Abstract
Cerebellar hemorrhage (CBH) is a frequent complication of preterm birth and may play an important and under-recognized role in neurodevelopment outcome. Association between CBH size, location, and neurodevelopment is still unknown. The main objective of this study was to investigate neurodevelopmental outcome at 2 years of age in a large number of infants with different patterns of CBH. Of preterm infants (≤ 34 weeks) with known CBH, perinatal factors, neuro-imaging findings, and follow-up at 2 years of age were retrospectively collected. MRI scans were reassessed to determine the exact size, number, and location of CBH. CBH was divided into three groups: punctate (≤ 4 mm), limited (> 4 mm but < 1/3 of the cerebellar hemisphere), or massive (≥ 1/3 of the cerebellar hemisphere). Associations between pattern of CBH, perinatal factors, and (composite) neurodevelopmental outcome were assessed. Data of 218 preterm infants with CBH were analyzed. Of 177 infants, the composite outcome score could be obtained. Forty-eight out of 119 infants (40%) with punctate CBH, 18 out of 35 infants (51%) with limited CBH, and 18 out of 23 infants (78%) with massive CBH had an abnormal composite outcome score. No significant differences were found for the composite outcome between punctate and limited CBH (P = 0.42). The risk of an abnormal outcome increased with increasing size of CBH. Infants with limited CBH have a more favorable outcome than infants with massive CBH. It is therefore important to distinguish between limited and massive CBH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebellar hemorrhage (CBH) is a common complication in very low birthweight infants. Reported incidences in infants born below 32 weeks gestation, and/or weighing less than 1500 g at birth, range from 2.2 to 19% [1,2,3], depending on the population studied and the imaging techniques used. The etiology of CBH is multifactorial. Potential risk factors are traumatic delivery and circulatory events related to prematurity, such as impaired cerebrovascular autoregulation, large patent ductus arteriosus, and other parameters of a compromised cerebral circulation [4,5,6,7].
Until recently, it was thought that the cerebellum was mainly involved in motor system functions [8]. However, in a retrospective, case-control design study, Limperopoulos et al. found cerebellar hemorrhagic injury in preterm infants to be associated with a high prevalence of long-term pervasive neurodevelopmental disabilities [2]. Other studies have shown that injury to the cerebellum not only affects motor functions, but also non-motor functions, including cognition, learning, and behavioral abilities [9, 10]. CBH may thus play an important and under-recognized role in the cognitive, learning, and behavioral problems known to affect survivors of extremely preterm birth [6, 11, 12].
In preterm infants with CBH, the size of the lesion may be of importance with respect to neurodevelopmental outcome. Three patterns of preterm CBH have been described [13, 14]; the first being massive CBH, mainly seen in the sickest and smallest infants (< 28 weeks gestation and/or < 750 g). These massive bleeds carry a high morbidity and mortality and are easily diagnosed with cranial ultrasonography (CUS), especially when the mastoid window is used [4, 15]. In surviving infants, massive CBH leads to severe cerebellar destruction and subsequent atrophy, and is associated with long-term and severe neurodevelopmental disabilities, such as cerebral palsy (CP) and motor, language and/or cognitive delays [1, 2, 11]. Associated supratentorial injury and/or cerebellar diaschisis may also play a role in this unfavorable outcome [5, 6]. Another pattern is small or punctate hemorrhages. These are small (≤ 4 mm) lesions that are beyond the scope of CUS but are frequently encountered as a chance finding on magnetic resonance imaging (MRI), which is often performed in very preterm infants around term equivalent age (TEA) [14]. These small CBH do not seem to lead to cerebellar atrophy and are associated with a more favorable prognosis. Tam et al. [16] found preterm infants with small CBH to have a 5-fold higher incidence of abnormal neurological examination at 3–6 years of age than those without CBH, but there was no significant difference in cognitive impairment. Steggerda et al. found no association between small CBH and neurodevelopmental outcome at 2 years of age [17]. The third, so far only rarely described pattern concerns “medium-sized” or limited hemorrhages that involve less than one third of the cerebellar hemisphere. These limited hemorrhages are mostly located at the convexity of one of the cerebellar hemispheres and may be diagnosed with CUS, especially if the mastoid fontanel is used as an additional acoustic window [13]. They occur in very preterm neonates, do not seem to cause acute clinical symptoms and their possible influence on outcome is not yet known, in addition, it is not known whether these limited hemorrhages may lead to cerebellar atrophy [14, 18]. As different parts of the cerebellum seem to be involved in different executive, affective, limbic, and sensorimotor functions, the relationship between location of the lesion(s) and neurodevelopmental and behavioral outcome is also worth investigating [11, 19,20,21,22].
Despite the increasing number of papers reporting about neonatal CBH, the number of included infants with CBH in these studies is limited. Therefore, drawing conclusions from associations between CBH size and location, and neurodevelopment is still a challenge. The main objective of this study was therefore to investigate and compare neurodevelopmental outcome at 2 years of age in a large number of infants with punctate, limited, and massive CBH. Other objectives were to investigate associations between:
location of CBH and neurodevelopmental outcome;
pattern of CBH and cerebellar atrophy;
pattern of CBH and perinatal factors.
Patients and Methods
In this retrospective, multi-center study, data from preterm infants ≤ 34 weeks gestation with MRI diagnosed CBH who were admitted to one of 6 tertiary neonatal centers (4 Dutch and 2 Italian) were collected and analyzed. The participating centers were selected based on their neonatal neuro-imaging protocols with special attention for cerebellar injury. Infants were born between 2003 and 2016 and included if at least one neonatal MRI examination had been performed. Exclusion criteria were as follows: (suspected) brain malformation, dysmorphic features or congenital anomaly suggestive of a genetic syndrome, metabolic disorder, chromosomal abnormality, and/or proven central nervous system infection.
The participating centers were Isala Women and Children’s hospital (IVKC), Zwolle, the Netherlands; Leiden University Medical Center (LUMC), Leiden, the Netherlands; University Medical Center Utrecht (UMCU), Utrecht, the Netherlands; Erasmus Medical Center (Erasmus MC), Rotterdam, the Netherlands; Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; and Istituto Giannina Gaslini, Genova, Italy. Infants were selected from an institutional MRI register and from existing databases of infants known to have cerebellar abnormalities.
As the study did not fall under the Medical Research Involving Human Subjects Act and clinically obtained anonymized data were used, the medical ethical committees of the participating centers waived an informed consent and ethical review procedure.
Patient Characteristics
Maternal, perinatal, and neonatal factors were retrieved from the medical records. Maternal factors included age, presence of pre-eclampsia, and use of antenatal steroids. Perinatal factors included gender, gestational age (GA), birth weight (BW), Z-score for BW according to Hoftiezer et al. [23], multiple birth, mode of delivery (i.e., breech extraction, instrumental delivery, cesarean section), umbilical cord pH, and Apgar score at 5 min. Neonatal factors were mechanical ventilation within the first postnatal week, high-frequency oscillation (HFO) ventilation within the first postnatal week, surfactant replacement therapy, hypotension (defined as need for inotropic support) within the first postnatal week, severe thrombocytopenia (defined as platelet count < 50 × 109/L) within the first postnatal week, and in-hospital mortality.
Neuro-imaging
At IVKC, LUMC, UMCU, Fondazione IRCCS Ca’ Granda and Gaslini, MRI was performed around term equivalent age (TEA) using a 1.5-T or 3.0-T MR system (Ingenia (IVKC), Achieva (LUMC, UMCU, Fondazione IRCCS Ca’ Granda and Gaslini), Philips Medical Systems, Best, The Netherlands. At Erasmus, MC MRI was obtained around the postmenstrual age (PMA) of 30 weeks, or, in infants born at GA > 30 weeks, as soon as possible after birth, using a 1.5-T GE EchoSpeed scanner (General Electrics Healthcare Technologies, Waukesha, WI). In UMCU, MRI was routinely done in all infants < 28 weeks gestation and in Fondazione IRCCS Ca’ Granda in all infants < 32 weeks gestation, while in the other hospitals MRI was performed if there was an indication, according to the local guidelines. T1-weighted, T2-weighted images (slice thickness 1.2 mm at Erasmus MC; 2 mm at IVKC, LUMC, UMCU, and Fondazione IRCCS Ca’ Granda; 3 mm at Gaslini) and, if available, susceptibility weighted images (SWI) were used for detection and scoring CBH. The SWI sequence was not available during the first years of the study and was only used since 2006 in some centers and since 2014 in all. Due to slice thickness of 3 mm for the T2-weighted images in Gaslini and therefore the limited ability to detect punctate CBH, especially before the SWI-era, only infants with limited or massive CBH were selected from this center. All available MRI examinations were screened for the presence of CBH by one of the investigators (V.B.). Subsequently, all MRI examinations with CBH were reviewed by 2 investigators: V.B. along with a neonatologist of each center (G.M.; S.J.S.; L.S.V.; J.D.; A.P., or M.F.). These neonatologists are experts with at least 10 years of experience in neonatal neuro-imaging. In case of disagreement, an expert from one of the other centers was consulted.
Pattern of CBH
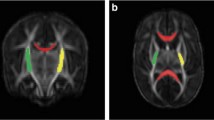
The classification was modified from the CUS classification by Meijler and Steggerda [18] and previous work by others [13, 24] (Fig. 1).
- 1.
Punctate CBH: one or more lesions ≤ 4 mm;
Infants with punctate CBH were subdivided into two groups: ≤ 6 lesions or > 6 lesions.
- 2.
Limited CBH: the lesion(s) being > 4 mm, but involving < 1/3 of the cerebellar hemisphere;
- 3.
Massive CBH: the lesion involving ≥ 1/3 of the cerebellar hemisphere.
MR images performed around TEA. a T2-weighted MR image and SWI of a punctate CBH located in the right cerebellar hemisphere (arrow). b Limited CBH at the convexity of the left cerebellar hemisphere. c Massive CBH located in the left cerebellar hemisphere, also leading to destruction and atrophy of that hemisphere
The pattern of CBH was determined based on the T2-W images (transverse planes). Foci of signal loss on T2-W images and/or SWI were considered to be hemosiderin deposits and thus (punctate) hemorrhages if there was no continuity with a vessel, suggesting a vascular structure.
For the MRI scans performed around TEA (PMA 38–44 weeks), the transcerebellar diameter (TCD) was measured on a transverse T2-W plane, at the largest cerebellar diameter. Because the TCD increases with increasing PMA at MRI, we corrected the measured value according to the equation developed by Kidokoro et al. [25]. Cerebellar atrophy was considered mild to moderate if the corrected TCD was < 50 mm, but ≥ 44 mm and severe if the corrected TCD was < 44 mm.
Location of CBH
We also categorized CBH according to location. Figure 2 shows a map of structural-function corresponding regions of the cerebellum on transverse and coronal planes. Region A comprises the convexity (lateral-posterior-inferior) hemispheric zones; region B, the anterior/medial hemispheric zones; and region C, the vermis. This map is based on previous works [19,20,21, 26].
Supratentorial Injury
Intraventricular hemorrhage (IVH) (classified according to Volpe [27]) was recorded based on neonatal ultrasound reports. White matter injury was graded according to Kidokoro [3]: grade 1: punctate lesions ≤ 3 mm in the periventricular white matter on one or both sides; grade 2: punctate lesions present within the corticospinal tract on both sides, or ≥ 3 lesions per hemisphere; grade 3: extensive lesions with high signal on T1-weighted images along the wall of the lateral ventricles; grade 4: cystic lesions in the periventricular white matter (this could be either cystic degeneration of periventricular hemorrhagic infarction or cystic periventricular leukomalacia).Ventricular dilatation (VD) was assessed on axial T2-weighted images at midventricular level. Bilateral ≥ 7.5 mm or one-sided ≥ 10-mm VD were considered severe. Based on these criteria, infants were categorized into presence or absence of severe supratentorial injury (i.e., IVH grades 3 and 4, WMI grades 3 and 4, and/or severe VD).
Follow-up
Results of the neurodevelopmental tests and neurological examinations performed between 18 and 36 months were retrieved from the medical files. In the four Dutch centers, a standard neurological examination and the Bayley scales of infant development (BSID-III American or Dutch edition) [28] were performed. As validation of the language scale of the BSID-III was not yet available during the entire study period in the Netherlands, only the cognitive and motor scales were tested. In addition, the Child Behavior Checklist (CBCL)—a questionnaire filled out by the parents—was used as a parameter of neurodevelopment [29]. Italian infants were examined and tested by means of a standard neurological examination and the Griffiths Mental Development Scales Revised (GMDS) [30]. The GMDS test comprises 5 subscales: locomotor, personal-social, hearing and speech, eye and hand coordination, and performance. The results of the neurological examination were considered abnormal when infants had cerebral palsy (hemi-, di-, or tetraplegia) and/or neurosensory hearing loss. Cerebral palsy was scored according to the Gross Motor Function Classification System (GMFCS) [31], a score ≥ 1 was considered abnormal. Information on cerebral visual impairment could not be consistently retrieved from the medical records and was therefore not included. A score of < − 1 SD for one of the subscales of the BSID-III or GMDS was considered abnormal, while a CBCL score above 60 was considered to be in the clinical range [29, 32, 33] and thus abnormal. If at least one of the outcome parameters (neurological examination, BSID-III or GMDS, CBCL) was abnormal, this was considered an abnormal composite outcome.
Firstly, each outcome parameter was compared between infants with punctate, limited, and massive CBH. Secondly, the composite outcome was compared between infants with punctate, limited, and massive CBH.
Statistical Analysis
Statistical analysis was performed using SPSS software (version 23.0, SPSS Inc., Chicago, IL, USA). Distribution of continuous variables was objectively assessed by means of the Shapiro-Wilk test. Continuous data are presented as median (range). Categorical variables are presented using frequency counts and percentages. Differences between variables were tested for significance using the Mann-Whitney U test when comparing two continuous variables, and the Kruskal-Wallis test when comparing three variables. For categorical variables, the Chi-square test or Fisher’s exact test was used. The contribution of maternal, perinatal, and neonatal variables on the pattern of CBH was analyzed using logistic regression. Neurodevelopmental outcome was related to size of CBH using logistic regression; adjustments were made for GA, gender, and presence of severe supratentorial injury. Significance and odds ratio (OR) with 95% confidence interval (CI) were calculated and a P value < 0.05 was considered significant. Infants with missing data were not included in analyses.
Results
Patients
Data of 218 infants with CBH were collected. The median GA at birth was 27.2 weeks (23.0–34.0 weeks) and median BW 958 g (400–2665 g). The median PMA at MRI was 41.3 weeks (27.7–64.1 weeks). One hundred forty-seven (67%) infants had punctate, 40 (18%) limited, and 31 (14%) massive CBH. Severe supratentorial injury was seen in 52% of the infants with punctate, 60% of the infants with limited, and 52% of the infants with massive CBH. There were no significant differences in incidences of severe IVH, WMI, or VD between the three groups (see Table 1). Ten infants died: two due to massive CBH and three due to severe supratentorial injury. The five other infants died due to systemic complications (i.e., sepsis, multi-organ failure, respiratory complications).
Perinatal Factors and Pattern of CBH
Table 1 shows perinatal variables for infants with punctate, limited, and massive CBH. Due to missing data P values for mode of delivery and umbilical cord pH were not representative and are therefore not shown. Infants with limited and massive CBH had a lower GA than infants with punctate CBH. They also experienced more respiratory difficulties: more infants with limited and massive CBH needed mechanical ventilation and surfactant replacement therapy. Lower than 5 min Apgar score was more often seen in infants with massive CBH than in the other two groups. Infants with massive CBH more often needed high-frequency ventilation, had lower BW, and more often severe thrombocytopenia than infants with punctate CBH. Analyzing the contribution of these variables by logistic regression revealed GA, Apgar score, and severe thrombocytopenia as independent contributors to massive CBH, while mechanical ventilation remained an independent factor for limited CBH.
Cerebellar Atrophy
Severe atrophy (TCD < 44 mm) at MRI around TEA (N = 141) was seen in 1% (1/102) of infants with punctate CBH, 26% (6/23) of infants with limited CBH, and in 75% (12/16) of infants with massive CBH. Mild to moderate cerebellar atrophy (TCD < 50 mm and ≥ 44 mm) was seen in 28%, in 48%, and in 19% of infants with respectively punctate, limited, and massive CBH. TCD was lower with increasingly larger CBH (P < 0.01; see Table 2).
Pattern of CBH and Neurodevelopmental Outcome
Follow-up was available for 177/208 (85%) of surviving infants (Table 3). The median corrected age at follow-up was 24.0 months (range 18.0–36.0 months). Infants with massive CBH had the highest percentage of abnormal results for neurological examination, BSID-III, GMDS, and CBCL (see Table 4). For all outcome parameters, except the BSID-III, there was a trend towards higher percentages of abnormal results with an increase in the size of CBH. As there was a striking difference between the test results of the GMSD and the Bayley-III, we analyzed the difference between Italian and Dutch children. Italian infants were significantly younger and had more respiratory difficulties, more often culture proven late onset sepsis and more surgery before TEA than Dutch infants (see Table 5).
A sub-analysis, performed in infants without severe supratentorial injury (N = 87), showed a trend towards higher percentages of abnormal results with increasingly larger CBH for an abnormal neurological examination and CBCL (Table 6). Overall, 33% of infants without severe supratentorial injury had an abnormal composite outcome. The composite outcome differed significantly between the three groups (P < 0.01) and again the percentages of infants with abnormal outcome increased with larger hemorrhages. Differences between the three groups remained significant (P 0.02) in infants without severe supratentorial injury.
Comparing massive and punctate CBH, logistic regression analysis demonstrated an increased risk of an abnormal composite outcome in infants with massive CBH, with an adjusted OR of 5.52 (95%CI 1.75–17.43; P < 0.01). Comparing massive with limited CBH, the adjusted OR was 4.09 (95%CI 1.09–15.28; P = 0.04). No significant differences were found for the composite outcome between punctate and limited CBH (P = 0.42).
In a sub-analysis of infants with punctate CBH, we analyzed the neurodevelopmental outcome of infants with ≤ 6 punctate lesions as compared to infants with > 6 punctate lesions. Thirty-nine infants (40%) with ≤ 6 punctate lesions had an abnormal composite outcome score compared to eight infants (36%) of infants with more than six punctate lesions (P = 0.79).
Location of CBH and Neurodevelopmental Outcome
Location A and location B were most frequently seen in infants with punctate and limited CBH. A combination of locations A and B, and of locations A, B, and C was most often seen in infants with massive CBH. Most infants (N = 119) had unilateral CBH. Although not significant, 67% of infants with bilateral limited CBH and 100% of infants with bilateral massive CBH had an abnormal composite outcome compared to respectively 48% and 74% of infants with unilateral limited and massive CBH (see Table 7). In none of the infants with limited CBH, location C (the vermis) was involved. For infants with punctate CBH, outcome did not differ between infants with and without vermis involvement. All infants with massive CBH and vermis involvement had an abnormal composite outcome (see Table 8). Due to the restricted number of infants per specific location, the relation between location and neurodevelopmental outcome could not be further investigated.
Discussion
We analyzed associations between perinatal factors, cerebellar atrophy, neurodevelopmental outcome, and pattern of CBH determined according to a newly defined MRI classification of CBH that includes punctate, limited, and massive CBH. Several studies have demonstrated an increased risk of an impaired neurodevelopmental outcome for infants with CBH [2, 10, 16, 34]. However, to the best of our knowledge, this is the first large sample study taking size of CBH into account. We demonstrated a higher risk of abnormal composite outcome with increasing size of CBH, infants with massive CBH having the highest chance of an unfavorable outcome. Even without severe supratentorial injury they still had a very high risk of an abnormal composite outcome. This is in accordance with the results of Limperopoulos et al. [2] and with data shown in the review of Hortensius et al. [12] who both demonstrated that cognitive, language and behavior sequelae occur frequently in infants with isolated CBH. However, in the systematic review of Hortensius et al. [12], an incidence of 43 to 75% for severe neurodevelopmental outcome in infants with isolated CBH was reported, which is much higher than the 38% found in this study. This may be explained by the number of infants with punctate CBH included in the analyses. In the systematic review, 15/126 infants with isolated punctate CBH were included, while in our study, 119/177 infants were included.
While the composite outcome did not differ significantly between infants with punctate and limited CBH, we found a significant difference in composite outcome between limited and massive CBH. These are important findings. So far, the outcome of infants with limited CBH has not been reported and previous studies, reporting on the neurodevelopmental outcome of infants with CBH, did not distinguish between limited and massive CBH. Combining these two patterns of CBH may have influenced the results in previous studies and may erroneously have suggested that limited CBH carries a similar high risk of an unfavorable prognosis as massive CBH. While we found an abnormal composite outcome in 40% of the infants with limited CBH, this percentage was lower than in infants with massive CBH (67%).
Severe cerebellar atrophy, using the corrected TCD, was seen in 75% of infants with massive CBH and in a minority of infants with limited or punctate CBH. Due to major growth and development during the second half of gestation, the cerebellum is particularly vulnerable to developmental disruption [4]. Also, without evident cerebellar injury, mild atrophy may develop in preterm born children. Cerebellar development may be disrupted by various factors, i.e., hemorrhage, toxic effects of hemosiderin deposition, or supratentorial injury [5, 6, 35]. Not all infants with CBH developed cerebellar atrophy. It may be interesting and clinically relevant to investigate the pathogenesis of cerebellar atrophy in infants with CBH and the possible influence of timing of the CBH in a next, prospective study.
The location of CBH may be of clinical importance in infants with limited or massive CBH. Two studies described a negative effect of vermis involvement in global developmental outcome [2, 9], but did not distinguish between limited and massive CBH. While in our study all infants with massive CBH and vermis involvement had an abnormal composite score, in none of the infants with limited CBH the vermis was involved. In contrast to Hortensius et al. [12], we found a difference in composite outcome between infants with uni- or bilateral CBH, with a more favorable outcome in those with a unilateral CBH. In that study and ours the number of infants with bilateral CBH was small, therefore no conclusions can be drawn on the relation between laterality and outcome. In infants with punctate CBH, neither the number of lesions nor the location (uni- or bilateral or vermis involvement) had an influence on the composite score.
Several factors have been associated with CBH, such as low GA, low BW, HFO ventilation, inotropic support, and severe IVH [5, 6, 17, 34]. We tried to identify factors that contributed to limited or massive CBH, when compared to punctate CBH. Mechanical ventilation was independently associated with limited CBH, while lower GA, lower Apgar score and severe thrombocytopenia were independently associated with massive CBH. Comparing limited and massive CBH, most factors were not significantly different between the two groups, suggesting that these may have a more similar pathogenesis.
We acknowledge several limitations of our study. Firstly, we compared infants with different patterns of CBH, but we did not compare them with a control group without CBH. Secondly, we retrospectively collected the data of infants with known CBH on MRI. Therefore, this is a selected group of preterm neonates: except for the infants in Utrecht and Milan, MRI was performed when clinically indicated. Infants without or with only minor infra- and supratentorial lesions are therefore underrepresented. However, we have reached our primary aim to compare infants with small, limited, and massive CBH.
Thirdly, the participating centers used different scan protocols. At Erasmus MC, infants were scanned around PMA 30 weeks, while at the other centers infants were scanned around TEA. This may have influenced the detection rate of small lesions: punctate white matter lesions often fade over time [36], the same may be true for punctate cerebellar lesions. However, we do not think this has influenced our results since in the majority of infants (68%), SWI was performed, enabling the detection of even tiny (remnants of) bleeds after a long period of time and we were still able to compare the different patterns of CBH. Furthermore, we examined cerebellar atrophy around TEA, while this may still develop after this age. Additionally, we only measured TCD and did not measure cerebellar volume and may thus have missed small alterations in cerebellar volumes.
Finally, different neurodevelopmental tests were used. In Italy, this was GMDS, while the Dutch centers used BSID-III. Although Picciolini et al. [37] reported that the BSID-III had a higher agreement with GMDS than the BSID-II, there are still differences between the two tests. Italian infants were significantly younger and suffered more neonatal morbidity than Dutch infants; this may partially explain the less favorable outcome in the Italian infants. Moreover, the maternal education level could not be found for more than half of the included infants. This may have influenced test results. In the Dutch centers, the language scale of the BSID was not yet validated and could therefore not be used. It is however well known that language may be affected by cerebellar injury [2]. This may be another explanation for the less favorable outcome of the Italian infants. All components of the composite outcome were allocated the same weight, but one could argue that for instance CP may impose a larger burden on the infant and its family than an abnormal CBCL score.
Although we collected a large population of infants, distributed over 6 centers, the number of infants was still too small to investigate the relation between the specific location of CBH and neurodevelopmental outcome.
Conclusion
Limited and massive CBH are associated with the same perinatal factors. The risk of an abnormal composite outcome increases with increasing size of CBH. Infants with limited CBH have a more favorable outcome than infants with massive CBH. It is therefore important to distinguish between limited and massive CBH. Future studies should focus on the relation between location of CBH and neurodevelopmental outcome, and between size of CBH and neurodevelopmental outcome at school age. In addition, the influence of perinatal factors and timing of CBH on subsequent cerebellar atrophy should be evaluated.
References
Steggerda SJ, Leijser LM, Wiggers-de Bruïne FT, van dG, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology. 2009;252(1):190–9.
Limperopoulos C, Bassan H, Gauvreau K, Robertson RL,J, Sullivan NR, Benson CB, Avery L, Stewart J, MD JSS, Ringer SA, Volpe JJ, duPlessis AJ Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007 Sep;120(3):584–593.
Kidokoro H. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics. 2014;134(2):444–53.
Volpe JJ. Volpe’s neurology of the newborn. Sixth edition. ed. Philadelphia, PA: Elsevier; 2018.
Limperopoulos C, Soul J, Haidar H, Huppi P, Bassan H, Warfield S, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116(4):844–50.
Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24(9):1085–104.
Tam EWY. Cerebellar injury in preterm infants. Handb Clin Neurol. 2018;155:49–59.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78.
Zayek MM, Benjamin JT, Maertens P, Trimm RF, Lal CV, Eyal FG. Cerebellar hemorrhage: a major morbidity in extremely preterm infants. J Perinatol. 2012;32(9):699–704.
Bednarek N, Akhavi A, Pietrement C, Mesmin F, Loron G, Morville P. Outcome of cerebellar injury in very low birth-weight infants: 6 case reports. J Child Neurol. 2008;23(8):906–11.
Brossard-Racine M, du Plessis AJ, Limperopoulos C. Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. The Cerebellum, 2015;14(2):151–164.
Hortensius L, Dijkshoorn ABC, Ecury Goossen G, Steggerda S, Hoebeek F, Benders MJNL, et al. Neurodevelopmental consequences of preterm isolated cerebellar hemorrhage: a systematic review. Pediatrics. 2018;142(5):e20180609.
Parodi A. Accuracy of ultrasound in assessing cerebellar haemorrhages in very low birthweight babies. Fetal and Neonatal. 2015;100(4):289–92.
Steggerda SJ, van Wezel-Meijler G. Cranial ultrasonography of the immature cerebellum: role and limitations. Semin Fetal Neonatal Med. 2016;21:295–304.
Limperopoulos C, Benson CB, Bassan H, Disalvo DN, Kinnamon DD, Moore M, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. 2005 Sep;116(3):717–24.
Tam EWY. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr. 2011;158(2):245–50.
Steggerda SJ, De Bruine FT, van dB, Rijken M, Leijser LM, Walther FJ, et al. Small cerebellar hemorrhage in preterm infants: perinatal and postnatal factors and outcome. Cerebellum. 2013 Dec;12(6):794–801.
Meijler G, Steggerda SJ. Neonatal cranial ultrasonography. 3rd ed. ed. Cham: Springer; 2019.
Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10(3):233–60.
Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. The Cerebellum, 2014;13(1):151–177.
Stoodley CJ, Limperopoulos C. Structure-function relationships in the developing cerebellum: evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med. 2016;21:356–64.
Klein AP, Ulmer JL, Quinet SA, Mathews V, Mark LP. Nonmotor functions of the cerebellum: an introduction. Am J Neuroradiol. 2016;37(6):1005–9.
Hoftiezer L, Hukkelhoven CWPM, Hogeveen M, Straatman HMPM, van Lingen R. Defining small-for-gestational-age: prescriptive versus descriptive birthweight standards. Eur J Pediatr. 2016;175(8):1047–57.
Martin RR. Massive intracerebellar hemorrhage in low-birth-weight infants. J Pediatr. 1976;89(2):290–3.
Kidokoro H. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol. 2013;34(11):2208–14.
Bolduc M, du Plessis A, Sullivan N, Guizard N, Zhang X, Robertson R, et al. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum. 2012;11(2):531–42.
Volpe JJ. Intraventricular hemorrhage in the premature infant--current concepts. Part II. Ann Neurol. 1989;25(2):109–16.
Bayley N. Bayley scales of infant and toddler development. 3rd edition ed. San Antonio, TX: Harcourt Assessment; 2006.
Achenbach TM, Rescorla L. Manual for the child behavior checklist. Preschool forms and profiles: Burlington VT: University of Vermont Department of Psychiatry; 2000.
Griffiths R, Huntley M. The Griffiths mental development scales-revised manual: from birth to 2 years. High Wycombe: Association for Research in Infant and Child Development; 1996.
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–23.
Dekker MC. Emotional and behavioral problems in children and adolescents with and without intellectual disability. J Child Psychol Psychiatry. 2002;43(8):1087–98.
van den Broek, AJ, Kok JH, Houtzager BA, Scherjon SA. Behavioural problems at the age of eleven years in preterm-born children with or without fetal brain sparing: a prospective cohort study. Early Hum Dev. 2010;86(6):379–84.
Dyet L, Kennea N, Counsell S, Maalouf E, Ajayi Obe M, Duggan P, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–48.
Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115(3):688–95.
Kersbergen K, Benders MJNL, Groenendaal F, Koopman Esseboom C, Nievelstein RAJ, van Haastert I, et al. Different patterns of punctate white matter lesions in serially scanned preterm infants. PLoS One. 2014;9(10):e108904.
Picciolini O, Squarza C, Fontana C, Giannì M, Cortinovis I, Gangi S, et al. Neurodevelopmental outcome of extremely low birth weight infants at 24 months corrected age: a comparison between Griffiths and Bayley Scales. BMC Pediatr. 2015;15:139.
Acknowledgements
We are grateful to I.C. van Haastert, O. Picciolini, P. Schiavolin, S. E. Sforza and S. Uccella for their helpful contributions to this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boswinkel, V., Steggerda, S.J., Fumagalli, M. et al. The CHOPIn Study: a Multicenter Study on Cerebellar Hemorrhage and Outcome in Preterm Infants. Cerebellum 18, 989–998 (2019). https://doi.org/10.1007/s12311-019-01053-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-019-01053-1