Abstract
The inhibitory mechanism named backward inhibition (BI) counteracts interference of previous tasks supporting task switching. For instance, if task set A is inhibited when switching to task B, then it should take longer to immediately return to task set A (as occurring in an ABA sequence), as compared to a task set that has not been just inhibited (as occurring in a CBA sequence), because extra time will be needed to overcome the inhibition of task set A.
The evidenced prefrontal and cerebellar role in inhibitory control suggests their involvement even in BI. Here, for the first time, we modulated the excitability of multiple brain sites (right presupplementary motor area (pre-SMA), left and right cerebellar hemispheres) through continuous theta burst stimulation (cTBS) in a valuable sham-controlled order-balanced within-subject experimental design in healthy individuals performing two domain-selective (verbal and spatial) task-switching paradigms. Verbal BI was abolished by prefrontal or cerebellar stimulations through opposite alterations of the basal pattern: cTBS on pre-SMA increased CBA reaction times, disclosing the current prefrontal inhibition of any interfering old task. Conversely, cerebellar cTBS decreased ABA reaction times, disclosing the current cerebellar recognition of sequences in which it is necessary to overcome previously inhibited events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Daily life events require frequent and often unexpected switching between different task sets. The term task set refers to the configuration of mental resources comprising representation of task-relevant stimuli, task-relevant responses, and corresponding stimulus-response mapping [1]. The changes are essentials to adaptively respond to a dynamic environment but they also have a cost which results in slower and/or less accurate performance on a given task A when it is preceded by a different task (e.g., task B–task A) (“alternating” or “switch” trial) compared to when it is preceded by a similar task (e.g., task A–task A). Such a difference between task repeat (A–A) and task switch (A–B) is known as switch cost. Task switching requires a number of executive functions, such as attention shifting, goal retrieval, task set reconfiguration, and working memory. Furthermore, successful switching between tasks involves the inhibition of the now-irrelevant competing task. Such an inhibition is referred to as backward inhibition (BI) [2]. BI is supposed to have the functional role of preventing previous and competing task representation to interfere with the execution of the current task. A stronger BI is thought to be related to a better task-switching performance, as it facilitates the activation of a new task set [2]. However, although highly functional to correct performance, BI makes it harder to switch back to a just inhibited task. For instance, if task set A is inhibited when switching to task B, then it should take longer to immediately return to task set A (as occurring in an ABA sequence), as compared to a task set that has not been recently inhibited (as occurring in a CBA sequence), because extra time will be needed to overcome the inhibition of task set A. Consequently, the reaction times (RTs) are longer on ABA sequences than on CBA sequences. This difference referred to as N–2 alternation cost represents the time needed for cognitive system to recover from inhibition of the previous task representation [3, 4].
While a number of studies investigated the neural correlates of cognitive processes involved in task-switching [5,6,7,8,9], the neural underpinnings underlying BI remain poorly studied. As it is well known, the presupplementary motor area (pre-SMA) is crucial in the cognitive control of actions requiring rapid updating, inhibition, switching, flexible action control, and working memory [5, 6]. Greater activity in pre-SMA has been shown in trials that are successfully stopped in comparison to trials with failed inhibition [10,11,12,13]. Consistently, a proposed pre-SMA function is the modulation of behavior when expecting a stopping stimulus [14,15,16,17]. Transcranial magnetic stimulation (TMS) studies have provided causal evidence for pre-SMA’s role in reactive inhibition of action [6, 18,19,20] and they indicated that switching from repetitive movements to new ones is impaired when pre-SMA activity is disrupted [7].
Coming back to BI, a reduced BI was reported in an unfortunately very small sample of patients with extensive damage to the right prefrontal cortex, suggesting the involvement of this wide area in the inhibition of no longer relevant task sets [21]. More recently, an fMRI study demonstrated that individuals who had a large BI effect exhibited increased activation of the supplementary motor area/premotor area and basal ganglia [22]. However, it is still uncertain whether in the process that inhibits a recently performed task, the pre-SMA is the only protagonist or it is an actor of a larger cast of brain areas.
An increasing amount of data suggests the involvement of the cerebellum in the inhibitory control [23, 24] also taking into account the wide bidirectional cortico-cerebellar projections and the existence of parallel segregated closed-loops between cerebellum and prefrontal areas [25, 26]. The importance of cerebello-frontal network in inhibitory control has been confirmed by the observation that the modulation of cerebellar plasticity by continuous theta burst stimulation (cTBS) induces functional changes in cerebello-prefrontal connectivity only during NoGo trials leaving unaffected Go trials [27]. The weakening of the cerebello-prefrontal functional link due to damage to the cerebellar cortex is associated with altered inhibitory performance [28]. Furthermore, lesion and functional neuroimaging studies supported the cerebellar involvement in task switching and remapping of responses [29,30,31,32]. In spite of this, up to now, no study has investigated the specific role of cerebellar circuitries in active inhibition of sequential events in which it is needed an inhibitory control upon a previous task set, as occurring in BI. In fact, within the many control processes (monitoring, inhibition, selection, error detection, attentional shift, and working memory) that BI encompasses, one of the most significant process is event sequencing, a supra-modal function in which a cerebellar role has been repeatedly proposed [33, 34]. Actually, the cerebellum provides a fast computational system for timing and sequencing the incoming patterns and outgoing responses so as to process the sequential information in an increasingly more efficient and adaptive manner [35]. Studies in healthy volunteers or in patients with focal cerebellar damage, as well as in animals with cerebellar lesions, indicated the cerebellar involvement in sequence detection and processing [36,37,38,39,40,41].
Starting from the role of pre-SMA and cerebellum in inhibiting and processing sequential events, the aim of the present research was to clarify the distinct roles of these structures in high-level inhibitory control during rapid task-switching as occurring during BI. To this aim, we separately applied cTBS on pre-SMA and cerebellar hemispheres in healthy adult subjects who performed two task-switching paradigms on verbal and spatial domains. We used the verbal and spatial paradigms since we recently demonstrated the material-dependency of BI process [42, 43], as it had been already reported for working memory [44], conflict resolution [45, 46], and attentional control [47, 48]. We chose the valuable within-subject experimental design in which the same subject performed two BI paradigms following sham or real cTBS applied on the right pre-SMA, left, or right cerebellar hemispheres. Currently, no study analyzed BI performances in different domains following neuromodulation of prefrontal or cerebellar areas in the very same individuals. We chose cTBS since this kind of non-invasive stimulation induces focal long-lasting effects in neocortical and cerebellar regions in spite of its brief application time. Applying cTBS to multiple sites, as well as investigating its effects on multiple domains in the same subject, represents a methodology infrequently used in the literature. Yet, such a methodological approach, although time-consuming and high-demanding, produces a stringent and reliable assessment of the effects. Indeed, it allows ruling out that the observed effects could be attributed to unspecific influence on the neural excitability, and unambiguously ascribing modified performances in one specific functional domain to the involvement of a specific neural circuit (and not of others).
Materials and Methods
Participants
Twelve healthy volunteers (5 women; mean age ± standard deviation, 25.13 ± 2.9 years) participated to this study. All subjects were right-handed as assessed by the Edinburgh Handedness Inventory [49]. All gave written informed consent for the study. All participants reported normal or corrected-to-normal vision, no history of neurological or psychiatric disorders, and no on-going medication. All subjects were naїve on the purpose of the study and they have never been exposed to TMS before. The experimental procedures were approved by the Ethics Committee of the Santa Lucia Foundation IRCCS according to the Declaration of Helsinki.
Task-Switching Paradigms
Participants were individually tested in a dimly lit testing room. Stimuli were presented on a 17-in. computer monitor placed at a distance of 60 cm. The experiments were programmed in E-Prime on a computer running the Microsoft operating system. Participants pressed one of two response buttons (“A” and “L” keys) on a QWERTY computer keyboard with their left and right index fingers, respectively. Given the material-dependency of the BI process [42], all participants performed two (one verbal and one spatial) task-switching paradigms in a counterbalanced order. For both paradigms, the following procedure was followed.
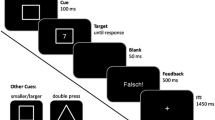
The tasks were explained to the participants who were told to respond as fast as possible and make as few mistakes as possible. The experiments started with a practice block of 21 trials through which the participants familiarized with the experimental setting and learned the task requests. Afterwards, the percentages of correct responses were calculated. The practice was considered successful when the percentage of correct responses was ≥ 80%. If not, the participant repeated new practice blocks until the criterion was reached. Each trial started with the presentation of a blank screen (500 ms), followed by the presentation at the center of the screen of a geometrical shape (visual cue) (1000 ms), and then by the presentation of the target stimulus (2500 ms). Both cue and target stimuli were presented with 7° width × 4° height visual angles in black on a pale gray background.
Each task-switching paradigm encompassed 174 trials in which pseudo-randomized series of non-alternating (CBA), alternating (ABA), and repetition (AA) sequences appeared. Repetition sequences were formed by two elements (AA) in order to avoid an excessive lengthening of the testing-time that could have exceeded the period of optimal cTBS effect. In a non-alternating CBA sequence, three different tasks were executed; in an alternating ABA sequence, the same task was performed for the first and third trial; in a repetition AA sequence, the same task was performed for two successive trials. The sequence of tasks was randomly created with the constraint that the number of trials for each of three tasks had to be counterbalanced. Since in a purely random (without replacement) selection procedure the probability to have two consecutive trials of the same task (AA) is higher than the probability to have three consecutive trials of different tasks (CBA) or a N–2 task repetition (ABA), the number of repetition sequences (AA) was necessarily higher that the number of non-alternating (CBA) and alternating (ABA) sequences. Thus, 42 ABA alternating, 41 CBA non-alternating, and 60 AA repetition sequences were presented. The occurrence of right and left responses was equally required in each task-switching paradigm. Given the pseudo-randomized presentation and lack of inter-trial interval, participants were unaware that different sequences were presented. The task was made up of three blocks of about 5 min each, with an inter-block interval of about 2 min. Thus, the experiment lasted about 16 min.
Verbal Task-Switching Paradigm
This protocol tested the BI effect in a paradigm that required to process verbal stimuli without tapping spatial components (Fig. 1a).
In each trial, the target stimulus was represented by a word written in Courier New font which appeared at the screen center and indicated the name of an animal (parrot, mouse, gorilla, elephant, frog, peacock, chick, bear). In each trial, the participants were asked to perform one of three tasks determining: (a) the number of legs of the animal (2-footed or 4-footed); (b) the actual size of the animal (small or large); (c) the presence of the tail of the animal (absent or present). The leg-number task was precued by a diamond shaped frame, the size task was precued by a square shaped frame, and the tail task was precued by a circle shaped frame. Participants had to press the left key for 2-footed, small, or no-tail task, and the right key for 4-footed, large, or with-tail tasks.
Spatial Task-Switching Paradigm
This protocol tested the BI effect in a paradigm that required to process spatial stimuli without tapping verbal or semantic components (Fig. 1b).
In each trial, the target stimulus was represented by an asterisk and participants were required to execute one of three tasks determining: (a) the vertical position of the asterisk from the screen center (up or down), (b) the horizontal position of the asterisk from the screen center (left or right); (c) the eccentricity of the asterisk from the screen center (close or far). The vertical position task was precued by a diamond shaped frame, the horizontal position task was precued by a square shaped frame, and the eccentricity task was precued by a circle shaped frame. The left-right lateralized target stimuli, as well as the number of left-right responses, were counterbalanced in order to avoid potential spatial-compatibility effects.
Parameters
As dependent variable, reaction times (RTs) were measured. Mean RTs for trials in which the task was the same as the task performed on N–2 trial (i.e., ABA task sequence), trials in which the task was different from the task required on N–2 trial (i.e., CBA task sequence) and trials in which the same task was repeated (i.e., AA task sequence), were computed for each participant and for both spatial and verbal paradigms. We compared RTs of the third trials of the alternating sequences (ABA) to those of the non-alternating sequences (CBA) to determine the BI effect. RTs on the repetition sequences were compared to those of the changing sequences to estimate the switch cost (see Supplementary Material). Only triplets for which participants responded correctly to all trials were used to compute the BI and switch cost effects.
The percentage of correct responses was also computed. However, because of the extended practice participants were submitted to, in both paradigms and in all experimental conditions, the behavioral performance resulted highly accurate and with a very low variability (96.55 ± 2.5%) also across conditions. For this reason, the accuracy scores were not statistically analyzed.
Continuous Theta Burst Stimulation
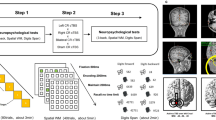
A MagStim Super Rapid magnetic stimulator (Magstim Company, Whitland, Wales, UK) connected with a figure-of-eight coil with a diameter of 70 mm was used to selectively deliver theta burst stimulation over the scalp sites corresponding to the right pre-SMA [50] or the posterior and superior lobules of the right or left cerebellar hemisphere [51] (Fig. 1c). We applied continuous theta burst stimulation (cTBS) over pre-SMA and cerebellar hemispheres considering that cTBS is able to activate plastic mechanisms in both regions, as already demonstrated as for motor evoked potentials [52], electroencephalographic recordings [53] and behavioral tasks [54]. Thus, through the selective disruption of cortical and cerebellar activities, the cTBS protocol allowed investigating the specific contribution of pre-SMA and cerebellar hemispheres to the BI effect. The magnetic stimulus had a biphasic waveform with a pulse width of about 300 μs. Three-pulse bursts at 50 Hz repeated every 200 ms for 40 s (600 pulses), and were delivered at 80% of the active motor threshold (AMT) [55, 56]. AMT was tested over the primary motor cortex at the start of each experimental session and defined as the lowest intensity that produced MEPs of 0.200 mV in at least five out of 10 trials when the subject made a 10% of maximum contraction using visual feedback [55, 56]. Stimulation intensities are reported in the Supplementary Materials. For the stimulation of right pre-SMA, the coil was positioned tangentially to the scalp with the handle pointing laterally to induce a medially directed current in the stimulated cortex [50]. For each participant, the stimulation site was determined at 15% of the distance between nasion and inion anterior to Cz [57, 58] and slightly shifted to the right (1 cm). For cerebellar cTBS, the coil was positioned tangentially to the scalp, with the handle pointing superiorly over the right or left cerebellar hemisphere using the scalp coordinates (1 cm inferior and 3 cm right or left to the inion) previously adopted [59, 60]. For sham cTBS, the coil was positioned in the same scalp coordinates used for real stimulations but angled away so that no current was induced in the brain resulting in an absence of biological effects [61]. Given in the present study different stimulation sites were targeted, the sham cTBS was applied over the right pre-SMA in 6 subjects, over the cerebellar vermis in 6 subjects. Since RTs of these two Sham sub-groups did not differ in both task-switching paradigms, we pooled all data in only one Sham condition (see Supplementary Materials for the related statistics).
Overview of the Procedure
Each participant underwent four cTBS sessions spaced by 1 week apart. In each weekly session, the subject performed both task-switching paradigms under the effect of cTBS delivered in counterbalanced order over the right pre-SMA, right cerebellar hemisphere (r-Cb), left cerebellar hemisphere (l-Cb), or Sham stimulation.
Statistical Analyses
To assess the modulation of BI following cTBS, we performed a three-way within-subject ANOVA on mean RTs including sequence (ABA and CBA), domain (spatial and verbal), and stimulation condition (pre-SMA, r-Cb l-Cb, Sham) as independent variables. Post hoc analyses were performed with the Duncan’s test, when appropriate.
Switch cost (SC) was computed as the RTs difference between trials in which the task changed and trials in which the task remained the same (see Supplementary Material for the statistics related to SC).
The effects of cTBS on SC were assessed by a three-way within-subject ANOVA on mean RTs with sequence (AA and CBA), domain (spatial and verbal) and stimulation condition (pre-SMA, r-Cb, l-Cb, Sham) as independent variables. Post hoc analyses were performed by Duncan’s test, when appropriate.
To assess the reliability of the results of the two above-described ANOVA designs, we ran two non-parametric bootstrap analyses. Four hundred bootstrap samples were formed by randomly, with replacement, drawing participants from the original sample (N = 12). The same ANOVA designs described above were run on each bootstrap sample. We then computed the average F values (Fboot) for each main effect and interaction. A significant Fboot suggested that the original effect did not depend on the specific group composition in terms of participants.
To rule out potential effects due to the relationship between the stimulation site and the responding hand, we carried out two three-way within-subjects ANCOVAs on BI and SC with the Sham condition used as covariate, and with domain (spatial and verbal), stimulation site (pre-SMA, r-Cb, l-Cb), and responding hand (left and right) as independent variables (see Supplementary Material for the related statistics).
Results
Task Inhibition
The results of three-way ANOVA on BI revealed the significant effect of the domain (F1,11 = 66.041; p = 0.00001; ηp2 = 0.857, power 0.99, Fboot1,11 = 88.36, p = 0.000001) with RTs on spatial task significantly faster than RTs on verbal task. The remaining main factors were not significant (sequence F1,11 = 2.640; p = 0.132; stimulation F3,33 = 0.721; p = 0.546). Importantly, the second-order interaction was significant (F3,33 = 3.607; p = 0.023; ηp2 = 0.247, power 0.74, Fboot3,33 = 5.22, p = 0.005).
Verbal Task-Switching
As for verbal task-switching (Fig. 2a), post hoc comparisons on significant interaction revealed that the RTs on ABA sequences were significantly (p < 0.05) slower than those on CBA sequences in Sham condition, indicating that in the absence of cTBS stimulation participants exhibited the typical BI effect. Interestingly, the pre-SMA stimulation significantly (p < 0.02) increased the RTs on CBA sequences when compared to Sham condition but it failed to modulate the RTs on ABA sequences (p = 0.28). Conversely, the RTs on ABA sequences were significantly reduced following both left (p < 0.005) and right (p < 0.0005) cerebellar stimulations when compared to the RTs of ABA sequences in Sham condition. RTs on CBA sequences were not modulated by the cerebellar stimulation (p = 0.14 and p = 0.11 for the left and right cerebellum, respectively). Scatterplots with the distribution of individual values were presented in the Supplementary Material section.
Spatial Task-Switching
As for spatial task-switching paradigm (Fig. 2b), post hoc comparisons revealed that the RTs on ABA sequences were not significantly different than those on CBA sequences in Sham condition (p = 0.93), revealing the absence of BI effect. However, pre-SMA stimulation selectively facilitated the ABA performance (p < 0.05) without affecting CBA performance (p = 0.29). Conversely, the cTBS applied over l-Cb induced a facilitatory effect on the CBA sequences (p < 0.05), without affecting RTs of ABA sequences (p = 0.53). Finally, the r-Cb cTBS significantly (always p < 0.001) reduced RTs on both CBA and ABA sequences in comparison to Sham condition. Scatterplots with the distribution of individual values were presented in the Supplementary Material section.
Other Statistics
The findings related to the contribution of prefrontal and cerebellar regions on the switch cost, as well as the relationship between stimulation side and responding hand, are described in the Supplementary Material section.
Discussion
The main result of the present research is that the cTBS applied over the pre-SMA or cerebellar hemispheres canceled the verbal BI with site-specific effects. Namely, in Sham (control) condition, we recorded longer RTs in the ABA in comparison to CBA sequences, consistently with the presence of an inhibitory process altering the cognitive configurations that were to be abandoned. This is the typical BI effect. It is known that the BI effect arises from the difference between RTs of ABA and CBA sequences, with the ABA RTs longer than the CBA RTs (Fig. 3a). Thus, any modification of this pattern (either decreasing the ABA RTs or increasing the CBA RTs) results in canceling BI effect.
The effects of stimulation on BI illustrated in (a) a graphical representation of backward inhibition (BI) effect expressed as relation between ABA and CBA sequences in the verbal task-switching paradigm. b Graphical representation of the effects of pre-SMA cTBS on BI. c Graphical representation of the effects of cerebellar cTBS on BI. (Cb, cerebellum)
Interestingly, cTBS applied on either pre-SMA or cerebellar hemispheres did cancel BI effect, although through an opposite alteration of the basal pattern. Namely, the pre-SMA stimulation elicited a significant increase in the RTs on CBA sequences (Fig. 3b), while cerebellar stimulation elicited a significant decrease in the RTs on ABA sequences (Fig. 3c). Once more, this finding emphasizes the importance of using multiple stimulation sites and remarkably demonstrates that the pre-SMA and cerebellum differently contribute to develop the BI effect. Interestingly, Meiran et al. [62] suggested that in the task-switching paradigm (although not specifically in the BI), the prefrontal regions subserve some components (i.e., congruency effect and residual cost), while more posterior areas subserve other factors (i.e., preparatory component). Notably, the different contribution of prefrontal and cerebellar areas in task switching has been previously described in patients affected by frontal or cerebellar focal lesions [29]. In line with previous findings suggesting the involvement of pre-SMA in the BI process [7], the present results indicate that pre-SMA acts whenever inhibition is required by promoting the switching from a previous to a successive task set in any N–1 trial. In other words, in the CBA sequences, the pre-SMA inhibits any interfering old task set to work on a new representation. It is commonly recognized that top-down activity arising from prefrontal areas is associated with control processes that select goal-related information, enhancing the representations that underlie the current behavior and inhibiting irrelevant or inappropriate information. Note that the BI is retained a conflict-solving process aimed at reducing the proactive interference from old to new representations. Ultimately, the difficulty in switching process induced by pre-SMA cTBS fits well into the general framework which considers the pre-SMA as a crucial node of the brain inhibitory networks [6, 15, 63,64,65,66]. As previously demonstrated by neuroimaging and stimulation studies, the pre-SMA, as well as the inferior frontal cortex and subthalamic nucleus, are recruited in numerous forms of executive control, as stopping an already initiated response, Go/NoGo task, and interference resolution task [67,68,69,70,71,72]. In particular, neuroimaging studies have consistently reported the activation of the right pre-SMA during successful response inhibition [5, 6, 10, 12, 73], and functional studies have shown that altering pre-SMA activity through TMS [19, 74,75,76,77], and cTBS [5, 78] affects the inhibitory control in a positive or negative manner. Furthermore, a very recent neurophysiological study on the age-related differences in overcoming BI effect confirms the involvement of frontal regions in two BI processes [79]. Namely, the suppression of the inhibitory effect of the N-1 trial on the N-2 trial is associated with right inferior frontal regions, while the response selection and conflict monitoring are associated with medial frontal regions [79].
In the light of the close functional link between frontal lobe and cerebellum [23, 26], we retained worth investigating the influence of cerebellar cTBS on BI effect. We found that the cTBS application over the right or left cerebellar hemisphere decreased RTs of ABA sequences, once more annulling the BI effect even if in an opposite way in comparison to the pre-SMA cTBS (Fig. 3c).
The faster ABA RTs following cerebellar cTBS suggest the specific contribution of the cerebellum in detecting the position of the single event within a sequence. In other words, cerebellar cTBS seems to alter the recognition of ABA as a sequence, in which it is necessary overcome the previously inhibited N–2 event. In light of this, the cerebellum may act by identifying the repetitive elements within the sequence, and making necessary the effortful overcoming the inhibition of the N–2 task back to being relevant. It is just this lacking recognition of the event order that makes an ABA sequence not different from a CBA sequence following cerebellar cTBS. Note that a remarkable feature of the BI is the event sequencing, since it is just the sequential order of representations that elicits BI. Sequencing skill should not be seen as a distinct cognitive function, but rather as a supra-modal function. The relationship with other cognitive functions, first of all working memory and timing, is not yet fully clarified. In fact, to act on a sequential order of events, the single inward sensory information has to be kept active in a (dedicated?) working memory system and compared with the successive incoming stimuli. So, the previous and ongoing representations are serially encoded, related, and processed to be inhibited or maintained depending on the situation. Not by chance, the cerebellar networks have been repeatedly given a role in sequencing incoming sensory patterns and outgoing responses in multifarious (motor, spatial, verbal, mnesic, behavioral, and cognitive) domains [34, 80,81,82] so that the cerebellar networks have been involved in sequencing virtually all abilities although to a greater or lesser extent [83,84,85]. In fact, the sequence-in/sequence-out mode of operation proposed as the key of the cerebellar function [36, 86] could well account for the involvement of the cerebellar networks in the BI. An intriguing interpretation of the role of the cerebellum in the executive processes is that the cerebellum accomplishes its sequencing function by first allowing the correct recognition of spatial and temporal relations among relevant information, then encoding and manipulating the ordered sequences of representations, eventually inhibiting the preceding and currently irrelevant (if not even interfering) information. This assumption entails that the cerebellum plays a role in mediating control processes and establishing the proper sequential mapping required to optimize a task in general, and to switch from a representation to another, in particular. Note that the present findings are quite compatible with recent functional neuroimaging studies indicating the involvement of the cerebellum in task switching [87].
Task-switching paradigms require abilities of cognitive flexibility to quickly disengage from and eliminate the detrimental effects of proactive interference and to quickly prepare for a new task, or both. Interestingly, the cerebellar networks are heavily involved in the cognitive flexibility abilities, as indicated by the observation that in the presence of cerebellar lesions it is very difficult to abandon a previously correct and now interfering representation and acquire a new one. In fact, although able to put into action fixed and repeating responses, subjects with cerebellar lesions are impaired in emitting behavioral responses requiring rapidly changing adaptations [41, 88,89,90]. Consistently, a number of studies have reported impaired sequencing functions [24, 36, 80, 85, 91] and task-switching deficits [29,30,31, 92] in patients with cerebellar lesions. Thus, the present findings indicating that the processing of sequential events is impaired by interfering with the cerebellar activity support the basic operational model of the cerebellum. As Schmahmann [93, 94] has advanced the term “dysmetria of thought” to define the nature of the cognitive impairment following cerebellar lesions, analogously the altered sequencing functions, we found following cerebellar cTBS may be regarded as a sort of “cognitive dysdiadochokinesia.”
Recently, it has been hypothesized that “pattern detection and prediction, and processing of anticipation are cerebellum-specific functions” so that the cerebellar sequencing can be considered “a trick for predicting the future” [37]. Here, we are advancing that cerebellar function in sequencing and in backward inhibition it is not only a trick for predicting the future but also a tool to avoid the interference of the past.
Potential Limitations
One potential limitation of the present study is the lack of BI effect in the spatial task-switching paradigm in Sham condition, although the spatial task had been designed with all the same features (type of training, number and category of sequences, presentation time, cues) but the nature of the material to be processed. We retain that this lack of spatial BI may be related to the prevailingly perceptual nature of the spatial task proposed, given the visual features of stimuli explicitly affording the response. In fact, a critical boundary condition for the BI to occur is the top-down selection of the task set, so that when target visual features suffice for triggering the response (in a bottom-up fashion) then the BI effect does not occur because a high-level elaboration is not needed [2, 95]. However, it is worth noting that the present spatial paradigm, although it did not evoke the BI, was able to elicit the switch cost (these data are described in details in Supplementary Materials), coherently with previous studies on spatial task-switching [96]. In fact, any task-switching performance depends on several other factors over the BI, as the task preparation time [97,98,99,100,101,102], the interval between the previous and the new task [103, 104], and the bivalence of the response-set [95]. When one factor does not work (as the spatial BI does here), the others still contribute to the switching performance. Consistently, in the absence of BI, for a sort of recency effect, it is possible that the task representation remains available in working memory longer for the more recently executed tasks (as in AB-A sequences) than for less recently executed tasks (as in CB-A sequences). Since the spatial task-switching paradigm did not evoke any BI effect in Sham condition, any interpretation of cTBS effects on spatial task-switching would risk being totally speculative. Thus, we retain that the performances related to spatial BI will require dedicated researches to enable various questions to be solved.
Another point of the study that deserves particular attention is the sample size (N = 12). In fact, notwithstanding the valuable within-subject design ensures a sufficient statistical power and limits the possible methodological confounds, it is still possible for the reported effect to be due to the rather small sample size, potentially raising a reliability issue. We were aware that the inference based on maximum likelihood estimators relies on an asymptotic distribution, which might not be appropriate for small samples, given the sampling distribution of the estimate is unknown [105]. Such an issue has been addressed through resampling statistic methods such as the bootstrap [106] which uses the data and computer power to estimate the unknown sampling distribution. At the heart of the bootstrap is the computation of the statistics of interest on multiple random selections with replacement of participants from the original samples (bootstrap samples). The bootstrap estimated statistic is less dependent on the specific composition of the sample than the original one. Thus, the adopted bootstrap resampling supports the robustness of present results.
Conclusions
The inhibition of a just executed task counteracts the potential interference from previously executed tasks so that an effective task-switching is supported. This inhibitory mechanism named backward inhibition results in a harder switching-back to a recently executed task than switching-back to a less recently executed task. The present results for the first time showed causal evidence of the distinct contribution of pre-SMA and cerebellar hemispheres in developing BI effect. Specifically, while the pre-SMA acts in the BI by inhibiting any interfering old task set to work on a new representation, the cerebellum allows recognizing the sequences in which it is necessary overcome a previously inhibited event.
References
Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, et al. Control and interference in task switching—a review. Psychol Bull. 2010;136:849–74.
Mayr U, Keele SW. Changing internal constraints on action: the role of backward inhibition. J Exp Psychol Gen. 2000;129:4–26.
Mayr U. Inhibition of action rules. Psychon Bull Rev. 2002;9:93–9.
Schuch S, Sommer A, Lukas S. Action control in task switching: do action effects modulate N - 2 repetition costs in task switching? Psychol Res. 2018;82:146–56.
Obeso I, Cho SS, Antonelli F, Houle S, Jahanshahi M, Ko JH, et al. Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul. 2013a;6:769–76.
Obeso I, Robles N, Marrón EM, Redolar-Ripoll D. Dissociating the role of the pre-SMA in response inhibition and switching: a combined online and offline TMS approach. Front Hum Neurosci. 2013b;7:150.
Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–92.
Shi Y, Meindl T, Szameitat AJ, Müller HJ, Schubert T. Task preparation and neural activation in stimulus–specific brain regions: an fMRI study with the cued task–switching paradigm. Brain Cogn. 2014;87:39–51.
Yin S, Wang T, Pan W, Liu Y, Chen A. Task–switching cost and intrinsic functional connectivity in the human brain: toward understanding individual differences in cognitive flexibility. PLoS One. 2015;10:e0145826.
Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack R. Triangulating a cognitive control network using diffusion–weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–52.
Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain––conjunction analyses of the stop–signal task. Neuroimage. 2010;52:1621–32.
JRR D, Ide JS, Luo X, CSR L. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–9.
Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–11.
Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, et al. Functional dissociation in right inferior frontal cortex during performance of go/no–go task. Cereb Cortex. 2009a;19:146–52.
Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop–signal task. J Neurosci. 2009b;29:15870–7.
Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with restraint: what are the neurocognitive mechanisms? J Cogn Neurosci. 2010;22:1479–92.
Sebastian A, Forstmann BU, Matzke D. Towards a model–based cognitive neuroscience of stopping—a neuroimaging perspective. Neurosci Biobehav Rev. 2018;90:130–6.
Cai W, George JS, Verbruggen F, Chambers CD, Aron AR. The role of the right presupplementary motor area in stopping action: two studies with event–related transcranial magnetic stimulation. J Neurophysiol. 2012;108:380–9.
Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. Neuroimage. 2009;44:537–45.
Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A. 2010;107:13240–5.
Mayr U, Diedrichsen J, Ivry R, Keele SW. Dissociating task–set selection from task–set inhibition in the prefrontal cortex. J Cogn Neurosci. 2006;18:14–21.
Whitmer AJ, Banich MT. Brain activity related to the ability to inhibit previous task sets: an fMRI study. Cogn Affect Behav Neurosci. 2012;12:661–70.
Picazio S, Koch G. Is motor inhibition mediated by cerebello–cortical interactions? Cerebellum. 2015;14:47–9.
Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, Leggio MG. The cerebellar cognitive profile. Brain. 2011;134:3672–86.
Barbas H, García-Cabezas MÁ. How the prefrontal executive got its stripes. Curr Opin Neurobiol. 2016;40:125–34.
Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. 2018;19:338–50.
Picazio S, Ponzo V, Koch G. Cerebellar control on prefrontal-motor connectivity during movement inhibition. Cerebellum. 2016;15:680–7.
Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–77.
Berger A, Sadeh M, Tzur G, Shuper A, Kornreich L, Inbar D, et al. Task switching after cerebellar damage. Neuropsychology. 2005;19:362–70.
Bischoff GA, Ivry RB, Grafton ST. Cerebellar involvement in response reassignment rather than attention. J Neurosci. 2002;22:546–53.
Dreher JC, Koechlin E, Ali SO, Grafman J. The roles of timing and task order during task switching. Neuroimage. 2002;17:95–109.
Peterburs J, Hofmann D, Becker MPI, Nitsch AM, Miltner WHR, Straube T. The role of the cerebellum for feedback processing and behavioral switching in a reversal–learning task. Brain Cogn. 2018;125:142–8.
Mauk MD, Medina JF, Nores WL, Ohyama T. Cerebellar function: coordination, learning or timing? Curr Biol. 2000;10:522–5.
Peterburs J, Blevins LC, Sheu YS, Desmond JE. Cerebellar contributions to sequence prediction in verbal working memory. Brain Struct Funct. 2019;224:485–99.
O'Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28:2252–60.
Molinari M, Petrosini L. Is sequence-in/sequence-out a cerebellar mode of operation in cognition too? Behav Brain Sci. 1997;20:259–60.
Leggio M, Molinari M. Cerebellar sequencing: a trick for predicting the future. Cerebellum. 2015;14:35–8.
Mandolesi L, Foti F, Cutuli D, Laricchiuta D, Gelfo F, De Bartolo P, et al. Features of sequential learning in hemicerebellectomized rats. J Neurosci Res. 2010;88:478–86.
Molinari M, Leggio MG, Solida A, Ciorra R, Misciagna S, Silveri MC, et al. Cerebellum and procedural learning: evidence from focal cerebellar lesions. Brain. 1997;120:1753–62.
Restuccia D, Della Marca G, Valeriani M, Leggio MG, Molinari M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch–negativity study. Brain. 2007;130:276–87.
De Bartolo P, Mandolesi L, Federico F, Foti F, Cutuli D, Gelfo F, et al. Cerebellar involvement in cognitive flexibility. Neurobiol Learn Mem. 2009;92:310–7.
Foti F, Sdoia S, Menghini D, Vicari S, Petrosini L, Ferlazzo F. Out with the old and in with the new––is backward inhibition a domain–specific process? PLoS One. 2015a;10:e0142613.
Foti F, Sdoia S, Menghini D, Mandolesi L, Vicari S, Ferlazzo F, et al. Are the deficits in navigational abilities present in the Williams syndrome related to deficits in the backward inhibition? Front Psychol. 2015b;18(6):287.
Palladino P, Mammarella N, Vecchi T. Modality–specific effects in inhibitory mechanisms: the interaction of peripheral and central components in working memory. Brain Cogn. 2003;53:263–7.
Akçay Ç, Hazeltine E. Domain–specific conflict adaptation without feature repetitions. Psychon Bull Rev. 2011;18:505–11.
Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends Cogn Sci. 2008;12:374–80.
Lin SH, Yeh YY. Domain–specific control of selective attention. PLoS One. 2014;9:e98260.
Stoppel CM, Boehler CN, Strumpf H, Krebs RM, Heinze HJ, Hopf JM, et al. Distinct representations of attentional control during voluntary and stimulus–driven shifts across objects and locations. Cereb Cortex. 2013;23:1351–61.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113.
Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre–supplementary motor areas. Nat Rev Neurosci. 2008;9:856–69.
Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol. 2007;98:145–52.
Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno S, et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008;119:2559–69.
Casula EP, Pellicciari MC, Ponzo V, Stampanoni Bassi M, Veniero D, Caltagirone C, et al. Cerebellar theta burst stimulation modulates the neural activity of interconnected parietal and motor areas. Sci Rep. 2016;31:36191.
Richard A, Van Hamme A, Drevelle X, Golmard JL, Meunier S, Welter ML. Contribution of the supplementary motor area and the cerebellum to the anticipatory postural adjustments and execution phases of human gait initiation. Neuroscience. 2017;358:181–9.
Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6.
Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–22.
Mantovani A, Simpson HB, Fallon BA, Rossi S, Lisanby SH. Randomized sham–controlled trial of repetitive transcranial magnetic stimulation intreatment–resistant obsessive–compulsive disorder. Int J Neuropsychopharmacol. 2010;13:217–27.
Ruitenberg MF, Verwey WB, Schutter DJ, Abrahamse EL. Cognitive and neural foundations of discrete sequence skill: a TMS study. Neuropsychologia. 2014;56:229–38.
Picazio S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Cerebellar contribution to mental rotation: a cTBS study. Cerebellum. 2013a;12:856–61.
Picazio S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Continuous theta burst stimulation (cTBS) on left cerebellar hemisphere affects mental rotation tasks during music listening. PLoS One. 2013b;8:e64640.
Koch G, Brusa L, Carrillo F, Lo Gerfo E, Torriero S, Oliveri M, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73:113–9.
Meiran N, Gotler A, Perlman A. Old age is associated with a pattern of relatively intact and relatively impaired task-set switching abilities. J Gerontol B Psychol Sci Soc Sci. 2001;56:88–102.
Duque J, Olivier E, Rushworth M. Top down inhibitory control exerted by the medial frontal cortex during action selection under conflict. J Cogn Neurosci. 2013;25:1634–48.
Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62.
Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–65.
Xu B, Sandrini M, Wang WT, Smith JF, Sarlls JE, Awosika O, et al. Pre-SMA stimulation changes task–free functional connectivity in the fronto–basal ganglia that correlates with response inhibition efficiency. Hum Brain Mapp. 2016;37:3236–49.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7.
Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17.
Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–31.
Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci. 2007;19:69–80.
Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–8.
Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–18.
Kenner NM, Mumford J, Hommer RE, Skup M, Leibenluft E, Poldrack R. Inhibitory motor control in response stopping and response switching. J Neurosci. 2010;30:8512–8.
Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, et al. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol. 2007;98:3638–47.
Picazio S, Veniero D, Ponzo V, Caltagirone C, Gross J, Thut G, et al. Prefrontal control over motor cortex cycles at beta frequency during movement inhibition. Curr Biol. 2014;24:2940–5.
Hsu TY, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJ, et al. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage. 2011;56:2249–57.
Jacobson L, Javitt DC, Lavidor M. Activation of inhibition: diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. J Cogn Neurosci. 2011;23:3380–7.
Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugmann S, Schuhmann T. The role of right prefrontal and medial cortex in response inhibition: interfering with action restraint and action cancellation using transcranial magnetic brain stimulation. J Cogn Neurosci. 2014;26:1775–84.
Giller F, Zhang R, Roessner V, Beste C. The neurophysiological basis of developmental changes during sequential cognitive flexibility between adolescents and adults. Hum Brain Mapp. 2019;40:552–65.
Leggio MG, Chiricozzi FR, Clausi S, Tedesco AM, Molinari M. The neuropsychological profile of cerebellar damage: the sequencing hypothesis. Cortex. 2011;47:137–44.
Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–9.
Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–13.
Bower JM. Is the cerebellum sensory for motor’s sake, or motor for sensory’s sake: the view from the whiskers of a rat? Prog Brain Res. 1997;114:463–96.
Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–42.
Leggio MG, Tedesco AM, Chiricozzi FR, Clausi S, Orsini A, Molinari M. Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain. 2008;131:1332–43.
Braitenberg V, Heck D, Sultan F. The detection and generation of sequences as a key to cerebellar function: experiments and theory. Behav Brain Sci. 1997;20:229–45.
von der Gablentz J, Tempelmann C, Münte TF, Heldmann M. Performance monitoring and behavioral adaptation during task switching: an fMRI study. Neuroscience. 2015;285:227–35.
Thach WT. On the specific role of the cerebellum in motor learning and cognition: clues from PET activation and lesion studies in man. Behav Brain Sci. 1996;19:411–31.
Thach WT. On the mechanism of cerebellar contributions to cognition. Cerebellum. 2007;6:163–7.
Steffener J, Gazes Y, Habeck C, Stern Y. The indirect effect of age group on switch costs via gray matter volume and task–related brain activity. Front Aging Neurosci. 2016;8:162.
Peterburs J, Desmond JE. The role of the human cerebellum in performance monitoring. Curr Opin Neurobiol. 2016;40:38–44.
Schweizer TA, Alexander MP, Cusimano M, Stuss DT. Fast and efficient visuotemporal attention requires the cerebellum. Neuropsychologia. 2007;45:3068–74.
Schmahmann JD. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci. 1998;2:362–71.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Neuropsychiatry Clin Neurosci. 2004;16:367–78.
Sdoia S, Ferlazzo F. Stimulus-related inhibition of task set during task switching. Exp Psychol. 2008;55:322–7.
Meiran N. Modeling cognitive control in task–switching. Psychol Res. 2000;63:234–49.
Meiran N. Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn. 1996;22:1423–42.
Hoffmann J, Kiesel A, Sebald A. Task switches under go/no go conditions and the decomposition of switch costs. Eur J Cogn Psychol. 2003;15:101–28.
Kiesel A, Hoffmann J. Variable action effects: response control by context–specific effect anticipations. Psychol Res. 2004;68:155–62.
Koch I. Automatic and intentional activation of task sets. J Exp Psychol Learn Mem Cogn. 2001;27:1474–86.
Meiran N, Chorev Z, Sapir A. Component processes in task switching. Cogn Psychol. 2000;41:211–53.
Monsell S, Sumner P, Waters H. Task–set reconfiguration with predictable and unpredictable task switches. MemCognit. 2003;31:327–42.
Allport A, Styles EA. Hsieh S. Shifting intentional set: exploring the dynamic control of tasks. In: Umiltà C, Moscovitch M, editors. Conscious and nonconscious information processing: attention and performance XV. Cambridge: MA:MIT Press; 1994. p. 421–52.
Altmann EM. Repetition priming in task switching: do the benefits dissipate? Psychon Bull Rev. 2005;12:535–40.
Dixon PM. Bootstrap resampling. John Wiley & Sons, Ltd: Encyclopedia of environmetrics; 2006.
Efron B, Tibshirani RJ. An introduction to the Bootstrap. London: Chapman & Hall/CRC; 1994.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All gave written informed consent for the study. The experimental procedures were approved by the Ethics Committee of the Santa Lucia Foundation IRCCS according to the Declaration of Helsinki.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 207 kb)
Rights and permissions
About this article
Cite this article
Picazio, S., Foti, F., Oliveri, M. et al. Out with the Old and in with the New: the Contribution of Prefrontal and Cerebellar Areas to Backward Inhibition. Cerebellum 19, 426–436 (2020). https://doi.org/10.1007/s12311-020-01115-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01115-9