Abstract
The cerebellum is thought to adapt movements to changes in the environment in order to update an implicit understanding of the association between our motor commands and their sensory consequences. This trial-by-trial motor recalibration in response to external perturbations is frequently impaired in people with cerebellar damage. In healthy people, adaptation to motor perturbations is also known to induce a form of sensory perceptual recalibration. For instance, hand-reaching adaptation tasks produce transient changes in the sense of hand position, and walking adaptation tasks can lead to changes in perceived leg speed. Though such motor adaptation tasks are heavily dependent on the cerebellum, it is not yet understood how the cerebellum is associated with these accompanying sensory recalibration processes. Here we asked if the cerebellum is required for the recalibration of leg-speed perception that normally occurs alongside locomotor adaptation, as well as how ataxia severity is related to sensorimotor recalibration deficits in patients with cerebellar damage. Cerebellar patients performed a speed-matching task to assess perception of leg speed before and after walking on a split-belt treadmill, which has two belts driving each leg at a different speed. Healthy participants update their perception of leg speed following split-belt walking such that the “fast” leg during adaptation feels slower afterwards, whereas cerebellar patients have significant deficits in this sensory perceptual recalibration. Furthermore, our analysis demonstrates that ataxia severity is a crucial factor for both the sensory and motor adaptation impairments that affect patients with cerebellar damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The connection between our movements and their sensory consequences is essential to our ability to adapt to changes in the environment. Because it takes time to process sensory feedback, we cannot rely on feedback alone to adjust our movements. Instead, the nervous system depends on an implicit understanding of how our bodies move in order to make predictions of the outcomes of motor commands and update these predictions on a trial-by-trial basis [1,2,3]. Previous work has shown that the association between our outgoing motor commands and their predicted sensory consequences is strongly linked to the cerebellum [4] and that disruption or damage to the cerebellum can lead to impaired updating of motor commands when sensorimotor prediction is necessary [5,6,7,8,9]. For instance, Lang and Bastian [5] used a simple weight-catching task to probe the ability of people with cerebellar damage to update their motor commands when catching a series of balls of different weights. While healthy control participants were able to rapidly adjust their movements based on an anticipation of the momentum of each ball, cerebellar patients had diminished anticipatory muscle activity and an inability to maintain their hand position; reflecting a deficit in the ability to predict the motor commands necessary to produce the desired sensory outcome. This inability of people with cerebellar damage to recalibrate their motor commands when the actual and predicted sensory consequences do not align has been shown across many types of movements [10], including eye movements [11], arm movements [12], and walking [13].
More recently, it has been shown that in addition to recalibration of motor commands, adaptation to motor perturbations also leads to a form of sensory recalibration which may include changes in felt hand position during reaching adaptation [14,15,16,17,18] or the perception of leg speed during walking adaptation [19, 20]. The cerebellum is known to be an important part of the brain circuit involved in motor adaptation; however, its influence on the associated sensory perceptual recalibration remains less clear. Relatively few studies have examined this type of sensory recalibration in patients with cerebellar damage, with mixed results: some have found that cerebellar patients have diminished ability to update their estimation of hand position following reaching adaptation tasks [21, 22], whereas others have shown no deficits in cerebellar patients compared with controls [23]. Importantly, these previous studies have utilized small [21, 22] or homogeneous [23] patient cohorts in terms of disease severity. For instance, Henriques et al. [23] specifically recruited patients with relatively moderate ataxia to avoid the possibility of having to adjust the experimental paradigm for more severely affected patients. However, it has been suggested that motor adaptation impairments are related to the severity of ataxia [13, 24]. This is likely the case for sensory perceptual recalibration as well. We are not aware of any studies that systematically investigated the influence of ataxia severity on motor or sensory recalibration in a large, diverse group of cerebellar patients, and doing so is an important next step to understanding the role of the cerebellum in sensorimotor adaptation.
Additionally, previous patient studies have only examined sensory perceptual recalibration in the context of hand-reaching, for instance, using visuomotor rotation tasks. Though hand-reaching paradigms are common in motor adaptation studies, they may not be the most effective method to study the associated sensory perceptual recalibration. Reaching adaptation tasks often rely on an interaction between visual and proprioceptive feedback. However, it is known that a visuo-proprioceptive mismatch alone is enough to elicit a sensory realignment even in the absence of movement errors [25], and this type of sensory realignment is not thought to be cerebellum-dependent [26]. In the context of reaching, it is thus difficult to dissociate the perceptual changes related to motor adaptation from those simply due to a mismatch between visual and proprioceptive feedback. Unlike reaching tasks that may require visual monitoring of the hand’s location relative to a target, walking is more reliant on proprioception alone, not typically depending on visual feedback of body kinematics. Walking tasks, as opposed to reaching, may thus be more effective for studying the influence of the cerebellum on the sensory perceptual recalibration associated with motor adaptation.
Here we sought to determine the role of the cerebellum in the sensorimotor recalibration of walking. First, we asked whether the cerebellum is required for sensory perceptual recalibration during locomotor adaptation. We studied patients with cerebellar damage using a split-belt treadmill paradigm, where the legs are independently driven to move at different speeds during walking. Alongside motor adaptation of step lengths, split-belt walking is known to induce a recalibration of leg-speed perception in healthy people such that the “fast” leg during adaptation feels slower afterwards, and vice versa [19, 20]. Given that patients with cerebellar damage are unable to fully adapt their motor patterns from split-belt walking [13], we hypothesized that alongside such motor adaptation deficits, cerebellar patients would also exhibit impaired recalibration of leg-speed perception. Next, we revisited data from previous cerebellar patient work [13, 27] to investigate the role of ataxia severity in motor and sensory perceptual recalibration deficits. We hypothesized that patients with severe ataxia would more likely exhibit sensory and/or motor recalibration impairments compared to those with milder ataxic symptoms.
Methods
Eight adults with cerebellar damage (cerebellar group; mean age 63 years old, range 32–69) and eight age-matched controls (control group; mean age 60 years old, range 32–72) participated in the first part of this study. Severity of ataxia was quantified by the International Cooperative Ataxia Rating Scale (ICARS) [28]. All cerebellar patients underwent a neurological examination to ensure that they did not have any extracerebellar signs such as spasticity, hyper-reflexia, rigidity, sensory loss, or weakness. When possible, we reviewed the MRI scans and genetic test results for each patient to confirm diagnosis. Control subjects were screened to ensure they had no previous diagnosis of any neurological or orthopedic condition. The characteristics of these participants are listed in Table 1.
In the second part of this study, data from 19 additional adults with cerebellar damage and nine healthy controls collected in a previous study [13] were re-analyzed and added to the data from part one to examine the specific sensorimotor and spatiotemporal deficits associated with cerebellar damage in a larger cohort of participants. This was done to determine the influence of ataxia severity on deficits in motor and sensory parameters, as well as further investigate the relationship between sensory and motor impairments following cerebellar damage. Here again, MRI and genetic test results were reviewed when possible, and all patients underwent a neurological examination to ensure that they did not have extracerebellar signs. The characteristics of these additional participants are listed in Table 2.
Split-Belt Treadmill
Participants walked on a custom split-belt treadmill (Woodway USA, Waukesha, WI) capable of moving each leg at different speeds. Speed commands for each belt were sent to the treadmill through either a custom MATLAB (The MathWorks, Natick, MA) program (during tied-belt or split-belt walking) or a custom Python program in the Vizard (WorldViz, CA, USA) development environment (during leg-speed perception assessments). Subjects were positioned with one leg on each treadmill belt, wore a safety harness, and held onto a handrail in front of the treadmill for all phases of the experiments.
Optotrak Motion Analysis
Kinematic data were collected during walking at 100 Hz using Optotrak (Northern Digital, Waterloo, ON, Canada). Infrared-emitting markers were placed bilaterally on the fifth metatarsal head (toe), lateral malleolus (ankle), lateral femoral epicondyle (knee), greater trochanter (hip), iliac crest (pelvis), and acromion process (shoulder). Limb angle was defined as the angle between a vertical line and the vector from the hip to the toe on an x-y plane. Limb angle is positive during flexion when the foot is in front of the hip and negative when the foot is behind the hip. Heel strike times were approximated using the maximum (positive) angle of the limb, and toe-off was approximated as the minimum (negative) limb angle [29].
Experimental Paradigm
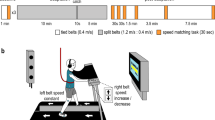
The general experimental paradigm for the first part of the study is shown in Fig. 1a. All subjects began with three baseline periods (1 min each) of tied-belt walking during which both treadmill belts moved 0.4 m/s. Subjects then walked for a 15 min adaptation period with split-belts, where the left belt moved at 0.4 m/s and the right belt moved at 0.8 m/s. After the first 10 min of adaptation, subjects experienced a 10 s “catch” trial where the belts were briefly set to tied-belt conditions (both belts 0.4 m/s). This catch trial gave us a glimpse of the amount of motor adaptation that had occurred up to that point. After the catch trial, subjects resumed split-belt walking for the remaining 5 min of adaptation. During post-adaptation, subjects walked with tied belts (both belts 0.4 m/s) for a total of 13.5 min. After each baseline period and at several time points during post-adaptation (0, 0.5, 1, 2.5, 6, 13.5 min), subjects performed a speed-matching task (described below) that assessed their perception of leg speed during walking.
Experimental paradigm and setup for part one of the experiment. a Experimental paradigm. White blocks indicate tied-belt walking (both legs 0.4 m/s), light gray blocks indicate split-belt walking (0.4:0.8 m/s), and dark gray blocks indicate the leg-speed perception task (see “Methods” for task details). Participants performed three baseline walking and leg-speed perception tasks, followed by 15 min of split-belt adaptation. Two-thirds of the way through the adaptation block, there was a 10 s “catch” trial where belts returned to tied-belt conditions. After adaptation, participants performed six leg-speed perception tasks separated by five tied-belt walking blocks of increasing length. b Leg-speed perception task setup. Participants were asked to walk on the treadmill and use a keypad to actively control the speed of the right leg to match that of the left leg, which was fixed at 0.4 m/s. Optotrak marker locations are indicated by blue circles. Participants wore headphones that played white noise to cancel auditory treadmill cues and a cloth drape to eliminate visual cues of the feet
The experimental protocol for the previously collected cerebellar patients and controls (part two) was slightly different. The slow and fast speeds were set at 0.5 and 1.0 m/s, respectively. Subjects first walked at baseline at the slow speed, then the fast speed, then again at the slow speed, before 10 min of adaptation with split belts (0.5:1.0 m/s). After adaptation, belts were tied at the slow speed for 4–5 min. Three patients were unable to sustain the fast tied-belt walking speed (1.0 m/s) and were tested at 0.4:0.8 m/s instead. After the first three or four strides of each period, subjects were asked whether they felt the two belts were moving at the same speed or two different speeds. If they felt the belts were moving at different speeds, they were asked to indicate which belt was moving faster. For additional details, see Morton and Bastian [13].
Leg-Speed Perception Task
To investigate changes in leg-speed perception due to split-belt walking, we designed a leg-speed perception task using the psychophysical method of adjustment (see Vazquez et al. [20]). A custom Python program was used to simultaneously control the treadmill, provide feedback of task timing, and collect input from subjects to update treadmill belt speeds during walking. Subjects were positioned on the treadmill and instructed to place their left hand on a handrail in front of the treadmill and their right hand on a small keypad (Fig. 1b). Vision of the legs was obstructed via an opaque drape, and auditory cues of speed from the treadmill motors were canceled via headphones playing white noise. Initially, the left leg was driven to walk at a constant speed of 0.4 m/s, while the right leg was not moving. Subjects were instructed to press up or down arrows on the keypad in front of them to adjust the speed of the right leg until they perceived it to match the speed of the left leg. Left leg speed was always 0.4 m/s to avoid introducing a declarative memory component to the task of remembering the speed. Subjects were given 45 s to complete the task and were provided feedback on the amount of time remaining via a television monitor in front of the treadmill. When the right leg was within the range 0.0–0.35 m/s, key presses resulted in speed increments of either 0.05, 0.055, or 0.065 m/s. These increments were varied with each iteration of the task so subjects were unable to simply count the number of key presses needed to reach the target speed. Once the speed passed 0.35 m/s, key presses resulted in a smaller change in speed, 0.005 m/s, to allow for fine control of speed as the right leg approached the target speed.
Data Analysis
Leg-Speed Perception Task
We recorded changes in right belt speed as the perception task was performed and defined the perception bias as the difference between the left and right belt speeds at the end of each 45-s trial. A value of zero indicates that the subject adjusted the speed of the right belt to perfectly match the left belt (i.e., accurate perception), whereas positive values indicate that the subject overshot the left belt speed (i.e., perceived the right belt to be moving slower than it was).
Motor Adaptation
Our primary outcome measure of motor behavior was step length difference, which has previously been shown to adapt robustly during split-belt treadmill walking [30]. Step length was calculated as the anteroposterior distance between the malleolus markers of each leg at heel strike. Slow step length (SL s ) refers to the step length measured at heel strike of the slow leg; fast step length (SL f ) refers to the step length measured at heel strike of the fast leg. Step length difference was defined as the fast minus the slow step length:
In the second part of the study, we additionally considered the spatial and temporal contributions to step length difference adaptation. In particular, it has previously been shown that step length can be altered by adapting spatial or temporal components of coordination, or a combination of both [31]. A new method was recently developed to quantitatively determine the specific contributions of spatial and temporal components of the overall step length difference adaptation [32]. That is,
With this model, the spatial contribution is due to differences in foot placement relative to the body, the temporal contribution is due to differences in step times, and the perturbation term is largely due to the treadmill belt speeds [32, 33] (see Finley et al. [32] for full derivations of spatial, temporal, and perturbation terms). Step length difference adaptation is thus an additive combination of spatial and temporal components to overcome the perturbation imposed by the treadmill.
Statistics
Ataxia Severity
In the second part of the study, the entire cohort of patients with cerebellar damage (n = 27) was divided into three groups based on degree of ataxia (i.e., total ICARS score, mild n = 9, moderate n = 10, severe n = 8; see “Results” for scores). Participants were divided with the goal of creating three groups of equal size. The group sizes are slightly unbalanced due to two subjects with the same ICARS score at the moderate/severe level who were both placed in the moderate group. ICARS scores between mild, moderate, and severe groups were compared using a one-way analysis of variance (ANOVA).
Leg-Speed Perception
To determine whether people with cerebellar damage have baseline changes in leg-speed perception, we averaged each subject’s three baseline perception bias values and compared these mean baseline values between cerebellar and control groups using an independent samples t-test. Each subject’s post-adaptation perception data was evaluated relative to his/her own baseline and is referred to as ∆Perception bias. Post-adaptation ∆Perception bias results were analyzed using repeated-measures ANOVA to assess differences between cerebellar and control groups across time. Subjects performed the leg-speed perception task six times during post-adaptation at set time points. However, some patients with cerebellar damage had difficulty performing the perception task at the final post-adaptation time point due to fatigue, so the first five time points are included in the ANOVA.
We were also interested in getting a broader picture of leg-speed perception changes in cerebellar patients who had wide-ranging ataxia severities. Thus, we combined the leg-speed perception data collected in the current experiment (where perceptual changes were quantified via the leg-speed perception task) with the previously collected data (where participants were asked whether they felt the belts were moving at the same or different speeds during post-adaptation). To combine these two datasets, we binarized the data from the current experiment to determine whether each participant experienced a significant sensory perceptual recalibration. Specifically, it was determined whether each subject’s initial post-adaptation perception bias was outside a 95% confidence interval calculated from his/her own baseline. If the post-adaptation result was outside this confidence interval, we considered that subject to have had a significant sensory perceptual recalibration. Analyzing the data in this manner allowed us to evaluate changes in perception for our entire cohort of participants, as the binarized data from part one of the experiment could be added to the data included in part two. We then used Fisher’s exact test to compare the ratio of patients experiencing a sensory perceptual recalibration in mild, moderate, and severe groups to the control group.
Motor Adaptation
In the first part of the study, step length difference for each participant was averaged during early adaptation (first 5 strides) and at the adaptation plateau (last 30 strides). Repeated-measures ANOVA was performed to evaluate changes in step length difference between cerebellar and control groups across these two time points. Adaptation was additionally quantified as the magnitude of the catch trial (i.e., mean step length difference during each participant’s catch trial). Catch trial magnitudes were compared between groups using an independent samples t-test.
In the second part of the study where we applied the model of Finley et al. [32], we refer to spatial, temporal, and step length difference terms as a percentage of the perturbation. For instance, a given subject may experience a perturbation of 200 mm and adapt with a 100-mm spatial contribution (50% of the perturbation) and an 80-mm temporal contribution (40% of the perturbation). This results in a total step length difference adaptation of 90% of the perturbation. By determining the percentage of the treadmill perturbation that was “corrected” for by each motor parameter, we could fairly compare motor behavior across subjects who were tested with slightly different paradigms (i.e., 0.4:0.8 m/s belt speeds or 0.5:1.0 m/s belt speeds). The percent corrected at the adaptation plateau for spatial, temporal, and step length difference terms were compared between control, mild, moderate, and severe groups using one-way ANOVAs.
Motor/Sensory Association
To determine whether the degree of motor adaptation was predictive of sensory perceptual recalibration deficits in cerebellar patients, we performed point-biserial correlations for the binarized sensory perceptual recalibration data (i.e., evaluating whether each patient recalibrated leg-speed perception) and the percent corrected for each motor parameter (spatial, temporal, and step length difference terms).
For all tests, post hoc analysis was performed using the Bonferroni correction when applicable. All data is reported as mean ± standard error unless otherwise specified.
Results
Experiment 1
Leg-Speed Perception
We found that cerebellar patients (n = 8) and control subjects (n = 8) had similar, accurate perception of leg speed at baseline as evidenced by a perception bias that was −0.008 ± 0.016 and −0.032 ± 0.016 m/s, respectively; t(14) = 1.068, p = .304. Thus, cerebellar damage alone does not appear to affect perception of leg speed during baseline (normal) walking. However, repeated-measures ANOVA demonstrated a significant effect of time (F (4,56) = 3.381, p = .015) and a time × group interaction (F (4,56) = 3.534, p = .012) for ∆Perception Bias (Fig. 2a). Post hoc analyses showed that control subjects had a significantly greater ∆Perception Bias immediately following adaptation compared to cerebellar patients (0.081 ± 0.020 vs. 0.000 ± 0.014 m/s, p = .005) that subsequently decayed back toward baseline values. In other words, cerebellar patients demonstrated significantly diminished recalibration of leg-speed perception after split-belt walking.
Leg-speed perception and motor adaptation results for part one of the experiment. a Post-adaptation leg-speed perception task performance. ∆Perception bias represents the change in perception bias compared to baseline. Control group (blue) demonstrated a significant recalibration of perception bias, whereas cerebellar group (red) did not. Five post-adaptation time points are shown, as the final post-adaptation leg-speed perception task was excluded due to fatigue in some cerebellar patients. b Step length difference during adaptation block. Both groups were initially perturbed by the treadmill, then adapted step length difference over time. Cerebellar patients tended to adapt less than the control participants; however, this difference was not statistically significant
Motor Adaptation
Step length difference during adaptation for cerebellar and controls groups is shown in Fig. 2b. All subjects could complete the walking task without difficulty. The amount of adaptation was quantified two ways. First, we analyzed changes in step length difference between early adaptation and the adaptation plateau. Repeated-measures ANOVA demonstrated a significant effect of time (F (1,14) = 27.130, p < .001) but no effect of group (p = .110) or time × group interaction (p = .261). In other words, both groups were able to adjust step length difference over the course of the adaptation period, and there were no significant differences in how the cerebellar and control groups adapted their walking patterns. Next, we compared catch trial magnitudes between the groups, with similar results. Catch trials were somewhat smaller in the cerebellar group compared to control (21.68 ± 27.08 vs. 71.56 ± 15.85 mm); however, this difference was not statistically significant; t(14) = −1.590, p = .134.
The results of Experiment 1 primarily demonstrate that cerebellar damage leads to impairment in the recalibration of leg-speed perception that normally occurs alongside locomotor adaptation. It was expected that patients would also have significantly decreased motor adaptation, as has been shown previously [13]. Though the group of patients tested here appeared to show slightly less adaptation than controls, this result was not significant and did not match the substantial impairment in locomotor adaptation previously seen in cerebellar patients.
Experiment 2
The discrepancy between the magnitudes of locomotor adaptation deficits seen in the present study compared to what was shown previously motivated us to assess a larger, more heterogenous cohort of participants to gain a broader understanding of how sensorimotor adaptation is affected with cerebellar damage. We therefore re-analyzed a group of cerebellar patients and control subjects used in previous work [13, 27] and added it to the data from the first part of this experiment to create a larger set of cerebellar (n = 27) and control (n = 17) participant data. This was done to answer two main questions: first, how does disease severity impact the ability of cerebellar patients to adapt to locomotor perturbations, and second, can a more detailed analysis of specific spatial and temporal characteristics of walking help us understand variability amongst individuals with ataxia?
Ataxia Severity
To answer these questions, we divided the overall cohort of cerebellar patients into groups based on ataxia severity (i.e., ICARS score). We first confirmed that mild, moderate, and severe groups had significantly different levels of ataxia (Fig. 3a) (F (2,26) = 64.684, p < .001; mild 13 ± 2, moderate 28 ± 2, severe 44 ± 2; all between-group post hoc tests p < .001). ICARS score ranges for each group were as follows: mild (3–19), moderate (22–35), severe (40–56).
Group results for part two of the experiment. a Comparison of ICARS score between mild, moderate, and severe cerebellar groups. All groups were significantly different from each other. b Comparison of motor adaptation parameters (step length difference, spatial contribution, temporal contribution) between groups. Percent corrected represents the percentage of the perturbation that was compensated for by each motor parameter. The mild group had no motor adaptation deficits compared with the control group, the moderate group had significantly reduced adaptation of the temporal component, and the severe group had deficits in all motor adaptation parameters. c Comparison of the ratio of participants in each group who experienced a recalibration of leg-speed perception. Total number of participants in each group is shown. Filled area represents participants who had a recalibration (e.g., 15/17 subjects in the control group); unfilled area represents participants who did not have a recalibration. Ratio of participants with leg-speed perception recalibration decreased with worsening ICARS score; moderate and severe groups had significantly lower ratios compared with the control group
Motor Adaptation
We next sought to determine whether ataxia severity played a role in the ability of patients to adapt to the split-belt treadmill, as well as the specific spatial and temporal characteristics of adaptation. Step length difference, spatial, temporal, and perturbation parameters for sample subjects from the control, mild, moderate, and severe groups are shown in Fig. 4. One-way ANOVA for group data (Fig. 3b) demonstrated that ataxia severity had a significant effect on all three motor adaptation parameters (step length difference F (3,43) = 14.593, p < .001; spatial contribution F (3,43) = 7.472, p < .001; temporal contribution F (3,43) = 7.956, p < .001). Post hoc tests showed that the mild group had no significant deficits and that the moderate group had deficits only in the temporal component (moderate vs. control p = .023). The severe group had deficits in step length difference due to decreases in both the spatial and temporal components (severe vs. control p < .001 for all parameters).
Motor parameters of sample subjects from the control, mild, moderate, and severe groups during adaptation. Step length difference (black), spatial contribution (green), temporal contribution (cyan), and perturbation (magenta) terms are shown. Adaptation is similar in control and mild cerebellar participants. However, the temporal component is diminished in the moderate participant, and the temporal, spatial, and step length difference parameters are all reduced in the severe participant
Leg-Speed Perception
We next compared the ratio of subjects in each group that experienced a change in leg-speed perception after split-belt walking (Fig. 3c). Fisher’s exact test demonstrated that both the moderate and severe groups had significantly lower ratios of individuals with sensory perceptual recalibration compared to control subjects (p = .008 and .004, respectively), while the mild group did not (p = .570). We should note that results from two participants (one mild and one moderate cerebellar patient) are not included, as their leg-speed perception results were missing from the dataset re-analyzed from Morton and Bastian [13].
Motor/Sensory Association
To determine the relationship between the degree of motor adaptation and the recalibration of leg-speed perception, we performed point-biserial correlations between motor and sensory parameters from cerebellar patients. There were no correlations between the recalibration of leg-speed perception and the percent corrected of any motor parameter (step length difference r pb = .208, p = .319; spatial contribution r pb = .129, p = .538; temporal contribution r pb = .271, p = .190). Thus, although cerebellar damage can lead to decreased motor and/or sensory perceptual recalibration, deficits in one domain were not predictive of deficits in the other.
Together, the results of Experiment 2 demonstrate that impairments in motor and sensory perceptual recalibration in patients with cerebellar damage are strongly dependent on the severity of ataxia. Step length difference and spatial contributions to motor adaptation do not exhibit significant impairments until severe ataxia occurs, whereas temporal contributions to motor adaptation and recalibration of leg-speed perception may begin to show deficits at a more moderate level. However, although cerebellar damage may lead to diminished motor and/or sensory perceptual recalibration, deficits in motor adaptation were not found to be predictive of deficits in leg-speed perception recalibration.
Discussion
The goal of this study was to determine the role of the cerebellum in the sensorimotor recalibration that occurs during locomotor adaptation. We showed that patients with cerebellar damage had significantly diminished recalibration of leg-speed perception after walking on a split-belt treadmill compared to healthy participants. In addition, we found that ataxia severity plays an important role in the degree of sensory and motor adaptation deficits that occur with cerebellar damage: people with mild ataxia did not show appreciable deficits, those with moderate ataxia had deficits in adapting temporal components of walking and showed impaired perceptual recalibration of leg speed, and those with severe ataxia had global deficits in walking adaptation and perceptual recalibration.
Our results support the hypothesis that the cerebellum is an essential part of the brain circuit involved in recalibrating sensory percepts during walking adaptation. That being said, the precise connection between motor adaptation, sensory perceptual recalibration, and the cerebellum is not fully understood. Our analysis in the second part of the study showed that people with ataxia (especially more severe ataxia) are likely to have deficits in motor and/or sensory perceptual recalibration. However, there was no correlation between the degree of motor adaptation and the likelihood of sensory perceptual recalibration deficits in cerebellar patients. We found that some patients were impaired in motor adaptation but not sensory perceptual recalibration, while others showed the opposite pattern. When ataxia was severe, patients were more likely to be impaired in both domains. This demonstrates that while both types of recalibration can be affected by cerebellar dysfunction, the mechanisms underlying motor and sensory perceptual recalibration have some degree of independence. Supporting this idea, it has been shown that motor and proprioceptive recalibration in reaching tasks have distinct generalization patterns [34, 35] and arise at different rates [36]. Henriques et al. [23] argued that sensory integration and recalibration may actually occur in the posterior parietal cortex and the cerebellum is not necessary for them to occur. The fact that most of our cerebellar patients showed no perceptual recalibration (6/8 in part one, and 14/25 in part two) is inconsistent with their conclusion, yet we agree that other brain areas must be involved in addition to the cerebellum. Future experiments will be needed to clarify whether the cerebellum is directly responsible or if cerebellar impairments subsequently lead to downstream deficits in sensory perceptual recalibration processes that may occur elsewhere in the brain.
We were somewhat surprised to find that the patients tested in the first part of this study only demonstrated a small, non-significant reduction in motor adaptation of step length difference. It is well-known that cerebellar damage generally produces substantial deficits in motor adaptation across a variety of tasks [13, 37,38,39]. In the same split-belt treadmill paradigm used in the present study, Morton and Bastian [13] found severe motor adaptation impairments in cerebellar patients compared to healthy participants. Yet, Hoogkamer et al. [24] recently found that adaptation of the symmetry of interlimb walking patterns such as step length was similar between cerebellar patients and healthy controls. In that study, it was suspected that this discrepancy with previous work was due to the relatively mild degree of ataxia of the patients tested (ICARS < 20) compared with the more severely impaired patients in the study by Morton and Bastian [13] (ICARS > 30). Here we confirmed the speculation of Hoogkamer et al. [24] that ataxia severity plays an important role in motor adaptation deficits. Specifically, adaptation of step length difference, including spatial and temporal components, was only impaired in patients with severe ataxia. People with moderate ataxia only showed deficits in the temporal component, and this did not lead to significant deficits in adapting step length. To better understand this result, an important consideration is that cerebellar degeneration is generally a chronic disease which may allow for partial compensation of developing motor impairments. Reaching experiments have shown that cerebellar patients tend to have a greater amount of forgetting at set breaks [39] which could indicate the use of conscious movement strategies to compensate for motor adaptation deficits. In the context of walking, we know that spatial components are more consciously modifiable than temporal features, and that conscious correction of step length symmetry primarily affects spatial, but not temporal, parameters of adaptation [31]. The results of part two of the present study demonstrate that although patients with mild-to-moderate ataxia may not express overall motor adaptation deficits, they may, in fact, have underlying impairments that can be largely masked by conscious strategies. Analyzing the temporal components of motor adaptation in patients with milder ataxia, however, may elucidate a feature that cannot be modified by compensatory strategies and thus may help identify adaptation deficits before they become clinically significant.
The specific impairments of spatial or temporal adaptation in patients with cerebellar damage may also relate to the affected cerebellar regions or pathways. We have previously suggested that spatial contributions to locomotor adaptation may involve intermediate/lateral cerebellar projections to cerebral structures via the thalamus, whereas temporal adaptation may occur through midline cerebellar projections to the brainstem and spinal cord [27, 31]. Many of the patients in the present study had diffuse cerebellar damage, making it difficult to associate spatial or temporal adaptation deficits with lesions in specific cerebellar regions. However, it is possible that patients with lesions localized to the vermis may have more significant impairment in the temporal components of adaptation, whereas those with damage in the cerebellar hemispheres may be more affected in the spatial domain. Future studies with more homogenous subsets of people with cerebellar damage may help elucidate the influence of specific cerebellar regions or pathways on the spatial and temporal components of locomotor adaptation.
Like motor adaptation, we also found that ataxia severity is linked to the development of deficits in sensory perceptual recalibration, as patients with moderate and severe ataxia were less likely to recalibrate leg-speed perception following split-belt walking. This result may help explain the contradictory results of previous studies that examined sensory perceptual recalibration in patients with cerebellar damage. Though Izawa et al. [21] and Synofzik et al. [22] found diminished updating of felt hand position in cerebellar patients following a reaching adaptation task, Henriques et al. [23] more recently demonstrated no sensory perceptual recalibration deficits in cerebellar patients compared to healthy participants. Patients in the study by Henriques et al. [23], however, had only mild-to-moderate ataxia (as evidenced by a mean score of 3.67 on the Scale for the Assessment and Rating of Ataxia), whereas Synofzik et al. [22] and Izawa et al. [21] studied patients with moderate-to-severe ataxia (mean total ICARS scores of 22 and 36, respectively). Based on the results of the current study, it is plausible that the negative result of Henriques et al. [23] may be related to the relatively mild degree of impairment of patients studied, and using a similar paradigm to test patients with more severe ataxia may have more likely shown decreases in the recalibration of sensory percepts.
Conclusion
In summary, we have shown that cerebellar damage leads to significant deficits in sensorimotor recalibration during walking. We first demonstrated that people with cerebellar damage have impaired recalibration of leg-speed perception that normally occurs during split-belt treadmill walking. Though future studies are necessary to determine whether the cerebellum is directly or indirectly involved in this type of sensory perceptual recalibration, we believe this result demonstrates that cerebellar integrity is necessary for it to occur. Additionally, we have shown that the pattern of sensorimotor adaptation impairment that occurs with cerebellar damage is strongly dependent on disease severity. With increasing severity, patients are more likely to show deficits in both motor adaptation and sensory perceptual recalibration.
References
Körding KP, Wolpert DM. Bayesian integration in sensorimotor learning. Nature. 2004;427(6971):244–7.
Vaziri S, Diedrichsen J, Shadmehr R. Why does the brain predict sensory consequences of oculomotor commands? Optimal integration of the predicted and the actual sensory feedback. J Neurosci. 2006;26(16):4188–97.
Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108.
Blakemore SJ, Frith CD, Wolpert DM. The cerebellum is involved in predicting the sensory consequences of action. Neuroreport. 2001;12(9):1879–84.
Lang CE, Bastian AJ. Cerebellar subjects show impaired adaptation of anticipatory EMG during catching. J Neurophysiol. 1999;82(5):2108–19.
Lang CE, Bastian AJ. Additional somatosensory information does not improve cerebellar adaptation during catching. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2001;112(5):895–907.
Miall RC, Christensen LOD, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol. 2007;5(11):e316.
Nowak DA, Hermsdörfer J, Rost K, Timmann D, Topka H. Predictive and reactive finger force control during catching in cerebellar degeneration. Cerebellum Lond Engl. 2004;3(4):227–35.
Nowak DA, Timmann D, Hermsdörfer J. Dexterity in cerebellar agenesis. Neuropsychologia. 2007;45(4):696–703.
Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21(6):628–33.
Straube A, Deubel H, Ditterich J, Eggert T. Cerebellar lesions impair rapid saccade amplitude adaptation. Neurology. 2001;57(11):2105–8.
Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91(1):230–8.
Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26(36):9107–16.
Cressman EK, Henriques DYP. Sensory recalibration of hand position following visuomotor adaptation. J Neurophysiol. 2009;102(6):3505–18.
Mattar AAG, Darainy M, Ostry DJ. Motor learning and its sensory effects: time course of perceptual change and its presence with gradual introduction of load. J Neurophysiol. 2013;109(3):782–91.
Ostry DJ, Darainy M, Mattar AAG, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci. 2010;30(15):5384–93.
Salomonczyk D, Cressman EK, Henriques DYP. Proprioceptive recalibration following prolonged training and increasing distortions in visuomotor adaptation. Neuropsychologia. 2011;49(11):3053–62.
Salomonczyk D, Henriques DYP, Cressman EK. Proprioceptive recalibration in the right and left hands following abrupt visuomotor adaptation. Exp Brain Res. 2012;217(2):187–96.
Jensen L, Prokop T, Dietz V. Adaptational effects during human split-belt walking: influence of afferent input. Exp Brain Res. 1998;118(1):126–30.
Vazquez A, Statton MA, Busgang SA, Bastian AJ. Split-belt walking adaptation recalibrates sensorimotor estimates of leg speed but not position or force. J Neurophysiol. 2015;114(6):3255–67.
Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci. 2012;32(12):4230–9.
Synofzik M, Lindner A, Thier P. The cerebellum updates predictions about the visual consequences of one’s behavior. Curr Biol CB. 2008;18(11):814–8.
Henriques DYP, Filippopulos F, Straube A, Eggert T. The cerebellum is not necessary for visually driven recalibration of hand proprioception. Neuropsychologia. 2014;64:195–204.
Hoogkamer W, Bruijn SM, Sunaert S, Swinnen SP, Van Calenbergh F, Duysens J. Adaptation and aftereffects of split-belt walking in cerebellar lesion patients. J Neurophysiol. 2015;114(3):1693–704.
Block HJ, Bastian AJ. Sensory weighting and realignment: independent compensatory processes. J Neurophysiol. 2011;106(1):59–70.
Block HJ, Bastian AJ. Cerebellar involvement in motor but not sensory adaptation. Neuropsychologia. 2012;50(8):1766–75.
Vasudevan EVL, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci. 2011;31(8):3055–65.
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145(2):205–11.
Malone LA, Bastian AJ. Spatial and temporal asymmetries in gait predict split-belt adaptation behavior in stroke. Neurorehabil Neural Repair. 2014;28(3):230–40.
Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94(4):2403–15.
Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103(4):1954–62.
Finley JM, Long A, Bastian AJ, Torres-Oviedo G. Spatial and temporal control contribute to step length asymmetry during split-belt adaptation and hemiparetic gait. Neurorehabil Neural Repair. 2015;29(8):786–95.
Long AW, Finley JM, Bastian AJ. A marching-walking hybrid induces step length adaptation and transfers to natural walking. J Neurophysiol. 2015;113(10):3905–14.
Cressman EK, Henriques DYP. Generalization patterns for reach adaptation and proprioceptive recalibration differ after visuomotor learning. J Neurophysiol. 2015;114(1):354–65.
Mostafa AA, Kamran-Disfani R, Bahari-Kashani G, Cressman EK, Henriques DYP. Generalization of reach adaptation and proprioceptive recalibration at different distances in the workspace. Exp Brain Res. 2015;233(3):817–27.
Zbib B, Henriques DYP, Cressman EK. Proprioceptive recalibration arises slowly compared to reach adaptation. Exp Brain Res. 2016;234(8):2201–13.
Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain J Neurol. 1996;119(Pt 4):1183–98.
Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93(5):2809–21.
Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103(4):2275–84.
Funding
This study was funded by the National Institute of Child Health and Human Development Grant HD040289.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Johns Hopkins Medicine Institutional Review Board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Statton, M.A., Vazquez, A., Morton, S.M. et al. Making Sense of Cerebellar Contributions to Perceptual and Motor Adaptation. Cerebellum 17, 111–121 (2018). https://doi.org/10.1007/s12311-017-0879-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0879-0