Abstract
The mechanism of perverted vertical responses during horizontal head impulse tests (HITs) requires further elucidation. A 47-year-old woman with a Chiari malformation showed alternating skew deviation, downbeat nystagmus with an increasing slow phase velocity, impaired smooth pursuit, and upward ocular deviation during horizontal HITs and corrective downward saccades in the presence of normal bithermal caloric tests and intact tilt suppressions of the post-rotatory nystagmus. These findings suggest dysfunction of the inferior cerebellum including the tonsil, nodulus, and uvula. We propose that disruption of signals from the medial part of the vestibulocerebellum, which normally inhibits the lateral and anterior canal pathways, may elicit an upward misdirection of the eye velocity during rapid horizontal head rotation. Otherwise, the Chiari malformation may have directly affected the brainstem structures involved in the direction matrix of the vestibulo-ocular reflex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The head impulse test (HIT) allows evaluation of the vestibulo-ocular reflex (VOR) during high velocity stimulation [1, 2]. Peripheral vestibular hypofunction gives rise to corrective saccades that occur in the same axis and in the opposite direction of the head rotation. While head impulse responses are mostly normal in central vestibular disorders, recent studies have described decreased [3,4,5] or increased [6] gains of the VOR, and inappropriate vertical (perverted) responses during horizontal HITs in cerebellar dysfunction [4, 7, 8]. Since the cerebellar flocculus sends inhibitory projections to the floccular target neurons that take part in the anterior and horizontal semicircular canal pathways, the flocculus has been considered critical for those abnormal HITs in central lesions [3, 9]. The perverted vertical responses during horizontal HITs, however, have been described in patients with diffuse cerebellar dysfunction of infectious, degenerative, or unidentified etiologies [4, 7, 9], and therefore have not permitted a localization of the responsible neural structure(s).
Chiari type 1 malformation consists of an elongation of the cerebellar tonsils and medial parts of the inferior lobes of the cerebellum enveloping the medulla and displacement of these structures into the upper cervical vertebral canal [10]. A variety of eye movement abnormalities have been observed in Chiari malformation, including horizontal spontaneous nystagmus, vertical upbeat or downbeat nystagmus, saccadic dysmetria, impairment of smooth pursuit, and positional nystagmus [2, 10, 11]. However, only a few studies have measured the VOR in Chiari malformation. A previous study reported spared horizontal VOR during low frequency (0.5 to 2 Hz) head motion in most patients with Chiari malformation [12]. Herein, we report upward deflection and downward corrective saccades during horizontal head impulses in a patient with Chiari malformation, which provoked speculation on the responsible structures for the perverted VOR during HITs.
Material and Methods
Case Reports
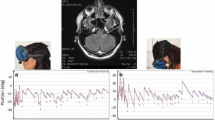
A 47-year-old woman had suffered from progressive dizziness, unsteadiness, and posterior neck pain for years. She also reported heaviness in the posterior neck and bilateral shoulders, and clumsiness of both hands. Examination showed spontaneous downbeat nystagmus (DBN) that increased during lateral gazes and with removal of visual fixation. She showed orthotropia in the straight ahead gaze, and hypertropia of the abducting eye during lateral gaze in each direction, but without abnormal heat tilt. The ocular motor ranges were full. Bedside HITs in each horizontal direction showed an inappropriate upward deviation and downward corrective saccades (Video). The patient also had dissociated sensory deficits primarily involving the pain and temperature sensation in the upper extremities, decreased biceps jerks, impaired position and vibration senses in the lower extremities, and unstable tandem gait. MRIs showed downward displacement of bilateral cerebellar tonsils and syrinx at the C4–5 level (Fig. 1), compatible with Chiari type I malformation.
Video. During horizontal head impulses, upward deflection of the eyes is followed by corrective downward saccades. EH: horizontal eye position, EV: vertical eye position, HV: horizontal head velocity (WMV 3294 kb)
Oculography
Eye movements were recorded binocularly at a sampling rate of 60 Hz using a video-oculography (SensoMotoric Instruments, Teltow, Germany). Spontaneous nystagmus was recorded both with and without fixation in the primary eye position. Eccentric gazes were induced in the horizontal (± 30°) and vertical (± 20°) planes. Head shaking nystagmus was evoked by horizontal head-shaking in a sinusoidal pattern at a rate of 2.8 Hz with an approximate amplitude of ± 10° for 15 s [13]. To induce positional nystagmus, the patients lay supine from sitting and turned their heads to either side while supine. The patient also had straight head-hanging and Dix-Hallpike maneuver in either direction [3].
Head Impulse Tests
Head and eye movements were measured during HITs using a magnetic search coil technique in a 70-cm cubic search coil frame (Skalar, Delft, The Netherlands) [14]. A scleral annulus ring was placed on the subject’s left eye after anesthetizing the conjunctiva. The patients were instructed to fixate on a red target placed 1.2 m in front of them. The head impulses were a passive, unpredictable, low-amplitude, and high-acceleration head rotation in the three planes of the both horizontal canals (HCs), right anterior canal (AC) and left posterior canal (PC), and left AC and right PC. A minimum of five impulses were applied in each direction. The gain of the VOR was calculated for each trial as the ratio of the peak velocity of the eye over the peak velocity of the head. Eye and head position signals were digitized at 200 Hz with an analog to digital converter and were displayed on a computer screen for eye motion monitoring during the tests. Digitized data were analyzed with MATLAB software (The Math-=Works Inc., Natick, MA, USA). Ten healthy subjects (seven men and three women, age 29–70 years, mean ± SD = 52 ± 14 years) served as controls [3]. We defined reduced responses of the eye velocity during HIT when the mean VOR gains were less than a mean-2SD of the control data (normal HC = 0.85 ± 0.07, normal AC = 0.86 ± 0.08, and normal PC = 0.87 ± 0.06) [14].
Other Neuro-otologic Tests
The caloric stimuli comprised alternate periods of irrigation for 30 s with 150 ml of cold (30 °C) and hot (44 °C) water. Horizontal VOR was also measured using the rotary chair system (ICS Medical, Schaumburg, IL, USA) [15]. To evaluate the VOR, the patient underwent sinusoidal oscillation about a vertical axis with a peak angular velocity of 50°/s at frequencies of 0.04 and 0.32 Hz. For the velocity step test, the participant was subjected to a series of velocity steps, first to the right and then to the left. In response to the stimuli, time constants (TCs) of the per- and post-rotatory nystagmus were calculated. To measure the tilt suppression of the post-rotatory nystagmus, forward head pitch was actively performed. The tilt suppression index (TSI) was calculated using the following formula, TSI (%) = (TC of post-rotatory nystagmus − TC of post-rotatory nystagmus after tilt suppression) / TC of post-rotatory nystagmus × 100. Normative data were obtained from 50 healthy volunteers whose ages ranged from 24 to 70 years (43.0 ± 15.4%) [15].
The subjective visual vertical (SVV) tilt was measured by seating the patient upright in a dark room and asking her to align a rod (10.0 cm long and 0.5 cm wide) vertically. The SVV tilt was considered abnormal when it exceeded normal values (− 2.4°~2.6° in both eyes; a negative value indicates a counterclockwise rotation from the patient’s perspective) [16]. Cervical and ocular vestibular-evoked myogenic potentials (VEMPs) were also performed. Detailed methods have been described in the literatures [3, 5, 17].
All experiments followed the tenets of the Declaration of Helsinki, and this study was approved by Institutional Review Board of Seoul National University Bundang Hospital.
Results
Nystagmus
Video-oculography documented spontaneous DBN with an increasing slow phase velocity (SPV) during visual fixation (3.7 ± 0.4°/s) (Fig. 2a). The DBN increased without a visual fixation in darkness (5.4 ± 0.8°/s), during convergence (7.9 ± 1.2°/s), during rightward (8.9 ± 1.0°/s), leftward (5.4 ± 0.4°/s) and downward (4.0 ± 1.0°/s) gazes, and in the straight head-hanging position (17.9 ± 6.5°/s) (Fig. 2b–d). Right beating spontaneous nystagmus was also noted in darkness without a visual fixation (0.8 ± 0.3°/s). Horizontal head shaking did not affect the spontaneous DBN.
The patient shows spontaneous downbeat nystagmus with an increasing slow phase velocity during visual fixation (3.7 ± 0.4°/s), which increases in darkness (5.4 ± 0.8°/s) (a). The downbeat nystagmus also increases during rightward (8.9 ± 1.0°/s) and leftward (5.4 ± 0.4°/s) gazes (b), and in the straight head-hanging position (17.9 ± 6.5°/s) (c). LH horizontal position of the left eye, LV vertical position of the left eye
The Vestibulo-ocular Reflex
Recording of HITs showed a delayed onset of the VOR for both HCs and catch-up saccades for left HC even though the velocity gains were within normal range for both HCs (Fig. 3). In contrast, the head impulse VOR gains for both ACs were increased with premature deceleration. The head impulse VOR appeared delayed for both PCs and decreased for left PC. Thus, the upward VOR gains were greater than the downward ones on both sides (ratio AC/PC gain = 1.67 on the right side and 2.05 on the left side).
Recording of the head impulse tests documents increased vestibulo-ocular reflex (VOR) gains and premature decelerations for both anterior canals (ACs) (right AC gain = 1.36, left AC gain = 1.38, normal AC = 0.86 ± 0.08, mean ± SD), decreased gain for left posterior canal (PC) (0.67, normal PC = 0.87 ± 0.06), and normal gains for right posterior and both horizontal canals (HCs, normal HC = 0.85 ± 0.07). Initiation of the VOR is delayed for both HCs and PCs, but was normal for both ACs. Normal range of VOR gain for each canal is determined by mean ± 2SD (HC = 0.71 to 0.99, AC = 0.70 to 1.02, PC = 0.75 to 0.99)
Sinusoidal harmonic accelerations showed normal gain and phase of the horizontal VOR without an asymmetry. During step velocity rotation, the average TCs of the per- and post-rotatory nystagmus were increased (TC of right beating nystagmus = 22.4 and 21.7 s and TC of left beating nystagmus = 18.7 and 20.4 s, normal TCs = 14.6 ± 3.6 s) with a normal tilt suppression of the post-rotatory nystagmus (TSI of right beating post-rotatory nystagmus = 53.7% and TSI of left beating post-rotatory nystagmus = 46.5%). Bithermal caloric tests were normal.
Other Vestibular Function Tests
The SVV was tilted counterclockwise (− 3.6°) from the patient’s perspective during binocular viewing (Fig. 4a). Cervical VEMPs elicited by sound stimuli and ocular VEMPs in response to vibratory stimuli were normal.
a The subjective visual vertical is deviated counterclockwise (− 3.5°) from the patient’s perspective during binocular viewing (normal = − 2.4°~2.6°). b Horizontal saccades are hypometric, more to the left, but without an abnormal upward eye deviation during the saccades. d At a peak target velocity of 10°/s, horizontal smooth pursuit is impaired in each direction (rightward gain = 0.56, leftward gain = 0.57, age-matched normal = 0.73 ± 0.08). e The downward smooth pursuit is impaired (downward gain = 0.36, age-matched normal = 0.50 ± 0.11), while the upward smooth pursuit is normal (upward gain = 0.88). LH horizontal position of the left eye, LV vertical position of the left eye
Saccades and Smooth Pursuit
Horizontal saccades were hypometric, more to the left, but without a directional bias in the vertical plane (Fig. 4b). Vertical saccades were normal. At a peak target velocity of 10°/s, horizontal smooth pursuit was impaired in both directions (rightward gain = 0.56, leftward gain = 0.57, age-matched normal = 0.73 ± 0.08) (Fig. 4c). The downward smooth pursuit was also impaired (downward gain = 0.36, age-matched normal = 0.50 ± 0.11), while the upward smooth pursuit was normal (upward gain = 0.88) (Fig. 4d).
Discussion
Our patient with a Chiari malformation showed upward eye deviation and corrective downward saccades during horizontal head impulses in addition to spontaneous and positional downbeat nystagmus with an increasing slow phase velocity, alternating skew deviation during lateral gazes, asymmetric head impulse VOR gains of the vertical semicircular canals (AC > PC), and asymmetric vertical smooth pursuit.
The upward deflection of the eyes was previously observed during yaw head impulses in patients with diffuse cerebellar degeneration [4, 7, 9]. This cross-coupled VOR during rotation of the head around an earth-vertical axis has been attributed to disinhibition of the AC pathway due to floccular dysfunction [7, 9]. Given that an isolated unilateral floccular lesion produced increased horizontal VOR gain during low speed stimulations and decreased gain during horizontal head impulses [3], the flocculus appears to serve for calibrating and facilitating the horizontal VOR during the HITs. In our patient, the preserved VOR gains for both HCs indicate mostly spared floccular function. Thus, the cross-coupled head impulse responses in our patient may be ascribed to other neural structure(s) affected by Chiari malformation.
Partial resection of the nodulus and uvula in monkeys abolishes the ability to orient the eye velocity vector to the shifted gravito-inertial acceleration vector [18]. Based on loss of the cross-axis VOR adaptation in experimental lesions involving the nodulus and uvula, there has been a speculation on the presence of collateral projections connecting the horizontal and vertica1 canal-ocular reflex pathways [9]. Given this hypothetical projection, the inferior cerebellar vermis may suppress the AC pathways via the inhibitory projections. When the inhibitory action of the inferior vestibulocerebellum is impaired in Chiari malformation, inappropriate upward eye velocity vector can be elicited during horizontal head impulses due to disinhibition of the collateral projections and resultant activation of the AC pathway. Of note, decompressive surgery with unilateral tonsillectomy produced new smooth pursuit deficits in four of ten patients with Chiari malformation and DBN in one of them, but without a change in head impulse responses [19]. Taken together with normal HITs in patients with an isolated hemi-tonsilar infarction [20], unilateral tonsilar dysfunction appears not enough to generate the cross-coupled responses during horizontal head impulses. Thus, factors such as bilaterality of the lesions or combined dysfunction of the nodulus-uvula complex seem to be required to produce vertical eye movements during horizontal head impulses in Chiari malformation.
The matrix of the VOR direction may be a function of the vestibular structures and their connecting fibers in the brainstem even though the cerebellum would also modulate this function. In our patient, the Chiari malformation may have directly affected the brainstem structures involved in the direction matrix of the VOR and may have distorted the directionality of the VOR. Thus, the cross-coupled horizontal VOR observed in our patient may also be ascribed to an alteration in the matrix of the VOR direction due to a direct effect of Chiari malformation onto the brainstem. Given the no cross-coupled responses during horizontal saccades, this alteration appears to have been specific for the VOR.
While the flocculus modulates the VOR through its direct connections with the brainstem vestibular and olivary nuclei, the cerebellar tonsil mostly participates in the control of pursuit eye movements [20]. Isolated unilateral tonsilar infarction in humans indeed impaired smooth pursuit in both horizontal and vertical directions [20]. Our patient with cerebellar tonsilar displacement also showed impaired smooth pursuit in the horizontal and downward directions.
Of note, the DBN in our patient had an accelerating slow waveform. Nystagmus with an increasing SPV has been occasionally found in acquired cerebellar disorders [21], including partial destruction of the uvula [22], and following a lumbar puncture in Chiari malformation [23]. A prior study of paraneoplastic cerebellar degeneration ascribed spontaneous DBN with an increasing SPV to inappropriate accentuation of neural integration that converts the eye velocity commands into position commands [21]. Since the brainstem neural integration system is inherently leaky, the cerebellum has been considered to improve the integration via a feedback loop [21]. In our patient with a Chiari malformation, the DBN of an accelerating slow waveform may also be attributed to unstable brainstem integration network owing to cerebellar dysfunction.
The spontaneous upward ocular drift and downbeat nystagmus in our patient was affected by head position changes with respect to gravity as well as by the eye-in-orbit position. The SPV of DBN increased by only about 0.3°/s during eccentric downward gaze in the head upright position, but increased by up to 12.5°/s during straight head hanging in darkness. Central positional DBN is mostly observed with lesions involving the nodulus and uvula [24]. Lesions involving these structures also cause alternating skew deviation and a tilt of the SVV [22], as shown in our patient. The ocular tilt reaction is also under the inhibitory control of the caudal cerebellum, possibly the nodulus [25]. The skew deviation and tilts of the SVV found in the nodular lesion appears to be due to an interruption of the inhibitory projections from the damaged nodulus to the graviceptive neurons in the ipsilateral vestibular nucleus [25]. Diffuse and symmetric cerebellar dysfunction results in increased variance rather than tilt of the SVV [26]. Thus, the counterclockwise tilt without an increase in the variance of the SVV observed in our patient indicates a partial and asymmetric damage of the nodular inhibitory projection. In addition to the ocular tilt reaction, the long-term alteration of this neural pathway induced by downward herniation of the caudal cerebellum and secondary compression of the brainstem vestibular structure(s) may have produced the perverted HITs found in our patient. Given the preserved tilt suppression of the VOR [27], however, impairment of the nodulus and ventral uvula, if any, should have been partial. A previous study on tilt suppression of post-rotatory nystagmus reported that patients with Chiari malformation show a behavior that was intermediate between that of normal subjects and the patients with midline cerebellar lesions [28].
In conclusion, the oculomotor findings observed in the present patient suggest impairments of the inferior cerebellar structures including the tonsil, nodulus, and uvula. We propose that disruption of signals from the medial part of the inferior cerebellum that inhibits the lateral and vertical canal pathways may have elicited an upward misdirection of eye velocity during rapid horizontal rotations. Otherwise, the Chiari malformation may have directly affected the brainstem structures involved in the direction matrix of the VOR and may have distorted the directionality of the VOR.
References
Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–9.
Kumar A, Patni AH, Charbel F. The Chiari I malformation and the neurotologist. Otol Neurotol. 2002;23:727–35.
Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. 2013;260:1576–82.
Jeong SH, Kim JS, Baek IC, Shin JW, Jo H, Lee AY, et al. Perverted head impulse test in cerebellar ataxia. Cerebellum. 2013;12:773–5.
Kim SH, Zee DS, du Lac S, Kim HJ, Kim JS. Nucleus prepositus hypoglossi lesions produce a unique ocular motor syndrome. Neurology. 2016;87:2026–33.
Choi JY, Kim JS, Jung JM, Kwon DY, Park MH, Kim C, et al. Reversed corrective saccades during head impulse test in acute cerebellar dysfunction. Cerebellum. 2014;13:243–7.
Walker MF, Zee DS. Cerebellar disease alters the axis of the high-acceleration vestibuloocular reflex. J Neurophysiol. 2005;94:3417–29.
Choi JY, Kim HJ, Kim JS. Recent advances in head impulse test findings in central vestibular disorders. Neurology. 2018;90:602–12.
Walker MF, Zee DS. Directional abnormalities of vestibular and optokinetic responses in cerebellar disease. Ann N Y Acad Sci. 1999;871:205–20.
Weber PC, Cass SP. Neurotologic manifestations of Chiari 1 malformation. Otolaryngol Head Neck Surg. 1993;109:853–60.
Guerra Jiménez G, Mazón Gutiérrez Á, Marco de Lucas E, Valle San Román N, Martín Laez R, Morales Angulo C. Audio-vestibular signs and symptoms in Chiari malformation type I. case series and literature review. Acta Otorrinolaringol Esp. 2015;66:28–35.
Salman MS, Sharpe JA, Lillakas L, Dennis M, Steinbach MJ. The vestibulo-ocular reflex during active head motion in Chiari II malformation. Can J Neurol Sci. 2008;35:495–500.
Choi KD, Oh SY, Park SH, Kim JH, Koo JW, Kim JS. Head-shaking nystagmus in lateral medullary infarction: patterns and possible mechanisms. Neurology. 2007;68:1337–44.
Choi KD, Oh SY, Kim HJ, Kim JS. The vestibulo-ocular reflexes during head impulse in Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 2007;78:1161–2.
Choi KD, Kim JS. Head-shaking nystagmus in central vestibulopathies. Ann N Y Acad Sci. 2009;1164:338–43.
Kim HJ, Lee SH, Park JH, Choi JY, Kim JS. Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol. 2014;261:121–9.
Choi SY, Lee SH, Kim HJ, Kim JS. Impaired modulation of the otolithic function in acute unilateral cerebellar infarction. Cerebellum. 2014;13:362–71.
Wearne S, Raphan T, Cohen B. Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol. 1998;79:2690–715.
Goldschagg N, Feil K, Ihl F, Krafczyk S, Kunz M, Tonn JC, et al. Decompression in Chiari malformation: clinical, ocular motor, cerebellar, and vestibular outcome. Front Neurol. 2017;8:292. https://doi.org/10.3389/fneur.2017.00292.
Lee SH, Park SH, Kim JS, Kim HJ, Yunusov F, Zee DS. Isolated unilateral infarction of the cerebellar tonsil: ocular motor findings. Ann Neurol. 2014;75:429–34.
Zee DS, Leigh RJ, Mathieu-Millaire F. Cerebellar control of ocular gaze stability. Ann Neurol. 1980;7:37–40.
Radtke A, Bronstein AM, Gresty MA, Faldon M, Taylor W, Stevens JM, et al. Paroxysmal alternating skew deviation and nystagmus after partial destruction of the uvula. J Neurol Neurosurg Psychiatry. 2001;70:790–3.
Barton JJ, Sharpe JA. Oscillopsia and horizontal nystagmus with accelerating slow phases following lumbar puncture in the Arnold-Chiari malformation. Ann Neurol. 1993;33:418–21.
Choi JY, Kim JH, Kim HJ, Glasauer S, Kim JS. Central paroxysmal positional nystagmus: characteristics and possible mechanisms. Neurology. 2015;84:2238–46.
Mossman S, Halmagyi GM. Partial ocular tilt reaction due to unilateral cerebellar lesion. Neurology. 1997;49:491–3.
Tarnutzer AA, Shaikh AG, Palla A, Straumann D, Marti S. Vestibulo-cerebellar disease impairs the central representation of self-orientation. Front Neurol. 2011;2:11. https://doi.org/10.3389/fneur.2011.00011.
Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985;228:199–202.
Hain TC, Zee DS, Maria BL. Tilt suppression of vestibulo-ocular reflex in patients with cerebellar lesions. Acta Otolaryngol. 1988;105:13–20.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2016R1D1A1B04935568).
Author information
Authors and Affiliations
Contributions
S.-H. K., as the first author, designed the study, interpreted and analyzed the data, and drafted the manuscript. H. J. K. acquired the data and made revisions of the manuscript and figures. J.-S. K., as the corresponding author, designed the study, interpreted the data, and made critical revisions of the manuscript.
Corresponding author
Ethics declarations
Disclosure
S.-H. K and H-J. K report no disclosure.
J.-S. K. serves as an associate editor of Frontiers in Neuro-otology and on the Editorial Boards of the Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, Journal of Vestibular Research, and Journal of Neurology and Medicine.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ᅟ
Rights and permissions
About this article
Cite this article
Kim, SH., Kim, HJ. & Kim, JS. Perverted Downward Corrective Saccades During Horizontal Head Impulses in Chiari Malformation. Cerebellum 18, 333–339 (2019). https://doi.org/10.1007/s12311-018-1000-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-018-1000-z