 1-year benefit of immediate TNFi treatment when compared with MTX in studies with double-blind randomized designs in terms of clinical efficacy (ACR50), joint damage (Sharp van der Heijde Score) and safety profile [9]. Our results demonstrated that the benefits of FL-TNFi therapy are retained over 2 years, at least in double-blind randomized studies. Data at Year 5 were insufficient to draw well-founded conclusions at that time point. Despite the similar objectives and conclusions of previous meta-analyses, they included a substantially different and incomplete range of studies.
We aimed to include a broad range of studies of heterogeneous design, as healthcare professionals may adopt different TNFi treatment strategies (e.g., early or non-first line/delayed) depending on local therapeutically and/or economically focused guidelines, as well as the individual preferences of patients [100]. A potential strength of our work is that we applied a thorough search for secondary publications to identify relevant long-term outcomes. Of the 13 study publications included in the meta-analysis, only 3 were primary publications, while 10 studies were secondary publications of the studies of interest. However, the heterogeneity of treatment strategies and designs, as well as insufficient reporting of outcomes in long-term studies represented a key methodological challenge for the quantitative synthesis of the results; therefore, caution is required when interpreting the results of our analysis. A possible limitation of our search strategy is that we relied on the systematic reviews of Singh et al. [7] and Cai et al. [8] for identifying studies involving MTX-naïve patients from the period before 2015. Therefore, potentially eligible studies that were not listed among the included or excluded trials in these two systematic reviews may have been missed in our study.
Although the benefits of FL-TNFi treatment versus other treatment strategies for most Year 2 efficacy outcomes were demonstrated in RCTs with a double-blind design, meta-regression analysis did not reveal an association between the percentage of the 2-year period that was double-blind and the effect size of efficacy outcomes. However, low risk of bias was associated with a greater effect size for several efficacy outcomes. The risk of bias and the percentage of total study time that was double-blind in the included studies were closely, but not fully, related. At Year 2, only two studies (COMET and PREMIER) were considered at low risk of bias. In both of these studies, 100% of the 2-year study period was double-blind. Although the same applied to OPTIMA, this study was categorized as having an unknown risk of bias due to partial unblinding of treatment for non-responders. For the remaining studies (categorized as low or unknown risk), the percentage of total study time that was double-blind varied from 0 to 77%. Although the number of studies included in the meta-regression was too low to detect statistically significant results, the consistently positive coefficients of characteristic features of double-blind parallel studies were concordant with our hypothesis that double-blind parallel designs have a greater ability to demonstrate the long-term benefits of starting immediate TNFi treatment versus other treatment strategies.
Data from open-label studies and extensions of double-blind RCTs suggest that first-line non-biologic treatment can be as effective as FL-TNFi treatment over a 2-year period. The initial benefit of first-line biological therapy can be offset by strategic treat-to-target protocols (as employed in BeSt, NEO-RACo, and IDEA) if both patients and rheumatologists adhere to tight disease management controls. However, adoption of treat-to-target recommendations in RA care is far from universal in Europe. A multi-country study (performed in France, Germany, Italy, Spain, and the UK in 2014) revealed that a treat-to-target approach was not adopted in almost half of patients with RA, and if applied it was mostly used in patients at ≥ 2 years since RA diagnosis and thus not in early RA [101]. For three RCTs (GO-BEFORE, C-OPERA and Quinn 2005), initial treatment differences between randomized arms were equalized during subsequent open-label extension phases. In the GO-BEFORE study, initiation of biological therapy was allowed in the control arm in the event of suboptimal response, while in C-OPERA and Quinn 2005, TNFi treatment was discontinued in all patients after the double-blind period. While pragmatic treatment escalations reflect clinical practice, the discontinuation of biologics in the absence of sustained clinical remission is unlikely in a real-life setting. In C-OPERA, non-responders moved to rescue treatment during the double-blind phase, and in the open-label extension phase of Quinn 2005, treatment escalation with csDMARD combinations was possible. In the COMET and OPTIMA studies, treatment de-escalation occurred during the double-blind phase in the FL-TNFi arms without symmetrical changes in the control groups. To mitigate the potential negative bias due to these treatment changes, the MTX withdrawal arm of COMET and patients in OPTIMA who were randomized to adalimumab withdrawal after responding to adalimumab were removed from our main analysis. Sensitivity analysis demonstrated that these changes significantly affected ACR70 and remission results at Year 2. Overall, these findings suggest that, in early RA, the choice of first-line treatment alone does not guarantee optimal long-term outcomes unless a proper disease management strategy is also employed.
In accordance with approved TNFi labels, we included patients with established RA in our analysis. However, recent research suggests that prevention of RA is possible in the earliest phase of this inflammatory disease, before a diagnosis can be established. Early treatment with effective DMARD therapy and subsequent achievement of remission in the early phase of RA (i.e., within the ‘window of opportunity’) may reverse the autoimmune response in some patients and lead to improved long-term outcomes [5, 102]. Two recent studies (EMPIRE and DINORA) have evaluated immediate TNFi treatment in early inflammatory arthritis. In both studies, biologic induction treatment was given until sustained remission was achieved, but for no longer than 1 year. Although the EMPIRE trial could not demonstrate a statistically significant difference between etanercept plus MTX and MTX monotherapy at Week 78 [103], the 2-year DINORA study provided encouraging evidence that even a short course of infliximab plus MTX was more effective than MTX alone or placebo in achieving sustained disease reversal [104]. Because we excluded patients with early inflammatory arthritis not fulfilling the criteria for RA diagnosis from our study, the patient population included in our analysis may not be the right population to demonstrate the preventive effects of early biologic therapy. It should be noted, however, that early inflammatory arthritis may resolve spontaneously in some patients, remain undifferentiated or may develop into an arthropathy other than RA. Although several prognostic factors of progressive erosive disease have been identified (e.g., number of swollen joints, acute-phase reactants, rheumatoid factor, ACPAs, or imaging signs) and should inform treatment decisions, the search is ongoing for better predictive models of optimal therapeutic strategies [105].
We hope that the results of ongoing studies will provide the necessary additional evidence to draw firmer conclusions regarding the optimal treatment strategy for patients with MTX-naïve early RA. As of July 2018, we identified 14 clinical trials involving treatment of 3459 such patients with biologic drugs that had not had results published in peer-reviewed journals [85,86,87,88,89,90,91,92,93,94,95,96,97,98]. Hence, this area is undoubtedly one in which the volume of research is rapidly growing. Eight of the 13 identified ongoing or completed studies (one study was stopped [98]) involve treatment with TNFi drugs, with seven assessing clinical efficacy; of these, six are focusing on induction of remission. These new studies may add valuable knowledge about identifying relevant target patient populations, and the optimal timing of biologic therapy, for RA prevention.
The apparently poor cost-effectiveness of originator TNFis has been noted by EULAR and in cost-effectiveness analyses performed by the National Institute for Health and Clinical Excellence and others, contributing to the limited use of biologic therapy in MTX-naïve early RA [11, 12, 106]. However, these analyses utilized biologic drug prices before approval of biosimilar TNFis. The introduction of biosimilar drugs offers hope that cost barriers for use in first-line settings will be overcome in the near future [16, 107]. Reliable data from new meta-analyses such as this one can be used to inform new cost-effectiveness analyses that accurately reflect recent changes to the current treatment landscape, including the introduction of biosimilars. The availability of up-to-date clinical and pharmacoecomic evidence may also ultimately lead to changes in treatment guidelines.
Conclusions
To our knowledge, this is the first systematic review and meta-analysis to provide evidence on the efficacy of biologic treatment over 1 year in MTX-naïve early RA. In double-blind studies, 2-year ACR50/ACR70 responses and remission rate outcomes were significantly improved with first-line TNFis compared with other treatment strategies. However, no significant differences in these outcomes were observed in open-label studies, including those with strict treatment protocols. Heterogeneity of studies and lack of publication standards hampered the analyses, and a possible limitation of our search strategy was that we relied on previous systematic reviews to identify studies published before 2015. Further good-quality evidence is needed if the efficacy of biologic therapy over 2 years is to be established in patients with early RA, and especially in those with inflammatory arthritis, who were not included in this analysis. The economic value of long-term TNFi in this setting also remains to be determined, although cost-effectiveness analyses based on the findings reported here are already underway. Due to the considerable price reduction in biologic therapy following the introduction of biosimilars, immediate TNFi treatment may become an attractive treatment option in early RA, due to its short-term efficacy benefits and the promise of offering disease reversal and sustained remission for a considerable proportion of patients.
References
Silman A, Hochberg M. Epidemiology of the rheumatic diseases. 2nd ed. New York: Oxford University Press; 2001.
Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33:679–707.
Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27:55–62.
Watson K, Symmons D, Griffiths I, Silman A. The British Society for Rheumatology biologics register. Ann Rheum Dis. 2005;64(Suppl 4):iv42–3.
van Nies JA, Krabben A, Schoones JW, Huizinga TW, Kloppenburg M, van der Helm-van Mil AH. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis. 2014;73:861–70.
Dennison EM, Packham J, Hyrich K. The BSRBR-RA at 15 years. Rheumatology (Oxford). 2016;55:2093–5.
Singh JA, Hossain A, Mudano AS, et al. Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017;5:CD012657.
Cai W, Gu Y, Cui H, et al. The efficacy and safety of mainstream medications for patients with DMARD-naive rheumatoid arthritis: a network meta-analysis. Front Pharmacol. 2018;9:138.
Donahue KE, Gartlehner G, Schulman ER, et al. Drug therapy for early rheumatoid arthritis: a systematic review update. Agency for Healthcare Research and Quality (US). Report No: 18-EHC015-EF. 2018.
van der Velde G, Pham B, Machado M, et al. Cost-effectiveness of biologic response modifiers compared to disease-modifying antirheumatic drugs for rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken). 2011;63:65–78.
National Institute for Health and Care Excellence. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. 2015. https://www.nice.org.uk/guidance/ta375/documents/rheumatoid-arthritis-adalimumab-etanercept-infliximab-certolizumab-pegol-golimumab-abatacept-and-tocilizumab-review-id537-appraisal-consultation-document2. Accessed Oct 11, 2018.
Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–77.
Putrik P, Ramiro S, Kvien TK, et al. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country’s wealth? Ann Rheum Dis. 2014;73:2010–21.
Pentek M, Poor G, Wiland P, et al. Biological therapy in inflammatory rheumatic diseases: issues in Central and Eastern European countries. Eur J Health Econ. 2014;15(Suppl 1):S35–43.
Baji P, Pentek M, Czirjak L, et al. Efficacy and safety of infliximab-biosimilar compared to other biological drugs in rheumatoid arthritis: a mixed treatment comparison. Eur J Health Econ. 2014;15(Suppl 1):S53–64.
Brodszky V, Baji P, Balogh O, Pentek M. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ. 2014;15(Suppl 1):S65–71.
The Center for Biosimilars. Biosimilar competition has led to consistent price reduction in Europe. 2017. https://www.centerforbiosimilars.com/news/biosimilar-competition-has-led-to-consistent-price-reduction-in-europe. Accessed Oct 11, 2018.
Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLoS ONE. 2015;10:e0138237.
Hobbs KF, Cohen MD. Rheumatoid arthritis disease measurement: a new old idea. Rheumatology (Oxford). 2012;51(Suppl 6):vi21–7.
DAS28.nl. Alternative validated formulae. 2018. http://www.das-score.nl/das28/en/difference-between-the-das-and-das28/how-to-measure-the-das28/how-to-calculate-the-das28/alternative-validated-formulae.html. Accessed Aug 28, 2018.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011. The Cochrane Collaboration. http://handbook.cochrane.org.
St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Bejarano V, Quinn M, Conaghan PG, et al. Effect of the early use of the anti-tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis Rheum. 2008;59:1467–74.
Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–90.
Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146:406–15.
van der Kooij SM, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2009;68:914–21.
Klarenbeek NB, Guler-Yuksel M, van der Kooij SM, et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis. 2011;70:1039–46.
Markusse IM, Dirven L, Han KH, et al. Continued participation in a ten-year tight control treat-to-target study in rheumatoid arthritis: why keep patients doing their best? Arthritis Care Res (Hoboken). 2015;67:739–45.
Akdemir G, Markusse IM, Dirven L, et al. Effectiveness of four dynamic treatment strategies in patients with anticitrullinated protein antibody-negative rheumatoid arthritis: a randomised trial. RMD Open. 2016;2:e000143.
Markusse IM, Akdemir G, Dirven L, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Intern Med. 2016;164:523–31.
Bergstra SA, Landewe RBM, Huizinga TWJ, Allaart CF. Rheumatoid arthritis patients with continued low disease activity have similar outcomes over 10 years, regardless of initial therapy. Rheumatology (Oxford). 2017;56:1721–8.
Emery P, Bingham CO 3rd, Burmester GR, et al. Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naive patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from C-EARLY, a randomised, double-blind, placebo-controlled phase III study. Ann Rheum Dis. 2017;76:96–104.
Weinblatt ME, Bingham CO 3rd, Burmester GR, et al. A Phase III study evaluating continuation, tapering, and withdrawal of certolizumab pegol after one year of therapy in patients with early rheumatoid arthritis. Arthritis Rheumatol. 2017;69:1937–48.
Atsumi T, Yamamoto K, Takeuchi T, et al. The first double-blind, randomised, parallel-group certolizumab pegol study in methotrexate-naive early rheumatoid arthritis patients with poor prognostic factors, C-OPERA, shows inhibition of radiographic progression. Ann Rheum Dis. 2016;75:75–83.
Atsumi T, Tanaka Y, Yamamoto K, et al. Clinical benefit of 1-year certolizumab pegol (CZP) add-on therapy to methotrexate treatment in patients with early rheumatoid arthritis was observed following CZP discontinuation: 2-year results of the C-OPERA study, a phase III randomised trial. Ann Rheum Dis. 2017;76:1348–56.
Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–82.
Emery P, Breedveld F, van der Heijde D, et al. Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomized study. Arthritis Rheum. 2010;62:674–82.
Durez P, Malghem J, Nzeusseu Toukap A, et al. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum. 2007;56:3919–27.
Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–93.
Genovese MC, Bathon JM, Martin RW, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–50.
Genovese MC, Bathon JM, Fleischmann RM, et al. Long term safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol. 2005;32:1232–42.
Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–83.
Emery P, Fleischmann RM, Doyle MK, et al. Golimumab, a human anti-tumor necrosis factor monoclonal antibody, injected subcutaneously every 4 weeks in patients with active rheumatoid arthritis who had never taken methotrexate: 1-year and 2-year clinical, radiologic, and physical function findings of a phase III, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res (Hoboken). 2013;65:1732–42.
Baker JF, Conaghan PG, Emery P, Baker DG, Ostergaard M. Validity of early MRI structural damage end points and potential impact on clinical trial design in rheumatoid arthritis. Ann Rheum Dis. 2016;75:1114–9.
Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res (Hoboken). 2016;68:744–52.
Baker JF, Conaghan PG, Emery P, Baker DG, Ostergaard M. Relationship of patient-reported outcomes with MRI measures in rheumatoid arthritis. Ann Rheum Dis. 2017;76:486–90.
Soubrier M, Puechal X, Sibilia J, et al. Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology (Oxford). 2009;48:1429–34.
Detert J, Bastian H, Listing J, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis. 2013;72:844–50.
Yamanaka H, Ishiguro N, Takeuchi T, et al. Recovery of clinical but not radiographic outcomes by the delayed addition of adalimumab to methotrexate-treated Japanese patients with early rheumatoid arthritis: 52-week results of the HOPEFUL-1 trial. Rheumatology (Oxford). 2014;53:904–13.
Takeuchi T, Yamanaka H, Ishiguro N, et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis. 2014;73:536–43.
Tanaka Y, Yamanaka H, Ishiguro N, et al. Low disease activity for up to 3 years after adalimumab discontinuation in patients with early rheumatoid arthritis: 2-year results of the HOPEFUL-3 Study. Arthritis Res Ther. 2017;19:56.
Nam JL, Villeneuve E, Hensor EM, et al. Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Ann Rheum Dis. 2014;73:75–85.
Bissell LA, Hensor EM, Kozera L, et al. Improvement in insulin resistance is greater when infliximab is added to methotrexate during intensive treatment of early rheumatoid arthritis-results from the IDEA study. Rheumatology (Oxford). 2016;55:2181–90.
Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr. 2006;84:1463–72.
Leirisalo-Repo M, Kautiainen H, Laasonen L, et al. Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomised, double-blind, placebo-controlled study (the NEO-RACo Study). Ann Rheum Dis. 2013;72:851–7.
Rantalaiho V, Kautiainen H, Korpela M, et al. Targeted treatment with a combination of traditional DMARDs produces excellent clinical and radiographic long-term outcomes in early rheumatoid arthritis regardless of initial infliximab. The 5-year follow-up results of a randomised clinical trial, the NEO-RACo trial. Ann Rheum Dis. 2014;73:1954–61.
Kuusalo L, Puolakka K, Kautiainen H, et al. Impact of physicians’ adherence to treat-to-target strategy on outcomes in early rheumatoid arthritis in the NEO-RACo trial. Scand J Rheumatol. 2015;44:449–55.
Kuusalo LA, Puolakka KT, Kautiainen H, et al. Intra-articular glucocorticoid injections should not be neglected in the remission targeted treatment of early rheumatoid arthritis: a post hoc analysis from the NEO-RACo trial. Clin Exp Rheumatol. 2016;34:1038–44.
Kuusalo L, Puolakka K, Kautiainen H, et al. Patient-reported outcomes as predictors of remission in early rheumatoid arthritis patients treated with tight control treat-to-target approach. Rheumatol Int. 2017;37:825–30.
Vaananen T, Vuolteenaho K, Kautiainen H, et al. Glycoprotein YKL-40: a potential biomarker of disease activity in rheumatoid arthritis during intensive treatment with csDMARDs and infliximab.Evidence from the randomised controlled NEO-RACo trial. PLoS ONE. 2017;12:0183294.
Kuusalo L, Puolakka K, Kautiainen H, et al. High burden of adverse events is associated with reduced remission rates in early rheumatoid arthritis. Clin Rheumatol. 2018;37:1689–94.
Horslev-Petersen K, Hetland ML, Junker P, et al. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis. 2014;73:654–61.
Andersen T, Hvid M, Johansen C, et al. Interleukin-23 in early disease development in rheumatoid arthritis. Scand J Rheumatol. 2015;44:438–42.
Axelsen MB, Eshed I, Horslev-Petersen K, et al. A treat-to-target strategy with methotrexate and intra-articular triamcinolone with or without adalimumab effectively reduces MRI synovitis, osteitis and tenosynovitis and halts structural damage progression in early rheumatoid arthritis: results from the OPERA randomised controlled trial. Ann Rheum Dis. 2015;74:867–75.
Greisen SR, Moller HJ, Stengaard-Pedersen K, et al. Macrophage activity assessed by soluble CD163 in early rheumatoid arthritis: association with disease activity but different response patterns to synthetic and biologic DMARDs. Clin Exp Rheumatol. 2015;33:498–502.
Horslev-Petersen K, Hetland ML, Ornbjerg LM, et al. Clinical and radiographic outcome of a treat-to-target strategy using methotrexate and intra-articular glucocorticoids with or without adalimumab induction: a 2-year investigator-initiated, double-blinded, randomised, controlled trial (OPERA). Ann Rheum Dis. 2016;75:1645–53.
Kragstrup TW, Greisen SR, Nielsen MA, et al. The interleukin-20 receptor axis in early rheumatoid arthritis: novel links between disease-associated autoantibodies and radiographic progression. Arthritis Res Ther. 2016;18:61.
Krintel SB, Dehlendorff C, Hetland ML, et al. Prediction of treatment response to adalimumab: a double-blind placebo-controlled study of circulating microRNA in patients with early rheumatoid arthritis. Pharmacogenomics J. 2016;16:141–6.
Heftdal LD, Stengaard-Pedersen K, Ornbjerg LM, et al. Soluble CD206 plasma levels in rheumatoid arthritis reflect decrease in disease activity. Scand J Clin Lab Invest. 2017;77:385–9.
Kristensen AM, Stengaard-Pedersen K, Hetland ML, et al. Expression of soluble CD83 in plasma from early-stage rheumatoid arthritis patients is not modified by anti-TNF-alpha therapy. Cytokine. 2017;96:1–7.
Ornbjerg LM, Ostergaard M, Jensen T, et al. Hand bone loss in early rheumatoid arthritis during a methotrexate-based treat-to-target strategy with or without adalimumab-a substudy of the optimized treatment algorithm in early RA (OPERA) trial. Clin Rheumatol. 2017;36:781–9.
Brahe CH, Ostergaard M, Johansen JS, et al. Predictive value of a multi-biomarker disease activity score for clinical remission and radiographic progression in patients with early rheumatoid arthritis: a post hoc study of the OPERA trial. Scand J Rheumatol. 2018. https://doi.org/10.1080/03009742.2018.1464206.
Sode J, Krintel SB, Carlsen AL, et al. Plasma microRNA profiles in patients with early rheumatoid arthritis responding to adalimumab plus methotrexate vs methotrexate alone: a placebo-controlled clinical trial. J Rheumatol. 2018;45:53–61.
Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet. 2014;383:321–32.
Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72:64–71.
Emery P, Smolen JS, Ganguli A, et al. Effect of adalimumab on the work-related outcomes scores in patients with early rheumatoid arthritis receiving methotrexate. Rheumatology (Oxford). 2016;55:1458–65.
Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37.
van der Heijde D, Breedveld FC, Kavanaugh A, et al. Disease activity, physical function, and radiographic progression after longterm therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol. 2010;37:2237–46.
Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health-related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. J Rheumatol. 2012;39:63–72.
Landewe R, Smolen JS, Florentinus S, Chen S, Guerette B, van der Heijde D. Existing joint erosions increase the risk of joint space narrowing independently of clinical synovitis in patients with early rheumatoid arthritis. Arthritis Res Ther. 2015;17:133.
Stephens S, Botteman MF, Cifaldi MA, van Hout BA. Modelling the cost-effectiveness of combination therapy for early, rapidly progressing rheumatoid arthritis by simulating the reversible and irreversible effects of the disease. BMJ Open. 2015;5:e006560.
Moller B, Everts-Graber J, Florentinus S, Li Y, Kupper H, Finckh A. Low hemoglobin and radiographic damage progression in early rheumatoid arthritis: secondary analysis from a phase III trial. Arthritis Care Res (Hoboken). 2018;70:861–8.
Quinn MA, Conaghan PG, O’Connor PJ, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:27–35.
clinicaltrials.gov. NCT03492658. 2018. https://clinicaltrials.gov/ct2/show/NCT03492658. Accessed July 25, 2018.
clinicaltrials.gov. NCT02504268. 2017. https://clinicaltrials.gov/ct2/show/NCT02504268. Accessed July 15, 2018.
clinicaltrials.gov. NCT00901550. 2012. https://clinicaltrials.gov/ct2/show/NCT00901550. Accessed July 15, 2018.
clinicaltrials.gov. NCT00480272. 2017. https://clinicaltrials.gov/ct2/show/NCT00480272. Accessed July 15, 2018.
clinicaltrials.gov. NCT03160001. 2018. https://clinicaltrials.gov/ct2/show/NCT03160001. Accessed July 15, 2018.
clinicaltrials.gov. NCT01491815. 2018. https://clinicaltrials.gov/ct2/show/NCT01491815. Accessed July 15, 2018.
clinicaltrials.gov. NCT02935387. 2018. https://clinicaltrials.gov/ct2/show/NCT02935387. Accessed July 15, 2018.
clinicaltrials.gov. NCT00523692. 2007. https://clinicaltrials.gov/ct2/show/NCT00523692. Accessed July 15, 2018.
clinicaltrials.gov. NCT01245452. 2013. https://clinicaltrials.gov/ct2/show/NCT01245452. Accessed July 15, 2018.
clinicaltrials.gov. NCT02837146. 2016. https://clinicaltrials.gov/ct2/show/NCT02837146. Accessed July 15, 2018.
clinicaltrialregister.eu. EUCTR2011-004017-17-GB. 2013. https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-004017-17/GB. Accessed July 15, 2018.
clinicaltrialregister.eu. EUCTR2010-023910-30-GB. 2011. https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-023910-30/GB. Accessed July 15, 2018.
cris.nih.go.kr. KCT0000089. https://cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=1201. Accessed July 15, 2018.
isrctn.com. ISRCTN49682259. 2015. http://www.isrctn.com/ISRCTN49682259?q=&filters=conditionCategory:Musculoskeletal%20Diseases. Accessed July 15, 2018.
Albert DA. Are all biologics the same? Optimal treatment strategies for patients with early rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. J Clin Rheumatol. 2015;21:398–404.
Hifinger M, Hiligsmann M, Ramiro S, et al. Economic considerations and patients’ preferences affect treatment selection for patients with rheumatoid arthritis: a discrete choice experiment among European rheumatologists. Ann Rheum Dis. 2017;76:126–32.
Taylor PC, Alten R, Reino JJG, et al. Factors influencing the use of biologic therapy and adoption of treat-to-target recommendations in current European rheumatology practice. Patient Prefer Adherence. 2018;12:2007–14.
Nagy G, van Vollenhoven RF. Sustained biologic-free and drug-free remission in rheumatoid arthritis, where are we now? Arthritis Res Ther. 2015;17:181.
Nam JL, Villeneuve E, Hensor EM, et al. A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: the EMPIRE trial. Ann Rheum Dis. 2014;73:1027–36.
Stamm TA, Machold KP, Aletaha D, et al. Induction of sustained remission in early inflammatory arthritis with the combination of infliximab plus methotrexate: the DINORA trial. Arthritis Res Ther. 2018;20:174.
Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2017;76:948–59.
Stevenson M, Archer R, Tosh J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20:1–610.
Dorner T, Strand V, Cornes P, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis. 2016;75:974–82.
Acknowledgements
Funding
The authors received no funding for the analyses reported here. The article processing charges were funded by Celltrion Healthcare Co., Ltd (Incheon, Republic of Korea).
At the time of publication of the current article, the authors are performing additional work related to the analyses reported here; funding for this work will be provided by Celltrion Healthcare Co., Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the article to be published.
Medical Writing, Editorial, and Other Assistance
Medical writing support for this article (including copyediting and fact checking) was provided to the authors by Rick Flemming, PhD CMPP and Emma Evans, PhD CMPP at Aspire Scientific Limited (Bollington, UK) and was funded by Celltrion Healthcare Co., Ltd.
Disclosures
László Gulácsi has received consultancy and lecturing fees from Astellas, BMS, Celltrion, Egis Pharmaceuticals, GSK, Hikma, Hospira, Lilly Hungaria Ltd, MSD Hungary, Pfizer, Roche, Sandoz and UCB. Zsombor Zrubka used to be a full-time employee of Egis Pharmaceuticals, Janssen Cilag, Sandoz and Pfizer. Valentin Brodszky has received grants and personal fees from Celltrion, Egis Pharmaceuticals, Pfizer and Sager Pharma. Fanni Rencz has received consultancy fees from Celltrion and Hospira. Rieke Alten has received honoraria from Celltrion. Zoltán Szekanecz has received consultancy and lecturing fees from AbbVie, Amgen, BMS, Lilly, MSD, Novartis, Pfizer, Roche and UCB. Márta Péntek has received grants and personal fees from Celltrion, Egis Pharmaceuticals, Merck, Pfizer and Sager Pharma.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
All data used in this systematic review and meta-analysis are available in the published sources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7498988.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gulácsi, L., Zrubka, Z., Brodszky, V. et al. Long-Term Efficacy of Tumor Necrosis Factor Inhibitors for the Treatment of Methotrexate-Naïve Rheumatoid Arthritis: Systematic Literature Review and Meta-Analysis. Adv Ther 36, 721–745 (2019). https://doi.org/10.1007/s12325-018-0869-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0869-8