Abstract
Cerebellar mutism syndrome (CMS) is a common surgical sequela in children following posterior fossa tumor (PFT) resection. Here, we analyze the neuropsychological features associated with PFT in children, focusing particularly on the differential profiles associated with the presence or absence of CMS after surgery. We further examine the effect of post-resection treatments, tumor type, and presence/absence of hydrocephalus on surgical outcome. Thirty-six patients diagnosed with PFT (19 with and 17 without CMS) and 34 age- and gender-matched healthy controls (HCs) were recruited. A comprehensive neuropsychological evaluation was conducted in all patients postoperatively and in HCs, including an assessment of general cognitive ability, motor skills, perception, language, memory, attention, executive functions, and academic competence. CMS was found to be a clinical marker of lower neuropsychological profile scores across all cognitive domains except auditory-verbal processing and visual memory tasks. PFT patients not presenting CMS exhibited milder impairment in intellectual functioning, motor tasks, reasoning, language, verbal learning and recall, attention, cognitive executive functions, and academic competence. High-grade tumors were associated with slower processing speed and verbal delayed recall as well as alterations in selective and sustained attention. Hydrocephalus was detrimental to motor functioning and nonverbal reasoning. Patients who had undergone surgery, chemotherapy, and radiotherapy presented impaired processing speed, verbal learning, and reading. In addition to the deleterious effects of PFT, post-resection PFT treatments have a negative cognitive impact. These undesired consequences and the associated tumor-related damage can be assessed using standardized, long-term neuropsychological evaluation when planning rehabilitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central nervous system (CNS) tumors are the second most common neoplasm in children and the most frequent solid tumor found in pediatric patients, and 60% to 70% of childhood CNS tumors develop infratentorially [1]. The overall incidence of pediatric brain tumor is approximately 4.5 per 100,000, with males comprising about 57% of the population affected [2]. Posterior fossa tumors (PFTs), which account for up to 60% of all childhood intracranial tumors [3], are typically classified by their histologic features, the three most common being medulloblastoma (40%), astrocytoma (20–35%), and ependymoma (10%) [1].
These three different tumor types typically require specific treatment regimens consisting of varying combinations of surgical resection, chemotherapy, and radiation therapy. However, radiation administered to the cranial region has been shown to have adverse effects on childhood neuropsychological and cognitive development, especially above certain dosage levels [4], and whole-brain irradiation and younger age were the most significant and consistent risk factors associated with poor cognitive outcome in children with brain tumors [5]. Furthermore, it has been reported that 5% to 40% of survivors exhibit significant, functionally disruptive neurocognitive impairments [6]. In addition to its role in motor skills and coordination, the cerebellum contributes to cognitive and executive functions. Children who undergo radiation therapy to treat cerebellar lesions are expected to develop deficits in cognitive functions such as intelligence, attention/executive function, and memory [7, 8].
Cerebellar mutism syndrome (CMS) is a syndrome that usually occurs after resection of a cerebellar tumor and is only rarely (roughly 1%) encountered in adults [9]. The syndrome commonly manifests 1 to 2 days after surgery, lasts for 1 day to several months, and can be followed by severe dysarthria before recovery [10]. The terms used to describe mutism caused by cerebellar disease have varied widely [11]. Thomale and Driever [12] listed the terms used in the literature, from most to least frequent: cerebellar mutism, posterior fossa syndrome, CMS, cerebellar mutism and subsequent dysarthria, and akinetic mutism. This list drew the attention of Gudrunardottir et al. [10], who pointed out the wide range of definitions, classifications, and systems used to grade symptoms, seeking to schematically organize all neurobehavioral, motor, and linguistic symptoms of this postoperative syndrome. The authors concluded that CMS was the most suitable term to cover all aspects of the condition, and it was thus decided to use this term over the others [10].
Regarding with Gudrunardottir et al. [10], the most widely accepted definition of mutism of cerebellar origin (and its associated symptoms) in children after cerebellar or fourth-ventricle tumor surgery states that postoperative pediatric CMS is characterized by delayed onset of mutism or reduced speech and emotional lability [13]. Additional common features include hypotonia and oropharyngeal dysphagia. CMS is frequently accompanied by cerebellar motor syndrome, cerebellar cognitive-affective syndrome, and brain-stem dysfunction including long tract signs and cranial neuropathies. Mutism is always transient in these cases but may require a prolonged recovery period. Speech and language may not return to normal, and other deficits of cognitive, affective, and motor function often persist [11]. On average, patients who experience CMS had lower cognitive scores at 1-year post-diagnosis and either demonstrated no significant recovery or continued to decline over time. The degree of the impairment varied depending on the skill measured. Processing speed was the most impaired skill among patients with and without CMS. Furthermore, patients with CMS exhibited impaired (> 2 SD below the mean value) processing speed and below-average intellectual ability at 1 year postoperatively, and scores remained low over time [14].
Depending on the definition used, CMS incidence figures range from 8% [3, 15] to 32% [16] among children with any kind of cerebellar tumor, compared to 24% [17] to 39% [18] in patients with medulloblastomas when a more precise definition of CMS is applied.
Known risk factors for CMS are brainstem tumor involvement, midline location, and certain types of tumors; the incidence of CMS in children with medulloblastoma is two to three times higher than for astrocytoma or ependymoma, but the biological mechanisms behind these associations are uncertain [17, 19,20,21]. Recently proposed risk factors for CMS include brainstem compression by the tumor, preoperative language impairment, low socioeconomic level of the family, and left-handedness [20]. Although the exact cause of CMS remains obscure, a recent study determined that significant risk factors for the syndrome are irritability in the pre- and postoperative period, midline localization of the tumor, maximum tumor size greater than 45 mm, histopathologic diagnosis of medulloblastoma, involvement of the superior cerebellar peduncles, and vermian incision and/or resection. Male gender also carries increased susceptibility to postoperative CMS. Transient ischemia and edema due to manipulation of the dentate nuclei, superior cerebellar peduncles, and the dentatothalamic pathway may contribute to the development of CMS [22]. The pathophysiological background of mutism may involve greater speech dysfunction mediated by crossed cerebello-cerebral diaschisis, a common finding during the mute period. Foremost injury to the bilateral dentatothalamocortical tract appears to be a critical component for the development of cerebello-cerebral diaschisis and subsequent mutism. Direct cerebellar injury is a likely reason for persisting deficits after the mute period. Minimization of injury to the dentatothalamocortical tract during surgery may be a promising measure in efforts to prevent mutism. Although mutism is generally thought to be transient, persistent impairment of verbal skills is common, with complete recovery of speech and language being infrequent in children affected by CMS [23,24,25].
Neuropsychological assessment provides important information about patients’ cognitive state, enabling us to identify the impact of surgical intervention and other treatments, not only in terms of intelligence quotient (IQ) but also concerning other neuropsychological functions essential to the ability to learn new information such as attention/executive function, memory, and information processing speed [26]. The aim of this research is to determine the neuropsychological features associated with PFT in children and their relationship with variables described in previous research, with particular focus on the presence or absence of CMS after surgery.
Materials and Methods
We assessed a group of 70 children (36 patients and 34 controls). The patient group comprised 36 children (26 boys, 10 girls) ranging in age from 4 to 18 years who had been treated for PFT in the Niño Jesús Children’s Hospital in Madrid, Spain, between 2015 and 2019. Their age at diagnosis was approximately 6 years, and the most common type of tumor was medulloblastoma. We collected data on demographics and past history, which appear in Table 1. Following surgery, the children were referred to the Clinical Neuropsychology Unit of our institution for assessment of postoperative neuropsychological consequences and to evaluate the suitability of different treatments. Each patient underwent an extensive formal neuropsychological assessment administered by a clinical psychologist specializing in clinical neuropsychology. This group of patients was compared to 34 healthy controls (HCs; 21 boys, 13 girls) in the same age range who had no neurological diseases or any other kind of disease or treatment impacting the CNS. Patients and controls were assessed using the same protocol.
Clinical and Neuropsychological Assessment
The full protocol is presented in Table 2. It is composed of standardized tests to assess several cognitive domains and in accordance with international recommendations [27]. The following domains were assessed:
general cognitive skills: Full Scale Intelligence Quotient (FSIQ), Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Working Memory Index (WMI), and Processing Speed Index (PSI);
motor functions: manual speed in the dominant and nondominant hand, and visuomotor coordination;
perception: Gestalt Closure and auditory processing
nonverbal skills: line orientation, emotion recognition of faces, visuo-constructional praxis;
reasoning: nonverbal and verbal reasoning;
language: receptive vocabulary, verbal comprehension, naming, word fluency/phonetic, and semantic association;
memory: memory of stories, verbal learning, delayed recall, facial recall, visual learning, and visual delayed learning;
attention: focused, selective, and sustained attention;
cognitive executive functions: planning, inhibitory control, flexibility, nonverbal fluency, and verbal operative memory; and
basic academic skills: reading/decoding, reading/understanding, writing, and arithmetic.
All tests were carried out in Spanish (native language). All of the normative scores were transposed to Z scores [mean (M) = 0; standard deviation (SD) = 1] to compare scores for all tests. Performance in cognitive domains was considered impaired if the score was > 1 standard deviation (SD) below the mean.
We compared controls and patients for such variables as tumor type, presence of hydrocephalus, surgical intervention, chemotherapy, and radiotherapy, with particular focus on the presence or absence of CMS.
Statistical Analysis
We used IBM SPSS version 25.0 (IBM Corp., Armonk, NY) for statistical analysis. Frequency, M, and SD were used to describe the sample characteristics. Nonparametric Mann-Whitney tests for independent samples were used to analyze the mean difference between HCs and clinical groups for all cognitive domains.
Results
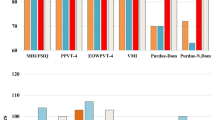
The neuropsychological results of the different groups are depicted graphically in Fig. 1. Firstly, significant differences between PFT patients and HCs were found in all domains assessed, with lower cognitive performance seen in the former (Table 3).
A comparison between PFT patients with CMS (Table 3) and PFT without CMS revealed that the former had significantly lower FSIQ, both overall and as reflected by the different indices that make up this score. Patients with CMS exhibited decreased motor functioning, nonverbal skills, attention, and executive functions; furthermore, all receptive and expressive linguistic processes and verbal memory were more impaired among these patients than in PFT patients without CMS. The only tasks in which patients with PFT (with or without CMS) and HCs performed similarly were those that require auditory-verbal processing and visual memory (learning and remembering).
When comparing patients with PFT who did not present CMS after surgery and individuals in the HC group (Table 3), overall impairments were also observed, though these were milder and less extensive than those in the PFT with CMS group. PFT followed by CMS was associated with lower intellectual capacity and worse execution in motor tasks, as well as impaired reasoning, verbal comprehension and fluency, verbal learning and recovery, attention, and cognitive executive functions. We also observed a greater number of preserved capacities among PFT patients who did not develop CMS; these patients had scores that were similar to those of the HC group in tasks assessing perceptual skills (visual and auditory processing), visuoconstructive praxis and spatial perception, receptive vocabulary, naming, narrative verbal memory, visual learning and delayed recall, planning, and nonverbal fluency. In a comparison between PFT with CMS (n = 19) and patients without CMS (n = 17) after PFT resection (Table 4), the former obtained lower scores in several cognitive domains. Specifically, we found significant differences in intellectual ability reflected by a lower FSIQ, as well as in VCI, WMI, and PSI scores. Significant differences were also observed in all tasks requiring motor skills, in some areas of receptive (receptive vocabulary) and expressive language (denomination), verbal memory (narrative and memory), and attention and cognitive executive functions, as well as academic skills that require reading comprehension.
Regarding the grade of the tumor (low grade (I, II) vs high grade (III, IV)), patients with high-grade tumors showed lower scores for the following variables: PSI (p = 0.019), verbal delayed recall (p = 0.045), selective attention (p = 0.019), and sustained attention (p = 0.031). We found that hydrocephalus had a negative impact on motor functioning, with significant differences in manual speed/dominant hand (p < 0.01), manual speed/nondominant hand (p < 0.01), and visuomotor coordination (p = 0.012) tasks as well as nonverbal reasoning (p = 0.007). Patients who had undergone chemotherapy and radiotherapy treatment showed lower scores in PSI, verbal learning, and reading compared to those who had received surgical intervention only.
Discussion
Previous studies [26, 28, 29] have found that children with PFT exhibit lower neuropsychological performance than the general population. In our research, children with PFT had a mean IQ of 89, and the group without CMS exhibited intellectual capacity scores that were not substantially lower than the overall population (FSIQ = 95), though these scores were significantly lower in the group with CMS (FSIQ = 81). The review by Hanzlik et al. [26], found that PFT patients had significantly lower scores than the normalized mean, at least one SD below. Moreover, Palmer et al. [30] reported a statistically significant loss of IQ of 2.55 points per year following diagnosis and treatment of PFT.
Existing research [25, 31,32,33] has shown that most patients develop speech deficits after surgery and/or treatment. Other studies [25, 32] have reached similar findings, reporting that patients with mutism presented more frequent speech deficits and slower speech rate than PFT patients without CMS, whereas non-mute patients did not differ from controls, as well as patients with CMS had poorer performance on several tasks compared to patients with no postoperative CMS. Schreiber et al. [14] reported that post-diagnosis trajectories of cognitive development over 5 years were worse in patients who experience CMS. Unlike previous studies, the children included in our study who had developed a PFT with no CMS showed significant differences when compared to HC children, and although these differences were milder and restricted, they also affected the intellectual competence, language, memory, attention, and executive functions of these children.
Despite continued evidence that radiation exposure has a negative impact on neurocognitive development in survivors of childhood CNS tumors, this therapeutic approach is often necessary to the survival of these patients. Studies have shown that tumor burden and the surgical intervention itself are associated with poorer performance in cognitive measures despite the fact that this detrimental effect has often been attributed to the radiation alone [26]. Di Rocco et al. [34] found significant deficits in IQ and attention among children with medulloblastomas at presentation, with significant improvement observed in these domains following surgery even before adjuvant therapy. There is still strong support for delaying radiation therapy for primary tumors in children younger than 3 due to the benefit seen in long-term outcomes [35]. Treatments following resection in PFT are detrimental to cognition but necessary for survival. As in previous studies [26, 28, 29, 36], we found that patients with high-grade tumors, who receive the most aggressive treatments, exhibit slower processing speed and greater weaknesses in delayed verbal memory and attentional processes compared to low-grade tumors that only require surgery.
Neuropsychological evaluation provides relevant information on the cognitive status of these patients. Further longitudinal studies are needed to broaden knowledge of the long-term impact of the brain damage caused by the tumor as well as the effects of treatment. It has been suggested that patients with CMS should undergo routine follow-up to monitor possible emotional, behavioral, and social problems over time [22]. On this issue, our findings suggest that although we can expect more severe impairments in children with CMS secondary to PFT treatment, all children with a PFT should be evaluated to determine the need for rehabilitative treatments, which can at least partially alleviate these disease- and treatment-related deficits.
Improvements made to the assessment and care of these children will provide a better understanding of the disease and its treatment. While there is no established treatment of mutism, early speech and rehabilitation therapy is recommended [37]. Long-term follow-up and rehabilitation after PFT should be tailored to patients’ individual needs, as a variety of functions, including memory, attention, executive functions, visuospatial skills, perceptual abilities, and communication skills may be impaired to varying degrees [25]. To achieve the best possible outcome for survivors and minimize the long-term consequences of CMS, new interventions must be developed to stimulate neurorecovery and plasticity, and their efficacy tested [38]. Particular attention should be paid to providing individual assessment and neuropsychological treatment for children who live with long-term consequences after PFT, as these measures may greatly improve their quality of life.
Recognizing and measuring both short- and long-term effects of PFT and treatments aimed at eliminating these tumors is the first step toward improving the quality of life of these children. These patients should be monitored to assess the development of cognition and emotion. As the survival rates of children diagnosed and treated for PFT increase, the appearance of neuropsychological deficits 5, 10, and 20 years later is becoming apparent. Rigorous study of the long-term outcomes in these children can be used to develop profiles for the disease, which could then be used to improve early intervention, design preventive measures, and more accurately predict disease and treatment outcome for patients and their families [26].
Conclusion
Patients with PFTs exhibit poorer neuropsychological performance than the general population. CMS is a common consequence following surgery to treat this type of tumor. Unlike previous research, we found the lowest performance in neuropsychological assessment among patients who developed CMS after surgery. We therefore consider CMS to be a clinical marker generally associated with extensive impairments across all cognitive domains.
Neuropsychological assessment provides valuable information about the impact of the type of tumor as well as the surgical resection and other treatments employed. Identifying impairments in cognitive domains is useful when managing and designing specific rehabilitation programs to improve performance immediately after surgery. Long-term neuropsychological follow-up is necessary to assess the effects of rehabilitation and the evolution of cognitive, behavioral, and emotional impairments.
References
Nejat F, El Khashab M, Rutka JT. Initial management of childhood brain tumors: neurosurgical considerations. J Child Neurol. 2008;23(10):1136–48.
Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-Oncology. 2012;14(Suppl 5):v1–49.
Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37(5):885–93.
Palmer SL, Leigh L. Survivors of pediatric posterior fossa tumors: cognitive outcome, intervention, and risk-based care. Eur J Oncol Nurs. 2009;13(3):171–8.
Tonning Olsson I, Perrin S, Lundgren J, Hjorth L, Johanson A. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol. 2014;51(4):515–21.
Reimers TS, Ehrenfels S, Mortensen EL, Schmiegelow M, Sonderkaer S, Carstensen H, et al. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol. 2003;40(1):26–34.
Aarsen FK, Paquier PF, Arts WF, Van Veelen ML, Michiels E, Lequin M, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol. 2009;27(21):3526–32.
von Hoff K, Kieffer V, Habrand JL, Kalifa C, Dellatolas G, Grill J. Impairment of intellectual functions after surgery and posterior fossa irradiation in children with ependymoma is related to age and neurologic complications. BMC Cancer. 2008;8:15.
De Smet HJ, Marien P. Posterior fossa syndrome in an adult patient following surgical evacuation of an intracerebellar haematoma. Cerebellum. 2012;11(2):587–92.
Gudrunardottir T, Morgan AT, Lux AL, Walker DA, Walsh KS, Wells EM, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. 2016;32(7):1195–203.
Catsman-Berrevoets C, Patay Z. Cerebellar mutism syndrome. Handb Clin Neurol. 2018;155:273–88.
Thomale UW, Driever PH. Inconsistent terminology for cerebellar mutism. Childs Nerv Syst. 2013;29(5):717–8.
Lanier JC, Abrams AN. Posterior fossa syndrome: review of the behavioral and emotional aspects in pediatric cancer patients. Cancer. 2017;123(4):551–9.
Schreiber JE, Palmer SL, Conklin HM, Mabbott DJ, Swain MA, Bonner MJ, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro-Oncology. 2017;19(12):1673–82.
Van Calenbergh F, Van de Laar A, Plets C, Goffin J, Casaer P. Transient cerebellar mutism after posterior fossa surgery in children. Neurosurgery. 1995;37(5):894–8.
Kotil K, Eras M, Akçetin M, Bilge T. Cerebellar mutism following posterior fossa tumor resection in children. Turk Neurosurg. 2008;18(1):89–94.
Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg. 2006;105(6 Suppl):444–51.
Wells EM, Khademian ZP, Walsh KS, Vezina G, Sposto R, Keating RF, et al. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr. 2010;5(4):329–34.
Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the posterior Fossa syndrome in children after cerebellar tumour surgery. Cortex. 2010;46(7):933–46.
van Baarsen KM, Grotenhuis JA. The anatomical substrate of cerebellar mutism. Med Hypotheses. 2014;82(6):774–80.
Reed-Berendt R, Phillips B, Picton S, Chumas P, Warren D, Livingston JH, et al. Cause and outcome of cerebellar mutism: evidence from a systematic review. Childs Nerv Syst. 2014;30(3):375–85.
Gora NK, Gupta A, Sinha VD. Cerebellar Mutism syndrome following midline posterior fossa tumor resection in children: an institutional experience. J Pediatr Neurosci. 2017;12(4):313–9.
Steinbok P, Cochrane DD, Perrin R, Price A. Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg. 2003;39(4):179–83.
Charalambides C, Dinopoulos A, Sgouros S. Neuropsychological sequelae and quality of life following treatment of posterior fossa ependymomas in children. Childs Nerv Syst. 2009;25(10):1313–20.
De Smet HJ, Catsman-Berrevoets C, Aarsen F, Verhoeven J, Marien P, Paquier PF. Auditory-perceptual speech analysis in children with cerebellar tumours: a long-term follow-up study. Eur J Paediatr Neurol. 2012;16(5):434–42.
Hanzlik E, Woodrome SE, Abdel-Baki M, Geller TJ, Elbabaa SK. A systematic review of neuropsychological outcomes following posterior fossa tumor surgery in children. Childs Nerv Syst. 2015;31(10):1869–75.
Wilson SJ, Baxendale S, Barr W, Hamed S, Langfitt J, Samson S, et al. Indications and expectations for neuropsychological assessment in routine epilepsy care: report of the ILAE Neuropsychology Task Force, Diagnostic Methods Commission, 2013-2017. Epilepsia. 2015;56(5):674–81.
Ronning C, Sundet K, Due-Tonnessen B, Lundar T, Helseth E. Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg. 2005;41(1):15–21.
Roncadin C, Dennis M, Greenberg ML, Spiegler BJ. Adverse medical events associated with childhood cerebellar astrocytomas and medulloblastomas: natural history and relation to very long-term neurobehavioral outcome. Childs Nerv Syst. 2008;24(9):995–1002 discussion 3.
Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–8.
Cornwell PL, Murdoch BE, Ward EC, Morgan A. Dysarthria and dysphagia as long-term sequelae in a child treated for posterior fossa tumour. Pediatr Rehabil. 2003;6(2):67–75.
Huber JF, Bradley K, Spiegler BJ, Dennis M. Long-term effects of transient cerebellar mutism after cerebellar astrocytoma or medulloblastoma tumor resection in childhood. Childs Nerv Syst. 2006;22(2):132–8.
Hudson LJ, Murdoch BE, Ozanne AE. Posterior fossa tumours in childhood: associated speech and language disorders postsurgery. Aphasiology. 1989;3:1–18.
Di Rocco C, Chieffo D, Pettorini BL, Massimi L, Caldarelli M, Tamburrini G. Preoperative and postoperative neurological, neuropsychological and behavioral impairment in children with posterior cranial fossa astrocytomas and medulloblastomas: the role of the tumor and the impact of the surgical treatment. Childs Nerv Syst. 2010;26(9):1173–88.
Sands SA, Oberg JA, Gardner SL, Whiteley JA, Glade-Bender JL, Finlay JL. Neuropsychological functioning of children treated with intensive chemotherapy followed by myeloablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54(3):429–36.
Quintero-Gallego EA, Gomez CM, Vaquero Casares E, Marquez J, Perez-Santamaria FJ. Declarative and procedural learning in children and adolescents with posterior fossa tumours. Behav Brain Funct. 2006;2:9.
Küper M, Timmann D. Cerebellar mutism. Brain Lang. 2013;127(3):327–33.
Lassaletta A, Bouffet E, Mabbott D, Kulkarni AV. Functional and neuropsychological late outcomes in posterior fossa tumors in children. Childs Nerv Syst. 2015;31(10):1877–90.
Acknowledgments
We thank Oliver Shaw and Biomedical Research Foundation of Niño Jesús Children’s Hospital for providing us a consultation service for English language editing and reviewing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cámara, S., Fournier, M.C., Cordero, P. et al. Neuropsychological Profile in Children with Posterior Fossa Tumors with or Without Postoperative Cerebellar Mutism Syndrome (CMS). Cerebellum 19, 78–88 (2020). https://doi.org/10.1007/s12311-019-01088-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-019-01088-4