Abstract
Introduction
The FDA recently approved three intragastric balloon (IGB) devices, ReShape, ORBERA™, and Obalon for treatment of obesity. Given the high cost, complication risk, and invasiveness of bariatric surgery, IGB treatment may present a safer and lower cost option for weight reduction. IGBs are generally placed in the stomach endoscopically for up to 6 months to reduce gastric capacity, enhance feelings of fullness, and induce weight loss. The mechanism of action likely involves stimulation of gastric mechanoreceptors triggering short-acting vagal signals to brain regions implicated in satiety. Balloon efficacy may be influenced by balloon volume, patient gastric capacity, and treatment duration.
Methods
This review focused on eight recent (2006–present) randomized controlled trials (RCTs) comparing percentage total body weight loss (%TBWL) between IGB and control groups including three reviewed by the FDA. %TBWL based on the reviewed studies was also compared with bariatric surgery and pharmacotherapy.
Results
Of the eight IGB studies, five had balloon treatment duration of 6 months. Efficacy at 6 months, based on a pooled weighted-mean %TBWL, was 9.7%, and the control-subtracted %TBWL was 5.6%. When one study without SDs was removed, the weighted mean %TBWL was 9.3 ± 5.7% SD, and control-subtracted %TBWL was 5.5 ± 7.8%, which was statistically greater than controls. IGB showed lower efficacy than bariatric surgery (median weight loss of 27% for Rouen-Y gastric bypass (RYGB). The control-subtracted %TBWL over 6 months of 5.5–5.6% is less than the most efficacious FDA-approved weight loss drug, Qsymia. At the recommended dose, Qsymia has a placebo-subtracted %TBWL at 6 months of approximately 6.6%. The weighted mean reported incidence of serious adverse events (SAEs) in the IGB group across all eight studies was 10.5%. Only six of the eight reviewed studies reported adverse events (AEs) in the IGB group, with a pooled reported incidence of 28.2%. Recently, the FDA reported new AEs including acute pancreatitis with ReShape and ORBERA™.
Conclusion
Based on the available evidence, it is unlikely that IGB use will supplant other forms of obesity treatment. The estimated cost of endoscopic balloon implantation and retrieval is US $8,150. Collectively, a relatively small control-subtracted %TBWL and the potential for serious complications makes IGB unlikely to become widely adopted. Given the recent FDA warning, IGB longevity on the market is questionable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the USA, 68% of adults are overweight (BMI = 25.0–30.0) or obese (BMI > 30.0) [1]. Currently, the only effective long-term intervention is bariatric surgery, which markedly reduces gastric capacity. However, such procedures are limited to candidates with a BMI ≥40 without comorbidities or ≥35 with comorbidities. The American Society for Metabolic and Bariatric Surgery reports an overall serious complication rate of 4% and mortality rate of 0.1% [2]. Recent FDA approval of three types of intragastric balloons (IGB) for treating obese patients with a BMI ≥30 offers a potentially safer and less invasive method for reducing gastric capacity and inducing weight loss. Although IGB may proffer lower serious complication risk than bariatric surgery, consensus on the effectiveness and safety of IGB treatment is lacking. Thus, this review evaluated the recent literature on IGB to assess the risk and benefits, with a focus on two of the recently FDA-approved IGB devices, ReShape and ORBERA™. Obalon, a swallowable IGB device, received FDA approval more recently and is discussed separately under “New and Future IGBs”.
IGB Development and Use
IGB as a treatment for obesity evolved from reports that, in certain cases, prolonged bezoar (indigestible mass) presence in the stomach leads to weight loss [3]. The Garren–Edwards Gastric Bubble (GEB; American Edwards Company) was the first FDA-approved IGB in the USA in 1985 [3]. The device was placed endoscopically, filled with 220 mL of air, and removed after 3 months. The GEB was subsequently withdrawn by the FDA in 1990 following reports of numerous complications [4,5,6,7]. Double-blind, sham-controlled studies showed lack of efficacy, likely due to insufficient volume. Adverse effects of GEB included small-bowel obstruction, secondary to unplanned deflation, gastric ulcers with GI hemorrhage, and gastric perforation, primarily due to premature deflation of the rigid polyurethane balloon [4,5,6,7]. For a review of devices used between 1990 and 2006, please see the Cochrane review [3].
Current IGB devices are delivered into the stomach to reduce gastric capacity and elicit feelings of fullness. In single balloon devices, the IGB is filled with saline solution to volumes of 400–700 cc, but the ReShape double balloon can be filled to a combined volume of 900 cc [8]. Methylene blue dye is added to alert patients to balloon leak or rupture by appearance of a blue color in the urine. Typically, IGB devices are placed and removed endoscopically under sedation or anesthesia. Following balloon placement, patients are placed on a liquid diet and provided antiemetics to alleviate nausea and vomiting. IGB devices are removed after 6 months, as risk of balloon deflation and possible migration increases substantially after this period [3].
The ORBERA Intragastric Balloon [Apollo Endosurgery, formerly the Bioenterics Intragastric Balloon (BIB)] has been the most commonly used IGB, approved for use in Europe in 1997 [9]. Clinical device surveillance based on reports from European practitioners between 2006 and 2013 revealed 3316 unspecified events/complaints representing 2.1% of 154,955 procedures [9]. A more detailed safety profile is still not available from Europe. The FDA approved ORBERA’s use in the USA in August 2015 on the basis of results of the ORBERA FDA Pivotal clinical trial and two non-US clinical trials in Australia and France [10]. Of these, the ORBERA Pivotal and the Australian trials are covered in this review, but the French study did not meet inclusion criteria.
Review of Literature
In 2007, the Cochrane Collaboration conducted an extensive literature review of the risks and benefits of IGB treatment [3]. The review included randomized or quasi-randomized controlled trials that (1) recruited overweight, obesity level I, II, III, and super-obese (BMI > 50.0) patients, (2) reported measures at balloon retrieval after at least 4 weeks, and (3) compared IGB to no treatment, or compared IGB plus diet, to diet only. Primary outcomes included weight loss, other anthropometric changes, and adverse effects. Other measures included patient motivation, delivery of IGB balloons, balloon volumes, and the use of H2 blockers. The search employed The Cochrane Library, MEDLINE, EMBASE, LILACS and databases of ongoing trials. A total of 220 met initial search criteria, but most were excluded for risk of bias in the results and in allocation concealment, leaving 16 studies, of which nine met full criteria. Analysis of these nine studies showed no significant difference in weight loss between IGB (without diet) vs no treatment, or IGB plus diet vs. diet alone. The review also noted high rates of minor and major complications, high cost, and weight regain after balloon removal [3]. Despite the negative findings of the Cochrane review, the FDA recently approved the ORBERA and ReShape IGB devices on the basis of three recent randomized controlled trial (RCTs) [3].
A 2015 meta-analysis (total n = 525) found significant mean weight loss of 8.9 kg, mean BMI reduction of 3.1, and %EWL of 21 favoring IGB in 6-month studies, and 1.5 kg and 1.2 BMI units for studies less than 6 months. %TBWL was not provided. The 6-month studies were considered modestly efficacious unlike those less than 6 months. Study selection criteria included RCTs comparing efficacy and safety of IGB with standard obesity treatment. Primary outcomes were weight loss, BMI, %EWL, and safety risks, indicating a high rate of non-serious, non-fatal minor complications [11].
In the same year, a meta-analysis by Moura et al. included nine RCTs comparing IGB plus diet vs. sham placement plus diet [12]. IGB (450–900 mL volume) plus diet was significantly more effective than sham plus diet in reducing BMI (1.1 units by t test; 1.4 units by meta-analysis after excluding one study with missing SDs), weight loss (2 kg by t test; 3.6 kg by meta-analysis) and control-subtracted %EWL (14.0 ± 26.5% SD significant by t test, but not by meta-analysis). %TBWL and control-subtracted %TBWL were not reported [12].
In a review limited to ORBERA (450–800 cc fill volume) in seven studies, Gaur et al. reported weight loss of 12.9 kg ± 0.8 SD by 3 months, and 16.0 ± 0.9 by 6 months, noting that 80% of final weight loss had occurred by 3 months [13]. In another nine studies (one overlapping), weight loss was 16.7 kg at balloon removal, 15.9 kg at 6 months following date of removal, and 8.7 kg at 12 months following removal (SDs not reported). Thus, on average, 52% of the weight loss was sustained at 12 months after balloon removal [13]. By comparison, however, following a 6-month diet only (750 kcal deficit) by Sacks et al., 66% of the weight loss was kept off at 18 months after the end of the diet [14].
Shoar and Saber [27] included 20 RCTs (n = 1195 subjects) evaluating change in weight and BMI, as well as %EWL and %TBWL at >3.0 months of IGB. They found a significant effect size favoring fluid- over air-filled IGBs and concluded that IGB appeared effective in the short term, but that the safety profile and long-term efficacy was questionable. Of the patients included in this meta-analysis, 24% received older, more experimental balloon devices employed during 1987–1995. A recent review chapter on IGB by Genco et al. primarily covers work by Genco “in press” or “not published”, and is thus not further considered [15]. Limitations of these recent reviews include significant risk of bias and potential author conflict of interest; inclusion of only one type of balloon in one review; inclusion of nonrandomized trials; heterogeneity in balloon volumes, patient characteristics, and dietary guidance; exclusion of trials with negative findings; use of outdated %EWL metric as an outcome measure, lack of comparison to other weight loss measures, and a lack of control-subtracted effect that we recommend (similar to placebo-subtracted effect used in drug studies).
Besides the stringent criteria for study inclusion, our review differs from others: First, it is the most recent, including discussion of three trials that led to the first FDA IGB device approval since 1985. Second, it considers two new swallowable devices, Obalon and Elipse, with Obalon receiving recent FDA approval. Third, it provides discussion of IGB potential mechanisms of action, which may stimulate improvements in existing and future devices. Fourth, it provides accurate assessment of IGB efficacy by using %TBWL and control-subtracted %TBWL, and compares findings to bariatric surgery and pharmacotherapy. Lastly, it includes the most recent FDA warnings on IGB safety. A formal meta-analysis was deemed inappropriate given the small number of studies meeting inclusion criteria and the apparent heterogeneity in sample size, patient characteristics, balloon volume, study duration, body weight criteria, and standard therapies. We do, however, report weighted effect sizes for studies of at least 6 months duration that included %TBWL or provided sufficient information for %TBWL calculation.
Methods
Search Strategy
A comprehensive literature search was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and made use of Ovid MEDLINE and Cochrane databases from 2006 to 2016. Key search words included “gastric balloon”, “intragastric balloon”, “stomach balloon”, “BIB”, “bioenterics”, “ReShape”, “dual balloon”, “single balloon”, “sham balloon”, “randomized controlled”, and “prospective”. Initial electronic search yielded 232 records of relevant studies, from which one duplicate was removed.
Eligibility
Retrieved study articles were reviewed on the basis of their titles and abstracts and screened to meet the following criteria:
-
1.
RCTs utilizing an IGB as an intervention and either sham, lifestyle modification, or pharmacological agent as a control. Single, double, and unblinded studies were included as investigators have noted that unblinding may occur on the basis of the presence or absence of IGB-related symptoms [8].
-
2.
At least one outcome measure on change in body weight, e.g., %EWL or %TBWL.
-
3.
Study duration of at least 3 months.
All review articles, nonrandomized clinical trials, pilot studies, and case reports were excluded. On the basis of these criteria, eight articles were reviewed.
Data Extraction
A modified version of the Cochrane Collaboration Data Collection Form for RCTs was used to assess whether articles met inclusion criteria based on study characteristics, population and setting, methods, participants, intervention groups, outcomes, results, and applicability. All eight articles met these criteria.
Outcome Measures
%TBWL is typically about half that of %EWL (when EW is approximately half of total TBW as in severely obese patients), a metric still used in bariatric surgery. %EWL is based on weight loss divided by “excess” weight and multiplied by 100. “Excess” weight (actual weight minus “ideal weight”) is based on the 1983 Metropolitan Life Insurance Company standard height–weight tables for “medium frame” men and women [16]. Karmali et al. recommended abandoning %EWL given that it is based on an outdated concept of “ideal” weight, difficult to explain, potentially misleading, and a barrier to comparing surgical and nonsurgical weight loss [16]. Except when there was insufficient data to calculate %TBWL or when %EWL was used as a study’s primary endpoint, we used %TBWL as the primary outcome measure. Safety was the secondary outcome based on SAEs and AEs.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Study Characteristics
The eight selected studies were published from 2006 to 2016. Sample sizes ranged from 22 enrolled (21 completers) to 326 enrolled (293 completers) obese or overweight subjects. Mean age ± SD ranged from 35.0 ± 6.6 to 45.9 ± 8.6 years, mean initial weight ranged from 95.1 ± 11.7 to 143.8 ± 31.2 kg, and mean initial BMI ranged from 35.0 ± 2.7 to 50.4 ± 7.8. IGB volumes ranged from 250 to 900 mL. The reviewed studies included four double-blind, randomized sham-controlled trials (including one crossover) [8, 17,18,19]. Of these four, only Ponce et al. used additional blinding measures including preventing between-subject contact, controlling medical records that might reveal assignment, using separate physicians for sham endoscopy, and blinding evaluators [8]. Even with these measures, Ponce et al. reported some patient unblinding beyond chance. Mathus-Vliegen and Eichenberger used endoscopic infusion of 500 mL saline directly into the stomach in the sham group [20] and reported that participants could not tell the difference between sham and true balloon at time of insertion. The remaining four studies reviewed included three unblinded RCTs and one randomized non-controlled trial [17,18,19,21,22,, 21]. Each study is summarized below in Table 1 in chronological order including study duration, experimental design, number of participants, group allocation, intervention type, type of dietary intervention, inclusion/exclusion criteria, and risk of bias based on the Cochrane Handbook [22]. The primary review outcomes %TBWL and control-subtracted %TBWL were calculated, when possible, unless already provided as in Farina 2012, Mathus-Vliegen and Eichenberger 2014, and Ponce et al. 2015 which provided %TBWL only. Most of this information is also provided in Table 1, along with study location, participant characteristics (age, BMI), number of completers, attrition rate, initial weight (kg), weight loss (kg), randomization procedure/allocation concealment, adverse events, and any other pertinent details.
Genco et al. (2006)
In a 6-month double-blind crossover RCT, 32 obese subjects were randomized to either 3 months BIB (500 mL saline) followed by 3 months sham balloon, or 3 months sham followed by 3 months BIB. Initial body weights were not provided, and thus %TBWL and control-subtracted %TBWL could not be calculated. Mean %EWL (study primary endpoint) was erroneously reported by study authors as 34.0 ± 4.8% SD (BIB) vs 2.1 ± 1% (sham), p < 0.001. Recalculation from the data in the article revealed that the actual %EWL at 3 months was 9.5% (SD could not be calculated) for BIB vs 2.1 ± 1% SD for sham. Prescribed diet in both groups included 1000 kcal with ≥1 g protein/kg of ideal weight.
Inclusion/exclusion Patients without medical or psychological contraindications precluding endoscopy or use of steroidal, anti-inflammatory or anticoagulant pharmacotherapy [23].
IGB AE incidence 0%
IGB SAE incidence 0%
Risk of Bias Summary Score 5* (*score includes bias due to incomplete outcome data and selective reporting per the error in %EWL noted above. Risk of bias scoring is explained below Table 1.)
Martinez et al. (2007)
In a 4-month double-blind RCT, 21 obese subjects were randomized to BIB (600 mL saline) or sham, both with dietary guidance. At 4 months, the calculated %TBWL was 8.8 ± 3.5% SD for BIB vs 6.4 ± 4.0% for sham, which did not differ significantly, p = 0.16, and thus the 4-month control-subtracted %TBWL was 2.4 ± 5.3%. Similarly, weight loss (kg) between groups did not differ significantly, 12.7 kg ± 5.6 SD for BIB vs 8.9 kg ± 9.2 for sham. Both groups were prescribed a low-fat hypocaloric diet.
Inclusion/exclusion Patients with no medical or psychological contraindications. Exclusions included GI structural abnormalities, high bleeding risk, persistent Helicobacter pylori (HP) infection, and concurrent pharmacological therapy potentially confounding the BIB effect [24].
IGB AE incidence Not reported
IGB SAE incidence 10%; persistent emesis (1 event)
Risk of Bias Summary Score 1
Farina et al. (2012)
In a 1-year randomized non-controlled study, 50 obese subjects were allocated to (1) 6 months of BIB (500 mL saline) followed by 6 months of pharmacotherapy (10 mg/day of sibutramine) (BIB/pharma; n = 15), (2) 6 months BIB followed by 6 months of continued lifestyle guidance only (BIB/lifestyle; n = 15), or (3) 6 months of pharmacotherapy (without BIB) followed by 6 months of continued lifestyle guidance without pharmacotherapy or BIB (pharma/lifestyle; n = 20). At 6 months, %TBWL was 14.5 ± 6.6% SD for BIB and 9.1 ± 6.7% for pharma, p = 0.036, and control-subtracted %TBWL was 5.4 ± 9.4% SD. At 6 months, the BIB group lost more weight, p < 0.05, than the pharma group. At 1 year, %TBWL was greater, p < 0.05, in those treated with either BIB/pharma (%TBWL = 15.8 ± 8.9 SD) or BIB/lifestyle (%TBWL = 14.3 ± 10.4) than with pharma/lifestyle (%TBWL = 8.0 ± 6.3). All groups were prescribed a standard diet with caloric deficit of 1000 kcal/day (to achieve 900–1500 kcal/day) and received lifestyle guidance at baseline and every 4 weeks.
Inclusion/exclusion Male and female obese subjects were recruited. Exclusions included diabetes mellitus, hypertension, glaucoma, cancer, other cardiovascular, endocrine, renal, or hepatic diseases, neurological or psychiatric disorders, drug or alcohol abuse, pregnancy, or lactation [18].
IGB AE incidence 40%; borderline hypertension (2 events), belching (2), heartburn (8)
IGB SAE incidence 0%
Risk of Bias Summary Score 6
Fuller et al. (2013)
In a 12-month unblinded RCT, 66 subjects were assigned to 12 months of behavior modification and initial 6 months of ORBERA (450–700 mL fill, based on “BMI and stomach anatomy”) or 12 months of behavior modification only. At 6 months, %TBWL for the ORBERA group was 14.2% vs. 4.8% for control (SDs not reported) (p < 0.0001), and thus control-subtracted %TBWL was 9.4%. At 12 months (6 months following balloon removal) %TBWL was 9.2% for ORBERA vs. 5.2% for control (p = 0.007) and thus control-subtracted %TBWL was 4.0%. The behavior modification program included guidance on food type and quantity, tailored exercise program, and compliance assessment.
Inclusion/exclusion Eligibility included BMI 30–40 for at least the past 2 years with metabolic syndrome (MS) and failed supervised weight reduction. Exclusions included conditions posing endoscopy risk, major surgery, participating in a formal weight loss program during prior 3 months, and conditions such as cerebrovascular or cardiopulmonary disease, uncontrolled hypertension, type 1 diabetes, psychiatric disorder, pregnancy, or a history of drug and alcohol abuse. Subjects were restricted from medication or supplements known to affect appetite and weight, or irritate the gastric lining [21].
IGB AE incidence 0%
IGB SAE incidence 9.6%; persistent emesis (1 event), abdominal pain (1), and gastroesophageal reflux (1)
Risk of Bias Summary Score 4
Mathus-Vliegen and Eichenberger (2014)
In a 26-week double-blind RCT, 40 obese subjects were randomized to BIB (500 mL) or sham for the first 13 weeks, followed by 13 weeks of BIB through week 26. At 13 weeks, %TBWL was 10.6 ± 3.8% SD (BIB) vs. 8.8 ± 4.9% (sham), and did not significantly differ between groups, p = 0.20. Control-subtracted %TBWL was 1.8 ± 6.2% SD. At 26 weeks, %TBWL was 14.2 ± 8.4% SD (BIB/BIB) vs. 15.8 ± 5.7% (sham/BIB), which did not differ, p = 0.67, and control-subtracted %TBWL for BIB/BIB was 1.6 ± 0.15% SD. All participants received biweekly guidance for a 1000–1500 kcal diet and exercise guidance was via self-help groups for aerobic fitness and aqua jogging.
Inclusion/exclusion Eligibility included age >18.0 years, with a 3-month stable BMI ≥32, failure to lose weight in a supervised weight loss program, and no history of gastrointestinal lesions, large hiatal hernia, or previous bariatric surgery [20].
IGB AE incidence 10.5%; balloon intolerance (2 events)
IGB SAE incidence 0%
Risk of Bias Summary Score 7
Mohammed et al. (2014)
In a 9-month unblinded RCT, 128 subjects were randomized to 6 months BIB (510–610 mL) plus diet and exercise, or diet and exercise only, and were followed for 3 months after balloon removal. At 6 months, %TBWL was 10.4 ± 1.0% SD (BIB) vs 4.6 ± 2.0% (control), p < 0.001; and control-subtracted %TBWL was 5.8 ± 2.2% SD. At 9 months, %TBWL was 13.2 ± 0.7% SD (BIB) vs 4.3 ± 2.1% (control), p < 0.001, and control-subtracted %TBWL was 8.9 ± 2.2%. The dietary and exercise regimen included a 1500 kcal/day diet and 45 min of walking five times per week.
Inclusion/exclusion Eligible participants were >20.0 years old, obese class I, II, and III based on WHO criteria. Exclusions included advanced chronic or psychiatric illness, pregnancy, liver disease, coagulopathy, renal impairment, endocrine and cardiopulmonary disease, abnormal macroscopic endoscopic lesions, gastric ulcers, cancer, hiatus hernia larger than 3 cm, grade C or D esophagitis, duodenal ulcers, previous GI surgery, smokers, drug or alcohol abuse, binge eaters, special diet for previous 6 months, drugs affecting appetite during the prior 2–4 weeks [17].
IGB Group AE incidence Not reported
IGB Group SAE incidence 7.1%; gastric ulcer (3 events), GI bleeding (3)
Risk of Bias Summary Score 3
Dayyeh et al. (2015) (FDA Reviewed)
In Apollo Endosurgery (non-peer-reviewed industry document) on “Directions for Use” reviewed by the FDA, Dayyeh et al. reported a 12-month multicenter, prospective, non-blinded RCT of 273 subjects who were randomized to receive 6-month ORBERA (400–700 cc fill) with a behavior management program (BMP) over 12 months, or 12 months of BMP alone (control). At 6 months %TBWL was 10.2 ± 6.6% (ORBERA) vs. 3.3 ± 5.0% (control), p < 0.001, and control-subtracted %TBWL was 6.9 ± 8.4% SD. At 9 months %TBWL was 9.1 ± 6.9% (ORBERA) vs 3.4 ± 5.3% (control), and control-subtracted %TBWL was 5.7 ± 8.7%, p < 0.001. The ORBERA group had a mean 26.5% EWL (95% CI 22.9–30.2%), which did not meet the study’s first co-primary minimum lower confidence level of 25% EWL. However, the second endpoint, minimum responder rate of at least 30% was met, with a mean of 45.6% of the ORBERA group (95% CI 36.7–54.8%) achieving at least 15% EWL above the control group. The weight difference between balloon and control groups diminished at 9 months and further at 12 months. BMP involved a 1000–1500 kcal/day diet with daily logs of food intake and exercise, and guidance for exercise and behavior change over 21 visits (9 in months 1–6, 12 in months 7–12). In a peer-reviewed publication on the same subjects, more details were given about fill volume (500–600 cm3) and methodology provided below, which were absent from the industry report [25].
Inclusion/exclusion Eligibility included ages 18–65 years with a BMI of 30–40, with obesity for more than the past 2 years. Exclusions included GI surgery (except uncomplicated cholecystectomy or appendectomy), GI obstruction, adhesive peritonitis or clinically significant hiatal hernia, history of esophageal or GI motility disorder, a patulous pyloric channel, delayed gastric emptying, history of inflammatory bowel disease, or a positive test for HP at screening [9, 25].
IGB AE occurrence 694 total reported events across 137 ORBERA subjects; nausea (132), emesis (115), abdominal pain (87), GERD (43), eructation (39), dyspepsia (32), constipation (32), upper abdominal pain (29), abdominal distension (27), dehydration (20), diarrhea (21), flatulence (18), impaired gastric emptying (14), abdominal discomfort (10), asthenia (7), postprocedural pain (8), headache (8), fatigue (7), halitosis (6), abdominal rigidity (5), gastrointestinal pain (5), vitamin B1 decreased (5), pharyngolaryngeal pain (5), esophagitis (4), hiccups (4), gastritis (3), anorexia (3), anemia (2), epigastric discomfort (2), fecal incontinence (2), migraine (2) and alopecia (2).
IGB SAE Incidence 10%; unspecified (8) and device intolerance (8).
Risk of Bias Summary Score 2
Ponce et al. (2015) (FDA Reviewed)
In a 48-week randomized, sham-controlled double-blind study, 326 obese subjects randomized to 750–900 mL saline-filled ReShape dual balloon (DUO) plus diet and exercise guidance or guidance alone (DIET). At 6 months, %TBWL was 7.6 ± 5.5% (DUO) vs 3.6 ± 6.3% (DIET), p < 0.001, and the 6 months control-subtracted %TBWL was 4.0 ± 8.4% SD. The results met the study primary endpoints: (1) a minimum advantage of 7.5% EWL for DUO above DIET at 24 weeks and (2) 35% of DUO participants achieved at least 25% EWL. For completers, the advantage was 15.6 ± 30.7% SD for DUO vs. DIET, and 54.5% of DUO achieved at least 25% EWL at 24 weeks. On the basis of intent-to treat analysis (ITT), the difference between groups was 13.8% EWL ± 44.8 SD, and 48.8% achieved at least 25% EWL at 24 weeks. Diet and exercise guidance was based on the National Heart, Lung, and Blood Institute Practical Guide [26].
Inclusion/exclusion Eligibility included ages 21–60; BMI 30–40 with one or more obesity-related co-morbidities, not at risk of pregnancy, and failure to lose weight during the prior 36 months in a medically supervised program. Exclusions were a history of or ongoing clinically significant GI or medical conditions which prevented use of the IGB or might confound study outcome assessment [8].
IGB AE incidence 36.9%; esophageal mucosal tear (1), gastroesophageal junction ulcer requiring transfusion (1), contained cervical esophageal perforation (1), and postretrieval pneumonitis requiring antibiotic treatment (1), gastric ulcers (65).
IGB SAE incidence 15.0%; “accommodative symptoms” (21) and “nonaccommodative symptoms” (7)
Risk of Bias Summary Score 5
Efficacy
During Balloon Placement
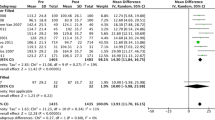
The reviewed studies ranged from 4 to 12 months in duration, with balloon removal varying between 4 and 6.5 months. For efficacy comparisons, we evaluated only the five studies with 6 months of balloon placement (Fuller et al., Dayyeh et al., Ponce et al., Farina et al., Mohammed et al.; see Table 1). The overall weighted mean %TBWL for IGB for the five studies was 9.7%, and for the control-subtracted %TBWL was 5.6%. When one study without reported SDs was removed, the weighted mean %TBWL for IGB in the remaining four studies was 9.3 ± 5.7% SD, and the control-subtracted %TBWL was 5.5 ± 7.8%. Review Manager version 5.3 (Cochrane Collaboration software) was used to combine data from the four trials. Weighted mean differences (and 95% confidence intervals) were used to pool data in continuous variables. Meta-analysis of control-subtracted %TBWL for the four studies as displayed in the Forest plot (see Fig. 1) showed a significant control-subtracted %TBWL for IGB of 5.5% (95% CI 4.3–6.8).
Comparison of control-subtracted percentage total body weight loss (%TBWL ± 95% confidence intervals) from four IGB studies using a random effect model (control-subtracted %TBWL = intervention group %TBWL minus control group %TBWL). The pooled weighted mean is represented by a black diamond; width of the diamond is the 95% CI and the center is the pooled point estimate. The size of the squares reflects the weights assigned to each study. The horizontal lines intersecting the squares represent the 95% CI [8, 9, 17, 18]. IV stands for independent variable
There was significant heterogeneity, however, as I 2 = 62% which is greater than 50%, and thus the meta-analysis result should be interpreted with caution.
The %TBWL values at 6 months for these four studies show lower efficacy than bariatric surgery (%TBWL of 27% for RYGB) [27, 28]. We use %TBWL only for this comparison because bariatric surgery cannot provide control-subtracted %TBWL (for obvious ethical reasons). The most efficacious current FDA-approved oral weight loss drug, Qsymia, showed an approximate 8.8% TBWL and a 6.6% control-subtracted %TBWL following 6 months of treatment at the recommended standard dosage (7.5/46 mg phentermine/topiramate). The higher dose of Qsymia (15/92 mg phentermine/topiramate) showed an approximate 11% TBWL and an 8.8% control-subtracted %TBWL [29] (although the swallowable Obalon device was not considered in the efficacy comparison, the control-subtracted %TBWL of 3.2 is considerably below that of 5.5% for the four IGB reviewed studies).
Follow-Up After Balloon Removal
Three studies in this review reported weight loss beyond time of removal at 6 months: two at 9 months, i.e., 3 months after balloon retrieval (Dayyeh et al. and Mohammed et al.), and one at 12 months, i.e., 6 months after retrieval (Fuller et al.). On the basis of these three studies, IGB efficacy in general appeared to diminish over time. In Dayyeh et al., control-subtracted %TBWL decreased from 6.9 ± 8.4% SD at 6 months to 5.7 ± 8.7% at 9 months [9]. Control-subtracted %TBWL in Fuller et al. decreased from 9.4% at 6 months to 4% at 9 months (SDs not reported) [21]. By contrast, however, in Mohammed et al., control-subtracted %TBWL appeared to increase between 6 and 9 months from 5.8 ± 2.2% SD to 8.9 ± 2.2% [17].
Safety
Adverse events across trials ranged from none (Genco et al.) to 28 SAEs (Ponce et al.). Only six of the eight reviewed studies reported adverse events (AEs) with a weighted mean incidence of 28.2%. The weighted mean reported incidence of serious adverse events (SAEs) across all eight studies was 10.5%, with three studies requiring early device removal (Fuller et al., Dayyeh et al., Mathus-Vliegen and Eichenberger) [19, 20, 21, 23, 25]. For further details, see Table 1. Mild AEs included dry mouth, constipation, and heartburn, while SAEs included persistent emesis, abdominal pain, GERD, ulcers, and perforation during endoscopy. Ulcers were reported in the studies by Ponce et al., Mohammed et al., and Shelby et al. While classified as a mild AE, untreated ulcers can become life threatening [30]. Closer monitoring for ulcers during IGB treatment is warranted. Although no life-threatening events or deaths were reported in the reviewed studies, incidents of cardiac complications/cardiac arrest and death have been reported with ORBERA (number not specified) [31].
Most recently, the FDA (February 9, 2017) warned medical providers about potential risks of fluid-filled balloons after receiving several dozen reports of IGB hyperinflation (reported as “overinflation”), with air or fluid in the stomach, resulting in device removal as early as 9 days following insertion. Symptoms of hyperinflation included intense abdominal pain, abdominal distension with or without discomfort, difficulty breathing, and/or vomiting. Cause for hyperinflation was cited as unknown by the FDA. We speculate that given incidents of hyperinflation of saline-filled silicone breast implants, IGB permeability may have resulted in fluid or air entry by osmosis [32]. Another possibility with regard to air is that anaerobic bacteria, which have been identified in breast implants, may also have been present in IGBs and released gas within the balloon [33, 34]. The FDA also received several reports of acute pancreatitis associated with ORBERA and ReShape, resulting in early device removal as well as hospitalization. Symptoms of acute pancreatitis included severe abdominal and back pain as soon as 3 days after implantation. Neither were listed as potential complications in the initial balloon labeling information. In the recently published article by Courcoulas et al. of the same patients as in the prior FDA-reviewed trial by Dayyeh et al., abdominal pain, abdominal distension, and vomiting were reported as mild, moderate, and severe AEs [19, 25]. Back pain was not distinctly reported, and may have been categorized as “postprocedural pain” or “gastrointestinal pain”.
Discussion
Prior to 2015, the FDA had not approved an IGB device since the Garren–Edwards Gastric Bubble in 1982. Influenced by evidence of improved efficacy and safety in newer IGB devices, the FDA approved the use of ReShape and ORBERATM, and the swallowable device, Obalon. Examining whether this decision was prudent, from a clinical and public health perspective is the focus of the present discussion.
Efficacy
Although three of the eight studies in this review could not be compared on our primary measures of %TBWL and control-subtracted %TBWL because of missing information or duration of less than 6 months, some qualitative comparisons can be made. Six of the eight studies showed a significant advantage of IGB vs. control; however, two studies on ORBERA found no significant effect: (1) Mathus-Vliegen and Eichenberger found no significant difference in weight loss between IGB and sham at 13 weeks [20]. A large and significant effect would be expected by this time according to a review of ORBERA studies by Gaur et al. which reported that 80% of weight loss occurred by 3 months [13]. (2) Similarly, at 4 months Martinez et al. also found no significant difference in weight loss between IGB and control (see Table 1) [24].
For the four studies included in the pooled-weighted effect, quantitative comparisons can be made. The %TBWL for IGB at 6 months ranged from 7.6 ± 5.5% SD to 14.5 ± 6.6%, with a weighted mean of 9.3 ± 5.7 SD and control-subtracted %TBWL at 6 months ranged from 3.2 ± 7.1% SD to 6.9 ± 8.4%, with a weighted mean of 5.5 ± 7.8 SD. The study by Ponce et al. showed the lowest control-subtracted %TBWL of 4% with the ReShape double balloon. Given that ReShape and ORBERATM necessitate invasive endoscopic placement and removal, come with risk for serious complication, and are relatively costly, they should at minimum demonstrate equivalent efficacy to the most efficacious pharmacological treatment or at least control-subtracted %TBWL of 6.6 as observed with the standard recommended dose of Qsymia (weight loss drug) at 6 months [35]. Only one study, by Dayyeh et al., met this criteria with a control-subtracted % TBWL of 6.9 ± 8.4% SD (see Fig. 1) [19, 25].
In contrast to the Cochrane group (2007), we found a significant pooled %TBWL in more recent devices, which are generally filled to greater volumes. Although IGB appears to provide statistically significant weight loss, it is of a much smaller magnitude than bariatric surgery and less than the most efficacious oral pharmaceutical agent, Qsymia. Although neither IGB nor Qsymia is currently covered by insurance, IGB costs, on average, US$8150, while Qsymia costs approximately US$135 for a 30-day supply, which translates to US$810 over 6 months [29, 36].
Safety
Of the eight studies reviewed, the highest complication rates were observed in the two pivotal FDA trials for ReShape and ORBERA. The ReShape 750–900 mL balloon study by Ponce et al. included 187 IGB treated subjects and had a 35% gastric ulceration rate [8]. The ORBERA study (500–600 mL) by Dayyeh et al. included 17 SAEs reported for 16 IGB-treated subjects or a 10% SAE incidence [19]. These balloons also had the greatest volumes, which may increase risk of complications. It is generally the case that the more invasive the treatment is (e.g., bariatric surgery), the greater the efficacy in a trade-off for safety.
The safety profile for IGB raises concerns about whether the modest weight loss efficacy warrants the potential risks associated with IGB. Besides the SAEs previously presented for the reviewed studies, the FDA recently warned healthcare providers about two other complications missing from the device’s directions for use, i.e., spontaneous balloon hyperinflation and acute pancreatitis, which may have gone undetected under the broad diagnosis of abdominal pain. It is possible that hyperinflation was the cause of acute pancreatitis.
Diagnosis of acute pancreatitis requires measurement of serum amylase and lipase, and confirmation with imaging or biopsy. The FDA should require that such blood tests be done at 3 months post IGB insertion to detect incipient pancreatitis. Left untreated, acute pancreatitis can result in pancreatic necrosis, sepsis, and death. Fecal samples for blood and/or endoscopy should also occur at 3 months to check for gastric ulcers. In Ponce et al., the gastric ulceration rate was an alarming 35%, and gastric ulcers left untreated can present significant complications including death [8, 37].
Mechanism of Action and Implications for Future IGB Development
Evidence suggests that IGB suppresses intake via vagal signaling through stimulation of gastric mechanoreceptors [38, 39]. Vagal signals project to the nucleus of the solitary tract (NTS) of the brain stem and then to the amygdala and insula among other brain regions [40]. In an fMRI study assessing brain activation during dynamic gastric distension in healthy subjects, volumes of 250–500 mL led to activation in the posterior amygdala, posterior insula, and precuneus [41].
Following acute gastric balloon distension to various volumes (0, 200, 400, 600, 800 mL on different days within the same subjects), Geliebter et al. found that volumes of at least 400 mL significantly reduced ad libitum liquid meal intake in lean and obese subjects by 0.4 mL for every milliliter of distension [42]. When the balloon was filled to 800 mL and quickly emptied, intake was similar to that for 0 mL volume, implicating a short-acting neural signal.
IGBs may also increase feelings of fullness by delaying gastric emptying. During a 2-month double-blind intragastric balloon (400 mL) crossover study, Geliebter et al. observed slower liquid meal gastric emptying at 60 min, at 1 month of balloon fill [43]. The slowing of solid meal gastric emptying was also observed at 1 and 3 months with BIB which then returned to normal at 6 months [13]. It is possible that emptying was slowed because the balloon created a partial obstruction, and that over time, gastric capacity increased to compensate for the balloon presence, resulting in a return to normal emptying.
Balloon efficacy may also be related to balloon volume. In the double-blind crossover study by Geliebter, there was no significant weight loss with balloon inflation to 400 mL at 4 weeks vs. empty balloon within the same subjects—a negative result also seen in a number of longer-term IGB studies [3, 43]. However, the 400-mL volume used in these early IGB studies only occupies about 21% of the gastric capacity based on an estimated capacity (milliliters) of 1920 ± 136 (SEM) in obese and 1017 ± 154 (SEM) in lean (p < 0.002) individuals, similar to Swedish findings [42, 44]. In rats, balloons occupying ~33% of gastric capacity resulted in significant weight loss (−16%, p < 0.05) and food intake (−27%, p < 0.0005) over 2 months [45]. By the end of 2 months, intake had returned to normal. The stomachs of the rats had hypertrophied, likely an adaptation to compensate for the space occupied by the balloon.
In a 3-month IGB study with 300 mL volume, 86 obese subjects were randomized to four conditions and the weight changes were (1) IGB only, −3.2 ± 4.1 kg SD; (2) IGB + diet, −5.1 ± 4.7 kg; (3) diet only, −6.9 ± 5.9 kg; and (4) no treatment, 0.6 ± 2.5 kg. The IGB only produced modest significant weight loss relative to no IGB, and the IGB + diet did not do better than diet alone [46]. Gastric capacity was measured in a subset of those receiving gastric balloons. Those with a larger gastric capacity lost significantly less weight than those with a smaller capacity. On the basis of this, a larger balloon volume may be advantageous in those with a larger gastric capacity. In a small subsample tested following balloon removal, there was suggestive evidence of enlarged gastric capacity.
IGB efficacy may also be enhanced by increasing treatment duration. Lopez-Nava et al. (non-randomized study) reported that a second ORBERA balloon placement, presumably for a 6-month period (not specified), after a 1-month interval led to an additional decrease in BMI of 2.5 ± 18.2 SD [47]. The smaller decrease in BMI the second time around (first 6 months’ BMI decrease of 6.5 ± 12.7) suggests adaptation to the balloon, possibly through an increase in gastric capacity.
One potential use of IGB would be in preparation for bariatric surgery. However, by itself, IGB does not address the dietary and behavior modification patients require for success after bariatric surgery. In a short-term period with IGB, patients may eat less and lose weight presurgery, but such weight loss could also be achieved by pharmacological agents.
New and Future IGBs
Both the Obalon and Elipse appear safer and less invasive than the other IGBs. The Obalon balloon (Obalon Therapeutics Inc.) is a swallowable capsule attached to a thin catheter. Once swallowed and position verified by fluoroscopy, the balloon is inflated with 250 mL of nitrogen gas, and the catheter is detached. After 3 months, the balloon is punctured, deflated, and removed by upper endoscopy [48]. In a 12-week uncontrolled pilot study, 17 adults received one, two, or maximally three 250-mL Obalon balloons combined with nutritional counseling, resulting in 5.0-kg weight loss, or 5.8% TBWL (SDs not provided), with no reported SAEs [49]. A second uncontrolled study for 3 months with only one 250-mL balloon was conducted in nine obese children, prescribed a diet and physical activity, and the weight loss was 5.7 kg ± 1.5 SD, with %TBWL of 5.8 ± 1.5 SD, without SAEs (see Table 1) [50].
In a 15-site, 6-month double-blinded RCT, 430 subjects were randomized to three Obalon capsules (250 mL nitrogen gas each) or three sugar-filled sham capsules. Both groups received 6 months of moderate intensity lifestyle counseling every 3 weeks by a dietitian blind to study assignment. Results were reported for those who swallowed at least two capsules and completed at least 18 weeks of lifestyle counseling. %TBWL for Obalon was 6.6 ± 5.1% SD and that for control was 3.4 ± 5.0%, and control-subtracted %TBWL was 3.2%, p = 0.035. Also, 62.1% of the IGB group achieved at least 5% TBWL. At 48 weeks, the IGB group regained 0.9% TBWL. A significant percentage of the IGB group experienced mild abdominal pain, nausea, and vomiting which resolved within 2 weeks. As Obalon is swallowed as a capsule, this study was not included in the earlier comparisons of endoscopic placed IGBs [48].
Another balloon capsule the Elipse (Allurion Technologies) is being investigated in the USA. It is filled with 550 mL water via a catheter, which is then detached, and remains in the stomach for approximately 4 months before it empties and passes through the GI tract [50]. Interim results for a 4-month uncontrolled study with Elipse in Europe were presented in 2015 [51]. The study enrolled 34 overweight subjects, and on the basis of 25 subjects, 10 kg were lost or 9.8% TBWL (SDs not given), and no SAEs reported (see Table 1). The Elipse was approved for the European Union in December, 2015, but to date, has not been approved by the FDA, and for that reason, it was not discussed above [52].
Development of devices that can be filled to an appropriate percentage of gastric capacity may also improve short-term efficacy. A liquid test meal could be used to estimate gastric capacity [53, 54]. In addition, development of a balloon that can fill intermittently in response to meal intake and then deflate may be less likely to result in adaptation and increased gastric capacity.
Given that the IGB studies reviewed above do not demonstrate convincing efficacy in comparison to surgery and pharmacological treatment, FDA approval may have been premature. The FDA appears to have based their approval for ORBERA, in part, on a less controlled study that did not qualify for this review [9]. The limited short-term nature of IGB and side effects warrants more consideration. Wider post-market studies will help determine whether the FDA made the right decision. The recent public statement alerting health providers to previously unforeseen and potentially severe complications suggests that these devices were not ready for FDA market approval, and future withdrawal from the market by the FDA is possible.
References
Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235. doi:10.1001/jama.2009.2014.
American Society for Metabolic and Bariatric Surgery. Metabolic and bariatric surgery: fact sheet. 2013. https://asmbs.org/wp/uploads/2014/05/Metabolic+Bariatric-Surgery.pdf. Accessed 18 Jul 2016.
Fernandes M, Atallah AN, Soares BGO, et al. Intragastric balloon for obesity. Cochrane Database Syst Rev. 2007;(1):CD004931.
Benjamin SB, Boyle TM, Agus SG, et al. Small bowel obstruction and the Garren-Edwards® gastric bubble: an iatrogenic bezoar. Gastrointest Endosc. 1988;34(6):463–7.
Hogan RB, Johnston JH, Long BW, et al. A double-blind, randomized, sham-controlled trial of the gastric bubble for obesity. Gastrointest Endosc. 1989;35(5):381–5.
Schapiro M, Benjamin S, Blackburn G, et al. Obesity and the gastric balloon: a comprehensive workshop. Gastrointest Endosc. 1987;33(4):323–7.
Fleisher A, Conti PS, McCray RS, et al. Jejunal entrapment of a gastric balloon. JAMA. 1987;257(7):930. doi:10.1001/jama.1987.03390070050019.
Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11(4):874–81.
Apollo Endosurgery Inc. ORBERA ™ intragastric balloon system (ORBERA ™). San Diego: Apollo Endosurgery; 2015. pp. 1–30.
FDA. Recently-approved devices—Obalon balloon system—P160001. Center for Devices and Radiological Health. http://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm520741.htm. Accessed 11 Oct 2016.
Zheng Y, Wang M, He S, Ji G. Short-term effects of intragastric balloon in association with conservative therapy on weight loss: a meta-analysis. J Transl Med. 2015;13:246.
Moura D, Oliveira J, De Moura EGH, et al. Effectiveness of intragastric balloon for obesity: a systematic review and meta-analysis based on randomized control trials. Surg Obes Relat Dis. 2016;12(2):420–9.
Gaur S, Levy S, Mathus-Vliegen L, Chuttani R. Balancing risk and reward: a critical review of the intragastric balloon for weight loss. Gastrointest Endosc. 2015;81(6):1330–6.
Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. Mass Med Soc. 2009. doi:10.1056/NEJMoa0804748.
Genco A, Maselli R, Casella G, Cipriano M, Redler A. Intragastric balloon treatment for obesity. In: Obesity, bariatric and metabolic surgery. Cham: Springer International Publishing; 2016. pp. 485–92. doi:10.1007/978-3-319-04343-2_50.
Karmali S, Birch DW, Sharma AM. Is it time to abandon excess weight loss in reporting surgical weight loss? Surg Obes Relat Dis. 2009;5(4):503–6.
Mohammed M. Effects of intragastric balloon versus conservative therapy on appetite regulatory hormones in obese subjects. Trends Med Res. 2014;1:1–5.
Farina MG, Baratta R, Nigro A, et al. Intragastric balloon in association with lifestyle and/or pharmacotherapy in the long-term management of obesity. Obes Surg. 2012;22(4):565–71. doi:10.1007/s11695-011-0514-y.
Abu Dayyeh BK, Eaton LL, Woodman G, et al. 144 A randomized, multi-center study to evaluate the safety and effectiveness of an intragastric balloon as an adjunct to a behavioral modification program, in comparison with a behavioral modification program alone in the weight management of obese subject. Gastrointest Endosc. 2015;81(5):AB147.
Mathus-Vliegen EMH, Eichenberger RI. Fasting and meal-suppressed ghrelin levels before and after intragastric balloons and balloon-induced weight loss. Obes Surg. 2014;24(1):85–94. doi:10.1007/s11695-013-1053-5.
Fuller NR, Pearson S, Lau NS, et al. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity. 2013;21(8):1561–70. doi:10.1002/oby.20414.
Higgins JPT, Green SE, Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley-Blackwell; 2011.
Genco A, Cipriano M, Bacci V, et al. BioEnterics® Intragastric Balloon (BIB®): a short-term, double-blind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes. 2006;30(1):129–33. doi:10.1038/sj.ijo.0803094.
Martinez-Brocca MA, Belda O, Parejo J, et al. Intragastric balloon-induced satiety is not mediated by modification in fasting or postprandial plasma ghrelin levels in morbid obesity. Obes Surg. 2007;17(5):649–57.
Courcoulas A, Dayyeh BA, Eaton L, et al. Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. Int J Obes (Lond). 2017;41(3):427–33.
NHLBI. The practical guide identification, evaluation, and treatment of overweight and obesity in adults. https://www.nhlbi.nih.gov/files/docs/guidelines/prctgd_c.pdf. Accessed 2 May 2017.
Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis. 2017;13(2):170–80.
Chang S-H, Stoll CRT, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149(3):275–87.
CONQUER Clinical Study | Qsymia® (Phentermine and Topiramate extended-release) Capsules CIV. https://qsymia.com/hcp/efficacy/study-2-conquer/. Accessed 8 May 2017.
Laursen SB, Leontiadis GI, Stanley AJ, Møller MH, Hansen JM, Schaffalitzky de Muckadell OB. Relationship between timing of endoscopy and mortality in patients with peptic ulcer bleeding: a nationwide cohort study. Gastrointest Endosc. 2017;85(5):936.e3–44.e3.
Abu Dayyeh BK, Kumar N, Edmundowicz SA, et al. ASGE bariatric endoscopy task force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82(3):425.e5–38.e5.
Robinson OG, Benos DJ, Mazzochi C. Spontaneous autoinflation of saline mammary implants: further studies. Aesthetic Surg J. 2005;25(6):582–6.
Seng P, Bayle S, Alliez A, Romain F, Casanova D, Stein A. The microbial epidemiology of breast implant infections in a regional referral centre for plastic and reconstructive surgery in the south of France. Int J Infect Dis. 2015;35:62–6.
Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A. Current concepts in the management of necrotizing fasciitis. Front Surg. 2014;1:36.
Haberman Associates. Vivus’ Qsymia (formerly Qnexa) approved by the FDA—the most efficacious weight-loss drug ever approved in the USA. Biopharmconsortium Blog. http://biopharmconsortium.com/2012/07/26/vivus-qsymia-formerly-qnexa-approved-by-the-fda-the-most-efficacious-weight-loss-drug-ever-approved-in-the-u-s/. Accessed 2 May 2017.
Shayani V, editor. Gastric balloon: insurance & costs. Bariatric Surgery Source. 2017. http://www.bariatric-surgery-source.com/gastric-balloon.html. Accessed 8 May 2017.
Cho Y-S, Kim H-K, Jang E-C, et al. Usefulness of the bedside index for severity in acute pancreatitis in the early prediction of severity and mortality in acute pancreatitis. Pancreas. 2013;42(3):483–7.
Ozaki N, Sengupta JN, Gebhart GF, et al. Mechanosensitive properties of gastric vagal afferent fibers in the rat. J Neurophysiol. 1999;82(5):2210–20.
Cubattoli L, Barneschi C, Mastrocinque E, Bonucci P, Giomarelli PP. Cardiac arrest after intragastric balloon insertion in a super-obese patient. Obes Surg. 2009;19(2):253–6. doi:10.1007/s11695-008-9606-8.
Mussa BM, Verberne AJM. The dorsal motor nucleus of the vagus and regulation of pancreatic secretory function. Exp Physiol. 2013;98(1):25–37. doi:10.1113/expphysiol.2012.066472.
Wang G-J, Tomasi D, Backus W, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39(4):1824–31.
Geliebter A, Westreich S, Gage D. Gastric distension by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48(3):592–4.
Geliebter A, Melton PM, Gage D, McCray RS, Hashim SA. Gastric balloon to treat obesity: a double-blind study in nondieting subjects. Am J Clin Nutr. 1990;51(4):584–8.
Granström L, Backman L. Stomach distension in extremely obese and in normal subjects. Acta Chir Scand. 1985;151(4):367–70.
Geliebter A, Westreich S, Gage D, Hashim SA. Intragastric balloon reduces food intake and body weight in rats. Am J Physiol. 1986;251(2):R794–7.
Geliebter A, Melton PM, McCray RS, et al. Clinical trial of silicone-rubber gastric balloon to treat obesity. Int J Obes. 1991;15(4):259–66.
Lopez-Nava G, Rubio MA, Prados S, et al. BioEnterics intragastric balloon (BIB). Single ambulatory center Spanish experience with 714 consecutive patients treated with one or two consecutive balloons. Obes Surg. 2011;21(1):5–9.
Shelby VS, James MS, George W, Steven E, Tarek I, Shayani H. The Obalon swallowable 6-month balloon system is more effective than moderate intensity lifestyle therapy alone: results from a 6-month randomized sham controlled trial. In: Digestive disease week. San Diego; 2016. http://www.ddw.org/program/online-planner.
Mion F, Ibrahim M, Marjoux S, et al. Swallowable Obalon® gastric balloons as an aid for weight loss: a pilot feasibility study. Obes Surg. 2013;23(5):730–3.
Nobili V, Corte CD, Liccardo D, et al. Obalon intragastric balloon in the treatment of paediatric obesity: a pilot study. Pediatr Obes. 2015;10(5):e1–4.
Machytka E, Chuttani R, Bojkova M, et al. Elipse™, a procedureless gastric balloon for weight loss: a proof-of-concept pilot study. Obes Surg. 2016;26:512–6.
Gaur S. Elipse system receives European marketing approval—Allurion Technologies. 2015. https://allurion.com/2015/12/08/elipse-receives-europe-marketing-approval/. Accessed 10 May 2017.
Galanti K, Gluck ME, Geliebter A. Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. Int J Eat Disord. 2007;40(8):727–32.
Geliebter A, Yahav E, Gluck M, Hashim S. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiol Behav. 2004;81(5):735–40.
Acknowledgements
This review was supported in part by the National Institute of Health, Bethesda, MD, USA grants R01 DK080153 (AG). No funding or sponsorship was received for the publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. A special thank you to Jenna Koroly, BA, for assisting with the completion of this article.
Disclosures
Chinara M. Tate has nothing to disclose. Allan Geliebter is a scientific advisor to Gelesis (Boston, MA), a company involved in developing obesity and diabetes treatments. He was a scientific advisor in 2003-5 to Fulfillium (Foster, CA), a company engaged in developing a gastric balloon, and received shares in the company, which is no longer active.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/5B48F060032605AF.
Rights and permissions
About this article
Cite this article
Tate, C.M., Geliebter, A. Intragastric Balloon Treatment for Obesity: Review of Recent Studies. Adv Ther 34, 1859–1875 (2017). https://doi.org/10.1007/s12325-017-0562-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-017-0562-3