Abstract
To develop a radiological score system to assess the severity of acute cerebellitis (AC) and to compare radiological severity score at the onset to cerebellar atrophy at follow-up. Clinical and MRI findings were recorded in 16 patients with AC. Radiological severity score considering topographic patterns, gray/white matter involvement, enhancement, tonsillar herniation or hydrocephalus development and clinical severity score taking into account clinical symptoms were assessed for each patient at the onset of the symptoms. Radiological and neurological sequelae were assessed at follow-up. At symptoms onset, clinical severity scale ranged from mild to severe and radiological severity score ranged from 3 to 7 with higher scores indicating a greater severity. The cut-off value of 5 for radiological score well segregated severe patients defined by clinical scale. A significant correlation between clinical scale and radiological severity scores (p < 0.001, r = 0.75) was found. At follow-up visit, all children developed cerebellar atrophy and 5 children showed neurologic sequelae while adults showed complete resolution without atrophy. Patients in whom atrophy was not observed had both older ages (p < 0.001) and a focal cerebellar involvement (p = 0.03). In patients with AC, radiological severity score may be a useful tool in evaluating clinical severity, but it is not capable to predict neither neurological sequelae nor evolution towards atrophy. Cerebellar atrophy, observed in children with AC, may be caused by several factors such as the age of patient and the extension of cerebellar involvement and it may be counterbalanced by neuronal restoring processes due to neuroplasticity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute cerebellitis (AC) is an inflammatory process involving cerebellum characterized by acute onset of cerebellar signs/symptoms (such as ataxia, nystagmus, or dysmetria) often accompanied by fever, nausea, headache, and altered mental status along with abnormalities on brain magnetic resonance imaging (MRI) [1,2,3]. AC is a pleiomorphic disease and its etiology, clinical presentation, and outcome is variable, involving both adults and children [1, 3].
AC often follows a systemic illness and the mean time between prodromal illness and the onset AC symptoms is about 1 week [4].
In children, AC is more often caused by an infectious pathogen, while in adults, para-infective and paraneoplastic causes are more common [3, 5]. Some reports highlight the link between immunity and AC but the causative agent cannot be isolated in nearly 50% of patients and several cases are classified as idiopathic AC [6, 7]. Respiratory tract infections, diarrhea and gastroenteritis, headaches, unspecified febrile illnesses, flu-like illnesses, systemic illness, and other infections may trigger AC symptoms [1].
Often AC affects children [3] at the age of 7 years and its incidence is of 5.9 patients per 100,000 per year [8], while a minority of cases happen in adults [3, 9, 10]. Pediatric AC usually has a monophasic course [1] and a good outcome with full clinical recovery in 50–86% of the cases but, in a minority of patients, the outcome may be unfavorable due to a fulminant course resulting in significant morbidity and also in mortality [11,12,13]. On the other hand, half of the adults with AC may show neurological sequelae [3].
Neuroimaging plays a leading role in the diagnostic work-up and in the assessment of patients with AC, guiding the management strategy [2, 10, 14]. MRI provides the key for diagnosis and it is a useful tool for follow-up [2, 3, 13, 15]. MRI shows a mixture of imaging features in patients with AC. About signal intensity changes, not only three main patterns of topographic distribution were reported (hemicerebellitis, bihemispheric cerebellitis, and cerebellitis with encephalitis) [1, 16] but also different gray/white matter and contrast enhancement (CE) patterns were described [11, 17].
At the onset of symptoms, bilateral diffuse hemispheric involvement was the most common imaging finding reported on non-CE-MRI [3], both in children and in adult patients. On the other hand, some differences were detected in CE patterns [17]. At the late stage, however, no difference on MRI between adults and children was reported [1, 18] . Brain computer tomography (CT) scanning is unrevealing in the acute setting, but CT is indicated mainly to exclude evidence of raised intracranial pressure, edema of cerebellar hemispheres, or brainstem compression [19,20,21].
Previous studies assessed separately either topographic distribution or the gray/white matter involvement [1, 11] but, to our knowledge, no MRI study systematically evaluated AC severity by combining cerebellar extension pattern, gray/white matter involvement, and contrast enhancement both in children and in adults with AC.
To this aim, we evaluated children and adults with AC taking into account MRI scans at the onset of symptomatology and at follow-up period.
The purposes of our study were as follows: (1) describing MRI findings at the onset of disease in order to develop a radiological severity score system; (2) comparing the radiological severity score at the onset of symptoms with clinical severity scale and with neurological sequalae; and (3) assessing radiological picture during the late follow-up and the evolution towards atrophy both in children and adults.
Materials and Methods
Patients
Patients were identified by reviewing hospital database and only patients admitted with cerebellar signs/symptoms and MRI confirmed AC were enrolled (Table 1). The following data were collected: sex, age, symptoms at disease onset, length of hospitalization, symptoms at discharge, and therapy.
Clinical severity scores based on signs/symptoms of both cerebellar (ataxia, dysmetria, balance disturbances, nystagmus, dysarthria) and extracerebellar (headache, vomit, hypotonia, behavioral changes, sensory changes) involvement were collected for all patients [22]. One point was given for each sign/symptom reported (Table 2). According to their initial symptoms, clinical severity of AC was divided into three categories defined: mild, moderate, and severe. A score 0–3 was described as mild category; a score 4–6 was described as moderate category; and a score 7–10 was described as severe category.
Neurological sequelae at follow-up visit were also recorded.
Imaging Technique
All MRI studies were performed with a 1.5 T system (Signa ® EXCITE™ HD; GE Healthcare, Milwaukee, WI, USA) using a conventional quadrature head coil. Axial T2-weighted imaging, fluid attenuated inversion recovery (FLAIR) imaging, diffusion-weighted images (a b-value of 1000 s/mm2 and a single b-0 acquisition), and contrast-enhanced axial T1-weighted imaging were obtained. ADC maps were calculated from isotropic DWI using FuncTool software, version AW 4.3, on a Sun GE AW 4.3 workstation (both from GE Healthcare).
Imaging Analysis
AC patients were categorized into the following groups according to topographic distribution of signal intensity changes on MRI: bihemispheric cerebellitis, hemicerebellitis, hemicerebellitis with encephalitic findings, and bihemispheric cerebellitis with encephalitic findings. Cerebellar alterations of gray matter (cortex, nuclei, brainstem involvement) versus both gray and white matter, enhancement and complications were also considered (Table 3).
An overall severity score was developed, assigning 1 point for each item. Scores ranged from 2 to 7, with higher scores indicating a severer radiological involvement. Follow-up MRI at 3 months and 6 months were also evaluated.
Statistical Analysis
Descriptive statistics were expressed as the median and interquartile range [IQR] for continuous variables if not otherwise specified.
Pearson correlation was used to compare age and the radiological severity score at the diagnosis.
Mann-Whitney test was used to assess the difference between the age and the radiological evolution towards atrophy in the late stages. Rank correlation was used to compare the radiological severity score and the clinical severity score at the onset, the radiological severity score and the pattern of diffuse or focal cerebellar involvement, the radiological severity score and the evolution towards atrophy in the late stages, the radiological severity score and the neurological sequelae.
χ 2 was used to compare the pattern of diffuse or focal cerebellar involvement with the evolution towards atrophy in the late stages.
About severity radiological score, receiver operating characteristic (ROC) curves analysis and area under the curves (AUC) were used to determine optimal cut-off values capable of differentiating patients with severer clinical pictures at the onset of disease.
Statistical significance was set at p < 0.05.
Statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS) for Windows version 25.0 (SPSS Inc., Chicago, IL, USA).
Results
The clinical, laboratory, imaging, and treatment data are summarized in Table 1.
Clinical Data
A total of 11 children and 5 adults were included in the study. At the time of disease onset, the median age of children was 5 years [IQR 4–7] while the median age of adults was 29 years [IQR 22–38]. The mean time between generalized signs/symptoms at onset (fever, rash, viral infections) and onset of cerebellar symptoms was 4 days (range 0–6 days).
According to clinical severity score, 2 patients were mild, 9 patients were moderate, and 5 patients were severe (Table 1).
Main Laboratory Findings in the Acute Phase of Acute Cerebellitis
All patients had laboratory studies and an extensive serological work-up. Lumbar puncture for cerebrospinal fluid (CSF) analysis was performed in 10 patients (8 children, 2 adults) showing pleocytosis in 6 patients (mean 52 cells/mmc, range 8–112) and elevated proteinorrachia in 5 patients (50–158 mg/dl). The CSF glucose was normal in all patients.
A definitive microbiological diagnosis was obtained in 14 out of 16 patients on CSF and/or blood serological tests (Table 1).
Magnetic Resonance Imaging Score in the Acute Phase
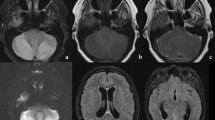
Brain MRI performed at the onset of symptoms revealed signal alterations with different topographic distribution patterns, a variable gray/white matter involvement, and presence of contrast enhancement (Fig. 1). The radiological severity score ranged from 3 to 7 with higher scores indicating a severer radiological involvement (Table 4).
MRI features to evaluate patients with acute cerebellitis. T2 FSE (a-b-c-d-e-h-i-k-l-p-q), T2 FLAIR (f-g-j), T1 FSE+mdc (m-n-o). Topographic patterns: hemicerebellitis (signal alterations in left cerebellar hemisphere in a–b), bilateral cerebellitis (diffuse signal changes in both cerebellar hemispheres in c–d), cerebellitis with encephalitis (bilateral cerebellar changes together with supratentorial signal alterations in e and arrows in f–g). Gray (GM) and white matter (WM) involvement: gray matter signal changes (cortex in h, dentate nuclei in i, dentate nuclei and brainstem in j) or combined gray and white matters signal alterations (k–l). Contrast enhancement: absent (m), present (pial in n and parenchymal in o). Complications: tonsillar herniation (white arrow in p–q) or hydrocephalus (head arrow in q).
Children showed a variable focal or diffuse cerebellar involvement while all adult patients showed a focal cerebellar pattern (Table 4). Diffusion-weighted imaging and ADC maps revealed cytotoxic edema in 2 children and vasogenic edema in the remaining patients.
Neurological Outcome at the Follow-Up
The clinical follow-up interval ranged from 14 days to 6 months.
By 3 months, adults and children showed differences about neurological sequelae, in particular, all adults had a normal neurological assessment while 5 out of 11 children showed neurologic sequelae consisting in hemiparesis, motor coordination disorders, dysarthria, and unbalancing persisting at 6 months (Table 1).
Magnetic Resonance Imaging Findings at the Follow-Up
Follow-up MRI scans were available in all patients (11 children and 5 adults) and interval ranged from 14 days to 6 months (Table 5). In children, at 10–14 days after the onset of symptoms, 3 patients showed MRI transient signal changes from corpus callosum to cerebellum; 1 child showed an increase of the cerebellar lesions; and 7 children showed a reduction of the cerebellar lesions. By 3 months after the onset of symptoms, cerebellar atrophy was reported in 8 children while 3 children showed a decrease of lesions. By 6 months, all children showed cerebellar atrophy. In adults, at 10–14 days after symptoms onset, 2 patients showed a complete resolution of the initial cerebellar lesions and 3 patients showed an improvement of radiological findings. In the later stages of follow-up, 4 out of 5 adults showed complete resolution of the signal alterations. None of adults developed cerebellar atrophy (Figs. 2 and 3) (Table 5).
Transient pattern in a child with acute cerebellitis. T2 FLAIR (a-d-g-h-j-l-n-p-r), T2 FSE (m-s), DWI (b-e-i-k-o-q), ADC (c-f). At the onset of symptoms, alterated signal intensity was shown in corpus callosum and bilateral dentate nuclei: increased in DWI (arrows in b–e) and restricted in ADC signal intensity. Fifteen days later, signal changes in corpus callosum and dentate nuclei ceased while signal intensity appeared in the cerebellar vermis (arrows in l). Three months later, resolution of cerebellar vermis alterations and atrophy development with widening of the folia and dilatation of the fourth ventricle was detected.
MR findings of cerebellitis during follow-up. T2 FSE (a-c-e-g-i-k-m-o-q), T2 FLAIR (b-d-f-h-j-l-n-p-r). Six years old child (a-b-c-d-e-f): at the onset, images revealed diffuse and high signal intensity on gray (cortex) and white matter of both cerebellar hemispheres; 15 days later, signal alterations were reduced, and slight dilatation of cerebellar folia was detected; at 3 months, parenchymal atrophy with gliosis appeared. Five-year-old patient (g-h-i-j-k-l): at the onset, images revealed high signal intensity on gray (cortex) matter of the right cerebellar hemisphere; 15 days later, signal alterations decreased; at 3 months, parenchymal atrophy of the right cerebellar hemisphere was seen. Twenty-year-old patient (m-n-o-p-q-r): at the onset, signal changes predominantly affecting the white matter of both cerebellar hemispheres were detected; 3 months later, signal alterations decreased while 6 months later, signal alterations disappeared without atrophy.
ROC curve analysis for severity radiological score showed that the score of 5 was the optimal cut-off value for radiological score and it was able to differentiate severer clinical conditions with the best combination of sensitivity (100%) and specificity (81.8%) (AUC) of 0.92, p = 0.008.
A positive correlation between clinical and radiological severity score at the onset of symptoms (p < 0.001, r = 0.75) was found.
Moreover, at 6 months, a significant difference between age and the evolution in atrophy (p < 0.001) along with a significant difference between the pattern of diffuse or focal cerebellar involvement and the evolution towards atrophy (p = 0.03) was found.
No correlation between age and radiological severity score, radiological severity score and neurological sequelae, radiological severity score and evolution towards atrophy, radiological severity score and the pattern of cerebellar involvement was found (p > 0.05).
Discussion
AC is a rare disease with a wide range in etiology, clinical presentation, radiological findings, and outcome that poses both diagnostic and therapeutic challenges [12]. MRI is a leading tool for diagnosis, and it may show a range of imaging appearances from generalized topographic patterns to selective signal changes in gray and white matters and it may detect blood-brain barrier breakdown by the presence of contrast enhancement. Moreover, MRI may be helpful in discovering intracranial complications [1, 11, 16, 19].
Previous MRI studies in AC assessed the severity of radiological framework according to either generalized topographic patterns or cerebellar gray/white matter signal alterations [1, 11, 16] and previous clinical studies did not find relationship between the radiological findings at the early stage of disease and the clinical severity [22].
We evaluated MR imaging of AC patients within 5 days of symptoms onset considering following features: topographic patterns, gray/white matter involvement, evidence of enhancement, and complications. In agreement with previous studies, our data show that bilateral cerebellar cortex involvement was the most common finding [3, 23] but it was often combined to white matter involvement or pial enhancement. On the other hand, signal alterations in supratentorial compartment, dentate nuclei, brainstem, and complications development were uncommon findings [1, 21, 24].
The first goal of this study was to evaluate imaging at the diagnosis in order to build a radiological score of severity capable of better classifying AC patients.
We compared the radiological severity score with the clinical severity scale at onset of symptoms and we found that the radiological severity score was able to differentiate between severe and mild/moderate patients on clinical severity scale with good combination of sensitivity and specificity. Thus, at the early stage of the disease, radiological severity score might be a useful tool for clinical classification of AC patients.
The second goal was to clarify, at the early stage of disease, the radiological pattern: monophasic or multiphasic. To this point, we evaluated the time course of MRI examinations, from symptoms onset to 15 days after onset, and we found a transient multiphasic pattern of inflammation only in few cases in whom corpus callosum and dentate nuclei were involved. It is conceivable that transient multiphasic signal abnormalities may reflect the involvement of cortico-ponto-cerebellar pathways that link, anatomically and functionally, the cerebrum and brain stem to the cerebellum, then the pathways involvement may or not spread to the cerebellum [25]. Previous studies suggested that these findings were not linked only to rotavirus infection [26, 27] and, accordingly, in our series, this association was not found. Because this multiphasic transient pattern may be also linked to other viruses, we confirmed that this pattern is caused more by the involved pathway than causative agent [24,25,26].
On the other hand, at the early stage of follow-up, in most of our cases, the acute cerebellar pattern did not show multiphasic transient changes in terms of topographic distribution. Usually, MRI findings detected at the onset of symptoms ameliorated substantially, until a complete resolution in some cases. Thus, in most patients, the inflammatory process is monophasic and seldom it shows a multiphasic pattern at the onset of symptoms. The multiphasic pattern may represent the expression of a unique pathologic process, with a variable course, often independent from the causative agent and from the severity of the radiological involvement.
The last aim was to assess MR findings of AC in the later stages of follow-up (between 3 and 6 months after symptoms onset). Previous studies reported reversal of the acute changes and, in some instances, a gradual development towards cerebellar atrophy with widening of the folia and dilatation of the fourth ventricle [1, 17, 28]; moreover, on FLAIR images, a slightly high intensities in atrophic cerebellar cortices were detected, reflecting AC-induced gliosis [12].
In adults, only few cases of cerebellar atrophy after AC were reported [18, 29]. We detected differences in the evolution of MRI framework in adults and children. In children, we found development of a progressive cerebellar atrophy, while in adults, the inflammatory process showed a complete resolution without atrophy. Both higher ages and focal involvement, as commonly observed in our patients, causing a reduced inflammatory response, might be the cause of the complete remission.
Previous studies reported a link between the gradual development of severe cerebellar atrophy and cerebellar ataxia while other studies did not and in patients with atrophy on MRI, neurologic examination may be normal [11]. Our data showed that cerebellar atrophy, severity radiological score, and neurological sequelae seem to be unrelated [2, 11, 30]; accordingly, although atrophy was present in all our children, they showed a substantial remission of symptoms.
Because a mismatch between neurological sequalae and atrophy may be detected in children, it is conceivable that cerebellar involvement occurring at early ages may activate plastic changes that are more pronounced than at older age as observed in children with diseases affecting central nervous system [31], and cerebellum [32] in whom neuroplastic changes may ameliorate or overcome initial impairments.
Our data suggest that radiological severity score may be useful in clinical classification of the AC patients at the early stage of the disease, but it is not linked to neurological sequelae or in predicting the evolution towards atrophy that may be mainly due to patient-linked factors such as age of symptoms onset or the development of neuroplasticity phenomena.
This study has some limitations. First, the small sample size and retrospective design may have caused potential selection bias, even though prospectively collected data were used. Second, the clinical score system, used in pediatric cohort, may have allowed an underestimation of clinical signs in adults. Further studies, in larger cohorts of patients, are warranted to confirm our conclusions.
Conclusion
MRI analysis of cerebellar findings may be helpful in classifying the clinical severity of AC at the onset of the disease. Radiological approach may reflect clinical severity at the onset of the disease but is not able to predict the radiological and clinical outcomes. The evolution of the radiological findings observed at the late follow-up in adults or in children might be due both to the age of patient and the neuroradiological pattern (diffuse/focal) and it may be influenced by neuroplasticity phenomena.
References
Emelifeonwu JA, Shetty J, Kaliaperumal C, Gallo P, Sokol D, Soleiman H, et al. Acute cerebellitis in children: a variable clinical entity. J Child Neurol. 2018;33:675–84.
De Bruecker Y, Claus F, Demaerel P, Ballaux F, Sciot R, Lagae L, et al. MRI findings in acute cerebellitis. Eur Radiol. 2004;14:1478–83.
Van Samkar A, Poulsen MNF, Bienfait HP, Van Leeuwen RB. Acute cerebellitis in adults: a case report and review of the literature. BMC Res Notes. 2017;10:610.
Bozzola E, Bozzola M, Tozzi AE, Calcaterra V, Longo D, Krzystofiak A, et al. Acute cerebellitis in varicella: a ten year case series and systematic review of the literature. Ital J Pediatr. 2014;40:57.
Tlili-Graiess K, Mhiri Souei M, Mlaiki B, Arifa N, Moulahi H, Jemni Gharbi H, et al. Imaging of acute cerebellitis in children. Report of 4 cases. J Neuroradiol. 2006;33:38–44.
Paketci C, Edem P, Okumus C, Sarioglu FC, Bayram E, Hiz S, et al. Herpes simplex virus-1 as a rare etiology of isolated acute cerebellitis: case report and literature review. J Neuro-Oncol. 2020;26:270–2.
Gruis KL, Moretti P, Gebarski SS, Mikol DD. Cerebellitis in an adult with abnormal magnetic resonance imaging findings prior to the onset of ataxia. Arch Neurol. 2003;60:877–80.
Nussinovitch M, Prais D, Volovitz B, Shapiro R, Amir J. Post-infectious acute cerebellar ataxia in children. Clin Pediatr (Phila). 2003;42:581–4.
Al-Shokri SD, Karumannil SA, Mohammed SS, Sadek MS. Post-Epstein-Barr virus acute cerebellitis in an adult. Am J Case Rep. 2020;21:e918567.
Donmez FY, Agildere AM, Tore HG, Ure S, Benli S. Abnormal diffusion-weighted imaging findings in an adult patient with acute cerebellitis presenting with a normal magnetic resonance imaging. J Comput Assist Tomogr. 2008;32:156–8.
Yildirim M, Gocmen R, Konuskan B, Parlak S, Yalnizoglu D, Anlar B. Acute cerebellitis or postinfectious cerebellar ataxia? Clinical and imaging features in acute cerebellitis. J Child Neurol. 2020;35:380–8.
Kornreich L, Shkalim-Zemer V, Levinsky Y, Abdallah W, Ganelin-Cohen E, Straussberg R. Acute cerebellitis in children: a many-faceted disease. J Child Neurol. 2016;31:991–7.
Alomani H, Arshad M, Elzonfly M, Aldakhil AA, Alharbi AH, Alasqah A, et al. Pediatric fulminant cerebellitis is still a fatal disease that we know little about! Two case reports and a literature review. Am J Case Rep. 2021;22:e928370.
Hacohen Y, Niotakis G, Aujla A, Siddiqui A, McCormick D, Bassi S, et al. Acute life threatening cerebellitis presenting with no apparent cerebellar signs. Clin Neurol Neurosurg. 2011;113:928–30.
Patel P, Rayamajhi S, Tokala H, Laird-Fick H. An unusual cause of altered mental status in elderly-acute cerebellitis: a case report and review. Case Rep Med. 2013;2013:653925.
Carceller Lechón F, Duat Rodríguez A, Sirvent Cerdá SI, Khabra K, de Prada I, García-Peñas JJ, et al. Hemicerebellitis: report of three paediatric cases and review of the literature. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2014;18:273–81.
Bakshi R, Bates VE, Kinkel PR, Mechtler LL, Kinkel WR. Magnetic resonance imaging findings in acute cerebellitis. Clin Imaging. 1998;22:79–85.
Adachi M, Kawanami T, Ohshima H, Hosoya T. Cerebellar atrophy attributed to cerebellitis in two patients. Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med. 2005;4:103–7.
Venkatesh M, Chakkalakkoombil SV, Duraipandi MB, Gulati R. Complicated acute cerebellitis with obstructive hydrocephalus and tonsillar herniation in a child. BMJ Case Rep. 2017;2017:bcr-2017-219337.
Cohen JE, Gomori M, Benifla M, Itshayek E, Shoshan Y. Acute pseudotumoral hemicerebellitis: diagnosis and neurosurgical considerations of a rare entity. J Clin Neurosci Off J Neurosurg Soc Australas. 2014;21:337–9.
Van Lierde A, Righini A, Tremolati E. Acute cerebellitis with tonsillar herniation and hydrocephalus in Epstein-Barr virus infection. Eur J Pediatr. 2004;163:689–91.
Lancella L, Esposito S, Galli ML, Bozzola E, Labalestra V, Boccuzzi E, et al. Acute cerebellitis in children: an eleven year retrospective multicentric study in Italy. Ital J Pediatr. 2017;43:54.
Hirayama K, Sakazaki H, Murakami S, Yonezawa S, Fujimoto K, Seto T, et al. Sequential MRI, SPECT and PET in respiratory syncytial virus encephalitis. Pediatr Radiol. 1999;29:282–6.
Kubota T, Suzuki T, Kitase Y, Kidokoro H, Miyajima Y, Ogawa A, et al. Chronological diffusion-weighted imaging changes and mutism in the course of rotavirus-associated acute cerebellitis/cerebellopathy concurrent with encephalitis/encephalopathy. Brain and Development. 2011;33:21–7.
Tang Y, Suddarth B, Du X, Matsumoto JA. Reversible diffusion restriction of the middle cerebellar peduncles and dentate nucleus in acute respiratory syncytial virus cerebellitis: a case report. Emerg Radiol. 2014;21:89–92.
Kizilkilic O, Karaca S. Influenza-associated encephalitis-encephalopathy with a reversible lesion in the splenium of the corpus callosum: case report and literature review. AJNR Am J Neuroradiol. 2004;25:1863–4.
Mori T, Morii M, Kuroiwa Y, Hotsubo T, Fuse S, Tsustumi H. Rotavirus encephalitis and cerebellitis with reversible magnetic resonance signal changes. Pediatr Int Off J Jpn Pediatr Soc. 2011;53:252–5.
Hayakawa H, Katoh T. Severe cerebellar atrophy following acute cerebellitis. Pediatr Neurol. 1995;12:159–61.
Karmon Y, Inbar E, Cordoba M, Gadoth N. Paraneoplastic cerebellar degeneration mimicking acute post-infectious cerebellitis. Cerebellum Lond Engl. 2009;8:441–4.
Hennes E, Zotter S, Dorninger L, Hartmann H, Häusler M, Huppke P, et al. Long-term outcome of children with acute cerebellitis. Neuropediatrics. 2012;43:240–8.
Pilato F, Dileone M, Capone F, Profice P, Caulo M, Battaglia D, et al. Unaffected motor cortex remodeling after hemispherectomy in an epileptic cerebral palsy patient. A TMS and fMRI study. Epilepsy Res. 2009;85:243–51.
Bonaventura E, Purpura G, Pasquariello R, Da Prato S, Di Lieto MC, Barsotti J, et al. Complex neurodevelopmental disorder in a preterm child with unilateral cerebellar hemorrhage. Appl Neuropsychol. Child. 2021;1–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
We declare that all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
Rosalinda Calandrelli declares that she has no conflict of interest. Marco Panfili declares that he has no conflict of interest. Huong Elena Tran declares that she has no conflict of interest. Cesare Colosimo declares that he is a scientific consultant for Bracco Diagnostics Inc. and Bayer HealthCare. Fabio Pilato declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Calandrelli, R., Marco, P., Tran, H.E. et al. A Novel Radiological Score System to Assess the Clinical Severity in Patients with Acute Cerebellitis. Cerebellum 22, 173–182 (2023). https://doi.org/10.1007/s12311-022-01377-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01377-5