Abstract
We sought to assess structural and functional patterns of cerebellum in multiple system atrophy (cerebellar type), and investigate the associations of structural and functional cerebellar gray matter abnormalities. We collected magnetic resonance imaging data of 18 patients with multiple system atrophy (cerebellar type) and 18 health control subjects. The gray matter loss across the motor and cognitive cerebellar territories in patients was assessed using voxel-based morphometry. And change in the connectivity between the cerebellum and large-scale cortical networks was assessed using resting-state functional MRI analysis. Furthermore, we assessed the relationship between the extent of cerebellar atrophy and reduced-activation in the cerebellar-cortical and subthalamo-cerebellar functional connectivities. We confirmed the gray matter loss across the motor and cognitive cerebellar territories in patients and found that the extent of cerebellar atrophy was correlated with decreased connectivity between the cerebellum and large-scale cortical networks, including the default, frontal parietal, and sensorimotor networks. The volume reduction in the motor cerebellum was closely associated with the clinical motor severity. A post hoc analysis showed reduced-activation in the subthalamo-cerebellar functional connectivity without the subthalamic nucleus atrophy. These results emphasized significant atrophy in the cerebellar subsystem and its association with the large-scale cortical networks in multiple system atrophy (cerebellar type), which may improve our understanding of the neural pathophysiology mechanisms of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple system atrophy (MSA) is a progressive neurodegenerative disease pathologically characterized by alpha synuclein-positive glial cytoplasmic inclusions (GCIs), which lead to the degeneration and death of neuron mainly in the striatum, cerebellum, and olivopontine structures [1]. Currently, MSA is mainly classified into a parkinsonian (MSA-p) and a cerebellar variant (MSA-c) [2]. In this study, we focused on MSA-c, which emphasizes on the cerebellum atrophy and dysfunction.
As a structure of the cerebello-thalamo-cortical circuit (CTC), the cerebellum is shown to exert a far-reaching influence on motor, cognitive and emotional functions, through its anatomical and functional interconnections with the basal ganglia and much of the cortical mantle [3, 4]. These heterogeneous functions have been suggested to indicate the existence of a “motor” cerebellum connecting to cortical motor regions, and a “cognitive” cerebellum connecting to prefrontal and parietal cortices [3, 5,6,7]. And the CTC circuit procession [6, 8, 9] suggested that the anatomically distinct cerebellar subregions are projected to unique cortical targets, which confirmed the heterogeneous structure and function of the cerebellum. From a network perspective, the cerebellar motor subsystem is consistently associated with the sensorimotor network, while the cognitive subsystem is functionally connected with large-scale cognitive-related networks, including the executive control, salience, and default mode networks (DMN) [10, 11]. Although cerebellar architecture is arguably more complex than two distinct subsystems [10], the division into motor and cognitive cerebellar subsystems might provide an important framework for understanding how alterations of the cerebellum’s structure and function in disease states.
Most neuroimaging studies focusing on the cerebellar atrophy and dysfunction patterns revealed changes in volume and functional connectivity pattern in the cerebellum, reflecting the pathological changes of MSA-c [12,13,14]. In a previous study, the disrupted motor network was reported associated with the cerebellum dysfunction in patients with MSA-c [15]. Recently, one study of MSA-c indicated the altered functional connectivity between cerebellum and large-scale cortical networks [16].These findings provided evidences for the hypothesis of “disconnection syndrome”[17]. However, no previous studies reported the structural and functional patterns of motor and cognitive cerebellar subsystems in patients with MSA-c. And the association between the structure and function of the cerebellar subsystems in MSA-c is unclear.
Here, we hypothesized that there were different disrupted structural and functional patterns of cerebellum subsystems in patients with MSA-c; in addition, the extent of gray matter loss would be associated with alterations in cerebellar-cortical resting-state connectivity. To realize this goal, we compared the cerebellar subregions atrophy between patients with MSA-c and health controls (HCs). And we investigated whether local volume reduction of cerebellar subsystems was associated with alterations in cerebellar-cortical functional connectivity. Furthermore, a post hoc analysis was conducted to determine subthalamic nucleus (STN) atrophy and the relationship between the cerebellar subgroups and STN, to identify whether the connectivity between cerebellar atrophic subregions and cerebellar input source might be affected. Finally, we explore the relationship between motor severity, connectivity, and atrophy of cerebellum in the patients.
Material and Methods
Participants
A total of 36 subjects were recruited from Dongfang Hospital of Beijing University of Chinese Medicine, including 18 patients with MSA-c and 18 healthy HCs (matched by age and educational level).
All subjects provided their written informed consent before being involved in the study. The research ethics committee of Dongfang Hospital of Beijing University of Chinese Medicine approved the study protocols.
The diagnosis of MSA fulfilled the criteria of probable MSA-c type defined by the American Academy of Neurology and the American Autonomic Society [2]. The inclusion criteria for HCs were as follows:
-
1.
Absence of neurological or psychiatric disorders including stroke, depression, or epilepsy.
-
2.
Absence of neurological deficiencies including visual or hearing loss.
-
3.
Absence of abnormal findings of brain MRI, such as infarction or focal lesions.
The subjects of hemorrhage, infarction, tumors, trauma, or severe white matter hyperintensity were excluded from the study. All individuals were evaluated by performing complete physical and neuropsychological examinations including mini-mental state examination (MMSE), Montreal Cognitive Assessment (MoCA), and Unified Multiple System Atrophy Rating Scale (UMSARS). The clinical examinations were performed on the day before undergoing functional magnetic resonance imaging (fMRI) scan.
MRI Acquisition and Analysis
Data Acquisition
MRI data acquisition was performed on a GE 3.0 T Discovery 750 scanner. Foam padding and headphones were used to control head motion and scanner noise. The data scan parameters of resting-state fMRI were as follows: repetition time (TR)/echo time (TE)/flip angle (FA) = 2000 ms/30 ms/90°, field of view (FOV) = 24 × 24 cm2, resolution = 64 × 64 matrix, slices = 36, thickness = 3 mm, gap = 1 mm, voxel size = 3.75 × 3.75 × 3 mm3, and bandwidth = 2232 Hz/pixel. For registration purposes, high-resolution anatomical images were collected using a 3D brain volume (BRAVO) T1-weighted sequence with the following parameters: TR/TE/inversion time (TI)/FA = 8150 ms/3.17 ms/450 ms/12°, resolution = 256 × 256 matrix, slices = 188, thickness = 1 mm, voxel size = 1 × 1 × 1 mm3.
Voxel-Based Morphometry Analysis
VBM analysis was performed using the Statistical Parametric Mapping software (SPM12;http://www.fil.ion.ucl.ac.uk/spm/software/spm12). Structural images of gray matter (GM), white matter and cerebrospinal fluid were segmented [18].
The initial affine of the GM concentration map was registered into the Montreal Institute of Neurology (MNI) space (http://www.mni.mcgill.ca/). Nonlinear warping of GM concentration images was performed using differential analytic registration (DARTEL) techniques and was resliced to a resolution of 1.5 mm3[19, 20]. To obtain the relative GM of each voxel, the GM concentration map was multiplied by a nonlinear determinant derived from spatial normalization. Finally, the GM images were smoothed using a Gaussian kernel of 8 mm × 8 mm × 8 mm full-width at half-maximum (FWHM) to compensate for residual between-subject anatomical differences. After spatial preprocessing, statistical analysis was performed using a smoothed normalized GM map.
The cerebellar subregions (CBMc and CBMm, respectively) were defined by using a validated probabilistic atlas of the human cerebellum available in the SUIT [21]. Bilateral Crus I and Crus II were defined as CBMc, and bilateral leaflets V, VI, VIIb, VIIIa, and VIIIb were defined as CBMm [3] (see Fig. 1). Extracting regional GM intensity measurements in contrast to the quiescent state pattern, creating the cognitive and motor cerebellar regions of interest CBMc and CBMm. The mean gray matter intensity value of non-zero voxels in the region of interest was extracted and averaged for each subject. The result represented value of cerebellar atrophy degree for each individual in the study. A voxel-wise general linear model (GLM) and family-wise error (FWE) correction were used for statistical analysis to compare GM intensity of cerebellar subregions between MSA patients and HCs. This analysis was also used in the published subthalamic nucleus (STN) defined by the middle-aged data set [22]. All statistical tests were two-sided, and a P value < 0.05 was considered statistically significant.
Resting-State Functional Connectivity Analysis
TheData Processing Assistant for Resting-State fMRI (DPARSF;http://rfmri.org/DPARSF) was used for preprocessing functional images. Briefly, preprocessing included data conversion, the first 10 volumes removal, slice timing correction, and head motion correction. To spatially normalize the fMRI data, the realigned volumes were spatially standardized into the MNI space using the EPI template. The functional images were resampled into a voxel size of 3 × 3 × 3 mm3. Then, the functional images were smoothed with a Gaussian kernel of 4 mm FWHM.
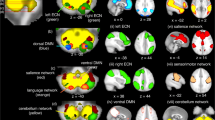
The putative resting-state network analysis was performed by using the group independent component analysis (ICA) of the GIFT software (http://icatb.sourceforge.net, version 1.3i). The number of individual components (ICs) was determined by using the minimum component length (MDL) standard [23]. The fMRI data of all participants was collected in a group, and the time dimension of the aggregated data set was reduced by principal component analysis (PCA) and estimated by IC using the Informax algorithm (with time course and spatial maps) [24, 25]. We selected the highly stable ICASSO toolbox [26], categorized it using a visual inspection of the average component map and its average power spectrum, by running Infomax 100 times and randomized and bootstrapped datasets under different initial conditions. Back-reconstructed for ICs and time courses of each participant were analyzed by using CICA3 [27]. The individual-level components were obtained from back-reconstruction and were converted into a z-score. The z-score reflected the degree of time series of a given voxel correlates with the mean time series of its belonging component. We mainly focused on 6 large-scale cortical networks [28]: anterior default network (aDMN), posterior default network (pDMN), left frontal parietal network (lFPN), right frontal parietal network (rFPN), dorsal attention network (dAN), sensorimotor network (SMN). Statistical analysis of individual ICA component maps of all subjects was performed by a random-effect single-sample t test, creating the sample-specific large-scale network masks (P < 0.05, FWE correction, cluster size > 100).
Finally, the processed images were imported into the functional connectivity toolbox in DPABI (http://rfmri.org/dpabi). Several covariates, including parameters of head motion, white matter, cerebrospinal fluid (CSF) signal, and global average signal were regressed by multiple linear regression analysis. After preprocessing, time band-pass filtering (0.01–0.08 Hz) of the fMRI data reduced the effects of a low-frequency drift and high-frequency physiological noise, such as respiratory and cardiac rhythms. We extracted blood oxygen level-dependent time processes from each of the nuisance-corrected source regions of interest and the similar processed the STN. And then, we analyzed the strength of each pair-wise connection across the regions of interest within network using the Pearson correlation. We normalized these values by using Fisher’s r-to-z transformation and then compared the differences of the values between HCs and patients by using Bonferroni-Holm correction for multiple comparisons[29].
Relationship Between Cerebellar Gray Matter Intensity and Resting-State Functional Connectivity
We explored the relationship between cerebellar GM atrophy and resting-state functional connectivity within network and the STN.
The correlation between the cerebellar subregions atrophy and cerebello-cortical connectivity in HCs was used as a baseline; the scores of patients group were directly compared with HCs.
The positive differences between patients and HCs implied a relative increase in connectivity accompanied with cerebellar atrophy, which might reflect a compensatory mechanism, while the negative differences between the patients and the HCs indicated a decreased functional cerebellum-cortical connectivity with the cerebellar subregions atrophy.
Relationship Between Motor Severity, Connectivity, and Cerebellar Atrophy
To determine whether a relationship existed between motor severity, atrophy, and connectivity, we analyzed the relationship between each cerebellar subregion (CBMm and CBMc) atrophy scores and the motor function as measured by UMSARS II by using a Pearson’s correlation. The relationships between the functional connectivity changes of each network and the UMSARS II were also examined [30].
Statistical Analysis
Continuous variables of demographics and neuropsychological scores were presented as mean ± standard deviation (SD) and compared by Student’s t tests, or Mann-Whitney U test based on distributional properties. Categorical variables were described as percentages and compared by chi-square tests. Spearman’s rank correlation test was performed to obtain the correlation coefficients between measured values or scores. The statistical tests were two-sided, and a P value < 0.05 was considered statistically significant. Analyses were conducted with SPSS version 25.0 (IBM Corp, Armonk, NY, USA).
Results
Demographic and Neuropsychological Tests
Clinical and demographic information for the subjects is shown in Table 1. No significant difference in gender, MMSE and MoCA scores was found between the MSA-c type and control groups. The patients exhibited significant higher UMSARS I and II scores.
Neuroimaging Analyses
Comparison of Cerebellar Atrophy in Patients Versus HCs by VBM Analysis
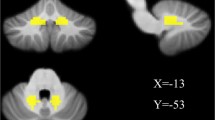
Atrophy in cerebellar subregions was apparent in patients relative to HCs. Specifically, one large and contiguous cluster was identified in the CBMc (voxel size: 10776; peak MNI co-ordinates: x = −7.5, y = − 67.5, z = − 34.5). Another large and contiguous cluster was identified in the CBMm (voxel size: 20158; peak MNI co-ordinates: x = −6, y = −67.5, z = − 34.5). After FWE correction, comparisons between overall mean gray matter intensity of patients compared with those of HCs revealed an overall volume loss of 16.59% in the CBMc (P < 0.001) and 18.74% in the CBMm (P < 0.001) (Fig. 2).
Voxel-based morphometry showing gray matter loss in the cognitive and motor cerebellar territories for MSA patients in comparison to controls. Areas of significant gray matter loss (red) in the cognitive and motor cerebellar territories, for patients with MSA versus control subjects. Results reported at P < 0.05, corrected for multiple comparisons (FWE)
Resting-State Functional Connectivity Analysis
To investigate functional connectivity alterations in the patients, seed based interregional correlation analysis was performed. We extracted several large-scale cortical networks including aDMN, pDMN, lFPN,rFPN, dAN, and SMN using the ICA method. Then, we selected the two subregions of the bilateral cerebellum and analyzed the functional connectivity between the cerebellar subsystem and cortical networks.
After FWE correction, assessments for the patterns of resting-state cortico-cerebellar connectivity revealed disrupted connectivity between the CBMc and pDMN (patients: mean r = 0.38 ± 0.05; HCs: mean r = 0.63 ± 0.07; t = − 3.025; P = 0.005),rFPN (patients: mean r = 0.31 ± 0.07; HCs: mean r = 0.60 ± 0.05; t = − 3.225; P = 0.003), and dAN(patients: mean r = 0.41 ± 0.07; HCs: mean r = 0.60 ± 0.07; t = − 2.040; P = 0.005) in MSA-c patients. In addition, after FWE correction, decreased connectivity was also observed between the CBMm and cortical networks pDMN (patients: mean r = 0.43 ± 0.05; HCs: mean r = 0.68 ± 0.05; t = − 3.518; P = 0.001), lFPN (patients: mean r = 0.67 ± 0.06; HCs: mean r = 1.03 ± 0.05; t = − 4.580; P = 0.000),rFPN (patients: mean r = 0.28 ± 0.06; HCs: mean r = 0.45 ± 0.05; t = − 2.087; P = 0.045),dAN (patients: mean r = 0.44 ± 0.07; HCs: mean r = 0.72 ± 0.07; t = −2.999; P = 0.005),and SMN (patients: mean r = 0.52 ± 0.06; HCs: mean r = 0.73 ± 0.06; t = − 2.399; P = 0.022) in the MSA-c patients (Fig. 3).
Functional connectivity from the cerebellum to the cortical networks. The resting state connectivity relationships between cerebellar subregions and large-scale cortical networks. The solid lines denote a loss of connectivity. pDMN = posterior DMN; lFPN = left frontal parietal network; rFPN = right frontal parietal network; Attention = attention network; SMN = sensorimotor network
Within the CBMm and STN, patients displayed decreased connectivity when compared to HCs (patients: mean r = 0.22 ± 0.06; HCs: mean r = 0.42 ± 0.07; t = − 2.151; P = 0.039, after correcting for FWE).
Relationship Between Cerebellar GM Intensity and the Resting-State Functional Connectivity
The extent of atrophy in the cerebellum was differentially correlated with specific impairments in resting-state connectivity between the cerebellar subsystem and the resting-state networks in patients, when compared with HCs.
Specifically, after Bonferroni-Holm correction, the extent of cerebellar atrophy was associated with a decrease in connectivity between the CBMc and the aDMN (r = − 0.547, P = 0.023), pDMN (r = − 0.496, P = 0.043), rFPN (r = − 0.519, P = 0.033). With regard to the CBMm network, cerebellar atrophy was related to a significantly decreased connectivity between the CBMm and the aDMN (r = − 0.637, P = 0.006), pDMN (r = −0.708, P = 0.001), rFPN (r = − 0.558, P = 0.020), SMN (r = − 0.506.9, P = 0.038), suggesting a potential relationship between atrophy and functional connectivity (please see Fig. 4).
The structure-function relationships from cerebellum to cortical network. Relationships between cerebellar atrophy and resting-state cerebellar functional connectivity. The solid lines denote a loss of connectivity. aDMN = anterior DMN; pDMN = posterior DMN; rFPN = right frontal parietal network; SMN = sensorimotornetwork
After FWE correction, there was no significant difference between HCs and patients with atrophy in the STN (P > 0.500). At the same time, the degree of cerebellar atrophy was found to be related to the decrease in connectivity between the CBMm and the STN (r = − 0.563, P = 0.019, after Bonferroni-Holm correction).
Relationship Between Motor Severity, Connectivity, and Cerebellar Atrophy
We observed an inverse correlation between the UMSARS II and the extent of cerebellar atrophy in the CBMm (r = − 0.527, P = 0.030). No other relationships between atrophy of CBMc and connectivity were significantly correlated with UMSARS scores (please see Fig. 5).
Discussion
The present study aimed at seeking the relationship of structural and functional cerebellar GM abnormalities in MSA-c. Our findings revealed a significant decreased volume in several cerebellum subregions in MSA-c patients, and showed the extent of cerebellar atrophy was related to reduced functional connectivity between cerebellar subregions and large-scale cortical networks including DMN, rFPN, dAN, lFPN, and SMN. Furthermore, we found abnormalities in motor performance to be associated with the extent of local cerebellar motor subsystem atrophy, which might be accompanied by decreased connectivity between the functional subsystems and large-scale cortical networks and STN. These results confirmed a great role of intrinsic structural and functional changes in the cerebellum in MSA-c and might improve our understanding of the neural pathophysiology mechanisms of MSA-c from the perspective of network.
We observed significant atrophy in cerebellar motor and cognitive subregions in patients with MSA-c. Structurally, most MSA neuroimaging researches have focused on the gray matter atrophy detected by the structural MRI [12, 14, 31,32,33]. Our study highlighted the atrophy of the cerebellar cognitive and motor subregions. The alpha synuclein-positive GCIs deposited in the cerebellum were found to destroy the neuron, which might lead to the atrophy of the cerebellum and cerebellar ataxia in patients with MSA-c. These findings might reflect the pathological changes of MSA- c [12, 14]. The novelty of this study was to explore the influence of cerebellar subregions loss on functional connectivity patterns within cerebellar-cortical networks in MSA-c. We did find a substantial impact of cerebellar atrophy on several decreased resting-state connectivity between cerebellar subsystems and large-scale cortical networks. The data on resting-state cerebello-cortical functional connectivity are consistent with numerous researches showing its reduced-activation[15,16,17, 34, 35].Our results demonstrate that the cerebellar GM volume loss may be an important determinant of alterations in some cerebello-cortical functional connectivity. Local volume reduction of cerebellar GM in the patients was related to a loss of connectivity between the motor cerebellum and aDMN, pDMN, and rFPN, as well as SMN, and between the cerebellar cognitive subsystem and aDMN and pDMN, as well as rFPN.
The rFPN is reported to play an important role in the executive control function, and the SMN was known involved in primary sensory and motor cortices[36]. We speculate that the disconnection between the motor cerebellum and these networks due to volume loss may be responsible for the control deficits and sensorimotor impairment of the disease. The DMN is known as a functional-anatomic network, which is involved in memory, self-reflection, and stream-of-consciousness processing [37]. Interestingly, we observed the DMN involved in the hypoactive cortico-cerebellar motor circuitry in MSA-c. This result suggests the complicated mechanism of local cerebellar atrophy effecting motor deficits, in which many cortical networks associated with several functions including not only motor but also emotion [38], behavior[39, 40], and cognition[41, 42] is involved.
Cerebellar atrophy in MSA has been previously reported to correlate with behaviors [43]. Consistent with this result, our findings show a close relationship between motor severity and cerebellar motor subregion atrophy in MSA-c. As we know, the scores of UMSARS II can reflect the motor function. These correlations suggest that cerebellar motor subsystem atrophy may account for motor dysfunction in MSA-c due to pathological loss of connectivity with large-scale cortical networks. Since the correlations between motor cerebellum and cortical networks occur in networks with no direct anatomical connections, we investigate the association of motor cerebellar atrophy with the major input structure, the STN [44]. In our study, we find reduced-activation in the subthalamo-cerebellar functional connectivity without volume reduction in STN, suggesting that motor cerebellum change might influence connectivity between the cerebellar subgroups and large-scale cortical networks through the STN. This result highlighted that atrophy of the cerebellar motor subregion may be an initiating step in the hypoactive cortico-cerebellar motor circuitry.
In contrast to the bidirectional changes in connectivity between local cerebellar atrophy and cerebellar-cortical connectivity [45, 46], in the current study, no cortical networks were observed over recruited by patients, in a compensatory attempt. Our results provide evidence of no network-level compensatory mechanism in MSA-c, which consistent with other MSA neuroimaging researches[16, 35, 47]. Given the over-activation in cortical functional connectivity patterns induced by cerebellar atrophy in PD [46] and healthy aging [45], it is possible that massive degeneration may deprive a compensatory mechanism of MSA, whereas surviving regions may provide a compensatory mechanism [48]. In MSA-c, a wide range of neurons were influenced by the degeneration in the cerebellum, suggesting that no regions are left to provide a compensatory mechanism. Our findings emphasize the role of cerebellar atrophy in pathological pattern in MSA-c, which is different from those in PD or normal aging.
Even though the decreased connectivity patterns between the cognitive cerebellum and several cortical networks including DMN and rFPN were found in the present study, no cognitive performance differences were observed related to cerebellar atrophy between patients and HCs. These findings are inconsistent with previous reports, which showed the cognitive deficits in relation to the cerebello-cortical network contributions [34] or cerebellar volume reductions accounting for cognitive deficits[43] in MSA. Unlike with pathology within the cerebral cortex in Alzheimer’s disease, selective neuronal loss is found mainly present in the cerebellum and basal ganglia in MSA[49], thus cognitive impairment is not available for all MSA patients. Although the cerebellum plays a crucial role in cognitive regulation [50], the structural and functional abnormalities in the cognitive cerebellum contributing to cognitive manifestations were not found in the present study. These results allow us to take the hypothesis that the cerebral cortex or cortical networks play a major role in the neurobiological mechanisms of cognitive impairment in MSA-c, and volume loss in cognitive cerebellum which account for decreased cerebello-cortical functional connectivity may be just a supplementary link in cognitive impairment. However, this hypothesis has yet to be verified.
Some of the limitations of our study are worth mentioning. First, in the current study, we mainly focused on the functional connectivity between cerebellar subregions and cortical networks. Further studies that simultaneously combine the resting-state and task-related fMRI, diffusion, perfusion, and metabolic data would be more helpful for a deep understanding of the MSA patients. Second, our study only focused on MSA-c. However, exploring MSA different subtype (parkinsonian and cerebellar variants) would provide valuable biomarkers for the early differential diagnosis of the disease. Third, MoCA and MMSE were administered as a measure of global cognitive performance without specific cognitive domains (eg. memory, mental speed, attention or executive function) being assessed, which might result in no significant difference in cognitive function being found between patients and HCs. Finally, a relatively small sample size was included in this study. Therefore, future longitudinal studies with a much larger sample are warranted to elucidate the progressive functional changes of the MSA patients and its relationship with the clinical performances.
Conclusion
Our findings reveal the relationship of atrophic and functional changes in the cerebellum in MSA-c. Furthermore, we have identified that cerebellar motor subsystem atrophy may account for motor deficit in MSA-c due to decreased connectivity with large-scale cortical networks and STN. These results not only provide compelling evidence that cerebellar atrophy is pivotal in neuropathological pathways of MSA-c, but also suggest the potential mechanism of cerebellar atrophy effecting motor dysfunction. These findings have important implications for the underlying neurobiology of MSA-c and added new evidence for the disconnection syndrome of MSA-c.
References
Krismer F, Seppi K, Wenning GK, Papapetropoulos S, Abler V, Goebel G, et al. Abnormalities on structural MRI associate with faster disease progression in multiple system atrophy. Parkinsonism Relat Disord. 2019;58:23–7.
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6.
Balsters JH, Laird AR, Fox PT, Eickhoff SB. Bridging the gap between functional and anatomical features of cortico-cerebellar circuits using meta-analytic connectivity modeling. Hum Brain Mapp. 2014;35:3152–69.
Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–77.
Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–63.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44.
O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–65.
Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–50.
Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–12.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–45.
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–94.
Deguchi K, Ikeda K, Kume K, Takata T, Kokudo Y, Kamada M, et al. Significance of the hot-cross bun sign on T2*-weighted MRI for the diagnosis of multiple system atrophy. J Neurol. 2015;262:1433–9.
Kim YM, Cheon CK, Yoo HW. Successful treatment with eliglustat in unexpected gastrointestinal involvement in a patient with type 1 Gaucher disease. Mol Genet Metab. 2017;120:S74.
Matsusue E, Fujii S, Kanasaki Y, Kaminou T, Ohama E, Ogawa T. Cerebellar lesions in multiple system atrophy: postmortem MR imaging-pathologic correlations. AJNR Am J Neuroradiol. 2009;30:1725–30.
You H, Wang J, Wang H, Zang Y-F, Zheng F-L, Meng C-L, et al. Altered regional homogeneity in motor cortices in patients with multiple system atrophy. Neurosci Lett. 2011;502:18–23.
Ren S, Zhang H, Zheng W, Liu M, Gao F, Wang Z, et al. Altered functional connectivity of cerebello-cortical circuit in multiple system atrophy (cerebellar-type). Front Neurosci. 2019;12:996.
Rosskopf J, Gorges M, Mueller H-P, Pinkhardt EH, Ludolph AC, Kassubek J. Hyperconnective and hypoconnective cortical and subcortical functional networks in multiple system atrophy. Parkinsonism Relat Disord. 2018;49:75–80.
Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–86.
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113.
Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802.
Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46.
Keuken MC, Bazin PL, Schafer A, Neumann J, Turner R, Forstmann BU. Ultra-high 7T MRI of structural age-related changes of the subthalamic nucleus. J Neurosci. 2013;33:4896–900.
Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28:1251–66.
Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51.
Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–81.
Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–22.
Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–95.
Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–37.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
Wenning GK, Tison F, Seppi K, Sampaio C, Diem A, Yekhlef F, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord. 2004;19:1391–402.
Chelban V, Bocchetta M, Hassanein S, Haridy NA, Houlden H, Rohrer JD. An update on advances in magnetic resonance imaging of multiple system atrophy. J Neurol. 2019;266:1036–45.
Dash SK, Stezin A, Takalkar T, George L, Kamble NL, Netravathi M, et al. Abnormalities of white and grey matter in early multiple system atrophy: comparison of parkinsonian and cerebellar variants. Eur Radiol. 2019;29:716–24.
Krismer F, Wenning GK. Multiple system atrophy: insights into a rare and debilitating movement disorder. Nat Rev Neurol. 2017;13:232–43.
Kawabata K, Hara K, Watanabe H, Bagarinao E, Ogura A, Masuda M, et al. Alterations in cognition-related cerebello-cerebral networks in multiple system atrophy. Cerebellum. 2019;18:770–780.
Wang N, Zhang L, Yang H, Liu H, Luo X, Fan G. Similarities and differences in cerebellar grey matter volume and disrupted functional connectivity in idiopathic Parkinson’s disease and multiple system atrophy. Neuropsychologia. 2019;124:125–32.
De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–67.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38.
Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–7.
Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–56.
Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30.
Liu X, Chen X, Zheng W, Xia M, Han Y, Song H, et al. Altered functional connectivity of insular subregions in Alzheimer’s disease. Front Aging Neurosci. 2018;10:107.
Zheng W, Liu X, Song H, Li K, Wang Z. Altered functional connectivity of cognitive-related cerebellar subregions in Alzheimer’s disease. Front Aging Neurosci. 2017;9:143.
Yang H, Wang N, Luo X, Lv H, Liu H, Li Y, et al. Cerebellar atrophy and its contribution to motor and cognitive performance in multiple system atrophy. Neuroimage Clin. 2019b;23:101891.
Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–54.
Kalpouzos G, Persson J, Nyberg L. Local brain atrophy accounts for functional activity differences in normal aging. Neurobiol Aging. 2012;33:623 e621–13.
O'Callaghan C, Hornberger M, Balsters JH, Halliday GM, Lewis SJ, Shine JM. Cerebellar atrophy in Parkinson’s disease and its implication for network connectivity. Brain. 2016;139:845–55.
Yang H, Wang N, Luo X, Lv H, Liu H, Fan G. Altered functional connectivity of dentate nucleus in parkinsonian and cerebellar variants of multiple system atrophy. Brain Imaging Behav. 2019a;13:1733–45.
Sako W, Abe T, Furukawa T, Oki R, Haji S, Murakami N, et al. Differences in the intra-cerebellar connections and graph theoretical measures between Parkinson’s disease and multiple system atrophy. J Neurol Sci. 2019;400:129–34.
Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127:2657–71.
Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75.
Acknowledgments
The authors sincerely thank the participants for their help and willingness to participate in this study.
Funding
This work was supported by the Beijing Municipal Natural Science Foundation, China(No.7182105), the National Natural Scientific Foundation of China (NO. 81571648), the Capital Health Research and Development of Special (NO. 2018-2-4202), the Dongfang Hospital of Beijing University of Traditional Chinese Medicine “1166” talent training project (NO. 030903010321).
Author information
Authors and Affiliations
Contributions
Dr. Zhiqun Wang and Dr. Zhigang Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hao Zhang, Shaozhen Ji, Zhigang Chen, Zhiqun Wang.
Acquisition, analysis, or interpretation of data: Hao Zhang, Shaozhen Ji, Shan Ren, Ming Liu, Weizheng Ran, Xinning Zang, Wenyang Tian.
Drafting of the manuscript: Hao Zhang, Shaozhen Ji, Zhiqun Wang.
Critical revision of the manuscript for important intellectual content: Shaozhen Ji, Zhigang Chen, Zhiqun Wang.
Statistical analysis: Hao Zhang, Zhiqun Wang.
Obtained funding: Zhiqun Wang.
Administrative, technical, or material support: Hao Zhang, Shan Ren, Ming Liu, Zhigang Chen, Zhiqun Wang.
Study supervision: Zhiqun Wang.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, H., Ji, S., Ren, S. et al. Cerebellar Atrophy in Multiple System Atrophy (Cerebellar Type) and Its Implication for Network Connectivity. Cerebellum 19, 636–644 (2020). https://doi.org/10.1007/s12311-020-01144-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01144-4