Abstract
Although the pathophysiology of essential tremor (ET), one of the most common movement disorders, is not fully understood, evidence increasingly points to cerebellar involvement. To confirm this connection, we assessed the everyday hand and finger movements of patients with ET, as these movements are known to be affected in cerebellar diseases. In 26 mildly affected patients with ET (compared to age- and gender-matched controls), kinematic and finger force parameters were assessed in a precision grip. In a second task, independent finger movements were recorded. The active finger had to press and release against a force-sensitive keypad while the other fingers stayed inactive. Finally, control of grip force to movement-induced, self-generated load changes was studied. Transport and shaping components during prehension were significantly impaired in patients with ET compared to controls. No significant group differences were observed in independent finger movements and grip force adjustments to self-generated load force changes. However, in the latter two tasks, more severely affected ET patients performed worse than less affected. Although observed deficits in hand and finger movement tasks were small, they are consistent with cerebellar dysfunction in ET. Findings need to be confirmed in future studies examining more severely affected ET patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential tremor (ET) is one of the most common, progressive movement disorders with an overall prevalence (all ages) of 0.9 % and a prevalence of 4.6 % in adults aged 65 years and older [1]. It may disable patients in performing activities of daily living, and it may lead to a reduced quality of life during the course of the disease [2]. A growing number of clinical studies demonstrate that ET symptoms go beyond the characteristic dominating postural and/or kinetic tremor. Non-motor symptoms can include mild cognitive changes [3], anxiety, depression [4], and hearing problems [5].
The pathogenesis is not completely understood. Clinical, electrophysiological, neuroimaging, and postmortem studies suggest cerebellar involvement. Neuroimaging reveals structural [6–8], functional [9, 10], and metabolic cerebellar changes [11]. It has been proposed that ET is a “Purkinjopathy” [12, 13]. In postmortem studies, cerebellar pathologies like torpedoes and axonal swellings in the cerebellar Purkinje cells were reported [14, 15]. Furthermore, Purkinje cell loss was observed [16]. Clinical and electrophysiological signs include disturbance in gait [17–19] and balance, speech disturbance [20], eye movement abnormalities [21], disturbed motor timing [22, 23], and motor learning [24]. Patients with ET have deficits in the temporal and force parameters of a precision grip [25]. In finger tapping and alternating hand movements, timing is disturbed [22, 23, 26].
Reaching, grasping, and moving an object and independent finger movements are indispensable for many activities of daily living. Hand and finger movements depend on cerebellar function. Patients with cerebellar diseases reveal deficits in prehension and in the precise regulation of grip forces according to object characteristics, dynamic loads, and independent finger movements [27–29]. While grasping an object, patients with cerebellar disorders show prolonged movement times, increased grip forces, disturbed timing of fingers involved in the precision grip, and increased grip aperture including multiple peaks. Patients with cerebellar disorders also reveal deficits in producing grip forces in anticipation of load forces generated through movement of an object [28]. This ability is thought to require an internal feed-forward model located within and/or updated by the cerebellum [30]. Cerebellar patients are slow and clumsy in independent finger movements [27].
To further investigate cerebellar involvement in ET, we (1) analyzed spatial, temporal, and dynamic parameters in a precision grip (2) assessed predictive grip force control in self-generated movements; and (3) studied the force enslaving effect in independent finger movements and forces. To estimate the effect of disease progression, all parameters were correlated to tremor and ataxia scores.
Materials and Methods
Participants
Twenty-six mildly affected ET patients (12 male, 14 female; mean age 59.5 ± 17.5 years, range 24–75 years, Table 1) diagnosed according to the criteria of Deuschl et al. [29] and 26 healthy controls who were matched as close as possible to gender and age (13 male, 13 female; mean age 55.7 ± 12.3 years, range 24–80 years) participated in the study. All participants were right-handed, determined by a laterality index higher than 50 % according to the Edinburgh handedness inventory [32]. An experienced neurologist (KS) performed a complete neurological examination and assessed impairment with the help of tremor and ataxia scales. Visual acuity has been assessed as part of the standard neurological examination. No pathologies were observed. To evaluate ataxia symptoms, the International Cooperative Ataxia Rating Scale (ICARS, total score max. 100, and “posture and gait” sub-score max. 34) was applied [33]. The degree of tremor was evaluated on the Clinical Rating Scale for Tremor [34], part 1 (clinical rating scale for tremor (CRST), total score: max. 80 and sub-scores “postural tremor of the upper extremities” and “intention tremor of the upper extremities”: max. 8 and 8, respectively). The CRST and ICARS were significantly correlated in the patient group (R = 0.488, p = 0.011). The family history for ET was positive in 61.5 %. Over 40 % (42.3 %) of ET patients reported tremor improvement when drinking a moderate amount of alcohol. Propranolol was taken for treatment of tremor by 23.1 %. Participants using other central acting drugs, e.g., gabapentin or primidone, were excluded. All participants gave written informed consent prior to participation. The study was approved by the local Ethics Committee of the University Clinic Essen.
Precision Grip
Procedure and Data Acquisition
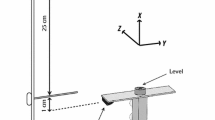
Participants were comfortably seated in a chair in front of a table so that their elbow could rest in 90° flexion, with their hands in front of their trunk. They were instructed to grasp with their right, dominant hand a custom-made object (height 90 mm, width 60 mm, depth 33 mm, incorporating sensors and with grasping surfaces covered by medium-grain sandpaper; for detailed description, see Brandauer et al. [35]). The hand was positioned at a marked starting point with thumb and index finger in contact (Fig. 1a). The object was 30 cm from the starting position. Participants were instructed to reach for the object after a verbal command, grasp its center with a precision grip (thumb, index and middle finger), lift it to a height of about 5 cm, and hold it for 3 s. Participants were instructed to move at a comfortable (“normal”) speed and a fast speed. Fourteen trials were tested in each condition. The first four trials were practice trials; the last ten trials were analyzed.
Illustration of the three experimental task conditions. a Precision grip: subject grasped the object at a distance of 30 cm from the starting position and lifted it about 5 cm. b Independent finger movements and forces task: participants repeatedly pressed and released one of the four fingers (the thumb was not included) between two predefined force levels. c Predictive grip force control task: participants moved the object with its grip surfaces vertical and parallel in front of their trunk
Data and Statistical Analyses
For the kinematic analysis of the task, an ultrasound based motion measurement system (CMS-HS, Zebris, Isny, Germany) and specially designed software (3DA) [36, 37] were used. Markers were attached to the fingernail of the index finger and thumb, and to the wrist proximal to the thumb, to register finger and transport movements in three-dimensional positional data. The sample rate of the spatial coordinates of each marker was at a frequency of 100 Hz with a spatial resolution of 0.1 mm. The following parameters were assessed: (1) peak grip aperture (in mm): maximum three-dimensional distance between the markers on the tips of index finger and thumb during the reach phase; (2) movement time of the wrist (in ms): time between movement start (velocity of the wrist >20 mm/s) and movement end (local minimum of the wrist velocity profile with the hand at the object); (3) straightness: ratio of the three-dimensional path of the wrist marker and the distance between the start and the end point; (4) grip formation time (in ms): time between grip opening (velocity of finger distance (VD) >15 mm/s) and closure (VD −20 mm/s); and (5) number of local peaks in the time course of grip aperture.
For the grip and load force analyses, the following parameters were obtained: (1) peak grip force (in N), (2) time to peak grip force (in ms): time between grip force start (grip force >0.1 N) and peak grip force, (3) finger touch latency (in ms): absolute time differences between touch of the thumb grip force sensor (grip force >0.1 N) and touch of the index/middle finger grip force sensor, and (4) peak negative load (in N) after grip initiation and before increase in load force.
A mixed-design analysis of variance (ANOVA) was calculated for the assessed parameters with the between-subject factor group (control, ET patients) and the within-subject factor condition (normal, fast). A p value < 0.05 was considered significant. The force and kinematic data were correlated with clinical rating scores of ataxia and tremor. Spearman’s correlation tests were used because values were non-normally distributed.
Predictive Grip Force Control
Procedure and Data Acquisition
Participants were instructed to use their dominant arm to move a custom-made object (60 × 26 × 60 mm; medium-grain sandpaper-covered grasping surfaces; total weight 500 g) with its grip surfaces vertical and parallel in a vertical plane in front of their trunk with amplitude of about 30 cm and at a frequency of 0.9 Hz (generation of movement-induced load changes; Fig. 1b). The object was grasped with the thumb on one side and the index and middle fingers on the opposite side. The demanded movement speed was coarsely indicated on a screen, but no feedback was provided. The examiner monitored the accurate movement execution. A practice trial of 11 s preceding four trials with duration of 22 s was performed. Sensors integrated in the object recorded grip force on each side (0–100 N, accuracy ± 0.1 N) and the linear vertical and horizontal accelerations tangential to the grasping surfaces (50 m/s2, accuracy ± 0.2 m/s2). The grip force of both sensors for each side was averaged. The load was calculated as the vectorial combination of the inertial loads in the vertical and horizontal directions and the gravitational load in the vertical direction (for more detailed information, see Brandauer et al. [26]).
Data and Statistical Analyses
For data analyses, the first two seconds of each trial were omitted to exclude any irregularities of the up and down movements at the start of the trial. The remaining 20 s were divided into two intervals of 10 s, each of which typically contained eight full cycles of load force and upward and downward movements, respectively. Similar to the previous task, a computer algorithm searched for local maxima and minima in the load force profiles. Around each load force peak, the maxima and minima of grip force were assessed. The load force amplitude, the mean load force, the mean grip force, and the ratio between grip force and load force maxima were calculated. A cross-correlation between grip and load force profile indicated coupling between the modulations of both forces. The maximum cross-correlation coefficient (Rx) indicated the precision of coupling, whereas phase shifts were measured by the time lag (Tlag) of Rx. If the value was positive, grip force modulation lagged behind load changes. The calculated measures were averaged across the eight intervals.
Group differences were assessed through t tests. The force data were correlated with clinical rating scores of ataxia and tremor. Spearman’s correlation tests were used. A p value <0.05 was considered significant.
Independent Finger Movements and Forces
Procedure and Data Acquisition
A custom-made instrumented object was equipped with four force sensors (0–100 N, accuracy ± 0.1 N, sampling rate 100 Hz; vertical distance between adjacent sensors 24 mm) and used as a four-finger keypad (for more detailed information, see Brandauer et al. [29]). Sensors were equipped with small disks 15 mm in diameter covered with 100-grit sandpaper. All fingers except for the thumb were placed on the sensors (Fig. 1c). Forearm and wrist lay in the sagittal plane on the table. Participants were instructed to perform regular and fast force changes of a given range with one instructed finger. A feedback of the applied force of the active finger was provided on the screen in form of a vertical bar that moved according to the applied forces. Two horizontal lines indicated the upper and lower force ranges. The other non-instructed fingers were to be kept on the sensors without being used. Three trials of 8 s each were performed with each finger. The first trial for each finger served as practice trial and was not included in the analysis.
Data and Statistical Analyses
To evaluate the applied forces of the active finger, a computer algorithm searched for local maxima and minima in the force profiles of the active finger. The force amplitude was calculated from adjacent maxima, and mean force during the interval was assessed. The first 500 ms of each of the trials was not considered for data analysis, so an interval lasted 7.5 s. The maxima, minima, and force amplitude of the non-instructed fingers were determined in a time window (one-half cycle) around each force peak of the active finger. The force amplitude of the non-instructed finger was described in relation to the force amplitude of the active finger. The mean force of the non-instructed fingers was calculated in the same manner as the active fingers.
A mixed-design ANOVA with the between-subject factor group (controls, ET patients) and the within-subject factor finger (fingers 2 through 5) was calculated to assess group differences between force developments of the active fingers. To determine group differences in the force enslaving effect, a mixed-design ANOVA was calculated for each active finger with the between-subject factor group (controls, ET patients) and the within-subject factor finger (the three non-instructed fingers). The force data were correlated with clinical rating scores of ataxia and tremor. Spearman’s correlation tests were used. A p value < 0.05 was considered significant.
Results
Precision Grip
To compare performance between groups, we applied a mixed-design ANOVA with condition (normal or fast speed) as within-subject factor. The movement involves three components. The transport component refers to movement of the hand from the starting point to the object. In the hand-shaping component, fingers are adjusted to the size of the object. The grip component includes the modulation of grip forces according to the object’s size and surface properties.
Transport Component
In ET patients, hand transport was slower compared to controls (movement time of the wrist, Fig. 2a; group effect, F (1,43) = 10.68, p = 0.002). In both groups, the hand transport time was slower in the normal speed condition compared to the fast condition (condition effect, F (1,43) = 238.84, p < 0.001, condition x group effect, F (1,43) = 0.260, p = 0.260). The straightness of the movement path toward the object did not differ between groups (Fig. 2b; group effect, F (1,42) = 0.370, p = 0.546) and deviated more from a straight line in the fast condition (condition effect, F (1,42) = 39.21, p < 0.001, condition × group effect, F (1,42) = 0.333, p = 0.567).
Hand Shaping
ET patients needed more time than the controls from the start of hand opening to object contact (grip formation time, Fig. 2c; group effect, F (1,42) = 8.04, p = 0.007). As expected, both groups presented a longer grip formation time in the normal speed compared to the fast condition (condition effect, F (1,42) = 237.23, p < 0.001, condition × group effect, F (1,42) = 1.290, p = 0.263). During grip aperture, ET patients presented significantly more peaks (Fig. 2d; group effect, F (1,45) = 7.45, p = 0.009). Both groups showed more local peaks in the normal speed condition than in the fast condition (condition effect, F (1,45) = 15.00, p < 0.001, condition × group effect, F (1,45) = 1.340, p = 0.253). Both groups applied a similar peak grip aperture (Fig. 2e; group effect, F (1,44) = 0.362, p = 0.551). The time between contact of the fingers on one side of the object and the thumb on the opposite site was not significantly different between groups (finger touch latency, Fig. 2f; group effect, F (1,50) = 0.051, p = 0.822). Both groups presented a shorter latency in the fast condition (condition effect, F (1,50) = 5.98, p = 0.018, condition × group effect, F (1,50) = 1.437, p = 0.236).
Grip Component
Time from grip force onset to maximal grip force did not differ between groups (time to peak grip force, Fig. 2g; group effect, F (1,50) = 2.078, p = 0.156). Time to peak grip force was longer in the normal speed compared to the fast condition (condition effect, F (1,50) = 57.37, p < 0.001, condition × group effect, F (1,50) = 0.040, p = 0.841). ET patients applied significantly lower maximal grip forces (Fig. 2h; group effect, F (1,50) = 5.73, p = 0.02). In both groups, maximal grip forces were higher in the fast condition (controls 9.75 N, ET patients 6.91 N) than in the normal speed condition (controls 7.67 N, ET patients 6.32 N). This difference was significantly less in the patient group (condition effect, F (1,50) = 13.95, p < 0.001, condition × group effect, F (1,50) = 4.357, p = 0.042). Peak negative load force did not vary between groups (Fig. 2i; group effect, F (1,50) = 2.908, p = 0.094).
Correlation Analysis Between Single Parameters in ET Patients
Most values were strongly correlated to each other, which was most apparent in the fast condition. ET patients with a slow performance in hand transport (movement time of the wrist) were also slow in grip formation time and in time to maximal grip force. Their path was less straight, and they showed more peaks during grip aperture. Their finger touch latency was increased. Participants with lower grip forces showed increased movement time of the wrist and grip formation time.
Correlation of Reach-to-Grasp Parameters and Tremor/Ataxia Scores
Clinically more severely affected ET patients (corresponding to a higher ICARS and CRST score) were more impaired in grip formation and hand transport. They presented more local peaks during grip aperture, and their movement path deviated more from a straight line to the object (straightness). Analysis revealed a significant correlation between the number of peaks with ICARS (p = 0.006, R = 0.569) and CRST (R = 0.476, p = 0.016) in the normal speed condition and with ICARS (R = 0.514, p = 0.014) in the fast condition (CRST, R = 0.369, p = 0.063). More specifically, the number of peaks was correlated to the postural tremor of the upper extremities sub-score of CRST (normal speed condition, p = 0.025, R = 0.448; fast speed condition, p = 0.052, R = 0.386). Also, the posture and gait sub-score of ICARS showed significant correlations (normal speed condition, p = 0.016, R = 0.477; fast condition, p = 0.039, R = 0.407). In the normal and fast conditions, straightness correlated with CRST [R = 0.463, p = 0.026 (normal speed condition) and R = 0.435 (fast condition)]. Straightness of the fast condition correlated with postural tremor (R = 0.438, p = 0.025; normal movement, p = 0.279). More severely impaired ET patients presented a larger peak negative load force in both the normal (ICARS, R = −0.475, p = 0.014; CRST, R = −0.397, p = 0.045) and fast conditions (ICARS, R = −0.444, p = 0.023; CRST, R = −0.436, p = 0.026). Peak negative load force was correlated with the intention tremor of the upper extremities sub-score of the CRST (normal speed condition, R = −0.616, p = 0.001, fast condition, R = −0.562, p = 0.003) and the posture and gait ICARS sub-score (normal speed condition, R = −0.530, p = 0.005; fast condition, p = 0.142).
In conclusion, patients with ET showed significant impairments in the hand transport and grip formation components. Their performance was slower than that of the control participants. Clinically more severely affected ET patients performed worse than those less affected.
Predictive Grip Force Control
Participants grasped an object with the thumb on one side and the index and middle fingers on the opposite side and moved it in a vertical plane in front of their trunk. Sensors recorded grip force and the linear vertical and horizontal accelerations (determining load forces). The maximum cross-correlation coefficient (Rx) and the time lag (Tlag) were calculated (for individual values, see Table 2). Group differences were assessed through a t test.
Load Force
Mean load forces and amplitudes of the load forces are dependent on the weight of the object and the acceleration of the movements. ET patients had significantly higher load force amplitudes than controls (T (49) = −2.050, p = 0.046), indicating that they performed the task faster and with higher accelerations, whereas mean load forces did not differ between groups (T (49) = 1.238, p = 0.222).
Grip Force
Mean grip forces and the grip force amplitude did not differ between groups (T (49) = 0.429, p = 0.670, T (49) = 0.393, respectively). No group differences were observed concerning adjustment of grip forces to load forces (grip force/load force ratio at maximal load force, T (49) = −0.697, p = 0.489).
Precision and Regulation of Grip Force
The cross-correlation coefficient was assessed to evaluate the precision and predictive regulation of grip forces according to load forces. A high coefficient indicates similar profiles of grip and load forces. These were comparable between ET patients and controls (T (49) = 0.774, p = 0.442). The time lag between grip and load force profiles was assessed to evaluate temporal regulation. Differences between groups were not observed (T (49) = −0.841, p = 0.404).
Correlation of Grip Force and Load Force Parameters to Tremor/Ataxia Scores
ET patients with a higher ataxia score applied higher mean grip forces than less-affected ET patients (mean grip force; ICARS, R = 0.554, p = 0.004). They were less able to adapt their grip forces to the self-generated load force profile (cross-correlation coefficient, ICARS, R = −0.440, p = 0.028). Mean load force correlated inversely with the postural tremor of the upper extremities sub-score of CRST (R = −0.415, p = 0.039), which indicates that ET patients with severe tremor performed the task slower.
In conclusion, on a group level, no significant differences between ET patients and controls were observed. However, more severely affected ET patients with ataxia symptoms performed worse in predictive control of grip forces than those less affected.
Independent Finger Movements and Forces
To evaluate independent finger forces, participants were asked to apply finger forces with one instructed (active) finger while the non-instructed (passive) fingers were to remain still. We examined the performance of the active fingers and the involvement of the passive fingers (force enslaving effect).
Active Fingers
The force alternation of the active finger was performed as fast as possible. Targets on the monitor instructed the amplitude. The actual force produced could deviate from the targets. Mean force, force amplitude, and number of force maxima were assessed (for values, see Table 3). Differences between groups were evaluated applying ANOVA with fingers as within-subject factor.
ET patients and controls applied almost identical mean forces (group effect, F (1,50) = 0.026, p = 0.872; finger effect, F (2.6,131.8) = 5.787, p = 0.002; finger × group effect, F (2.6, 131.8) = 0.450, p = 0.693). In both groups, the highest forces were produced with the little finger and lowest forces with the ring finger. Amplitude of forces varied significantly between fingers without significant differences between both groups (group effect, F (1,50) = 0.026, p = 0.873; finger effect, F (3,150) = 59.66, p < 0.001; finger × group effect, F (3,150) = 0.101, p = 0.959). The number of maxima did not differ between groups but varied significantly between fingers (group effect, F (1,50) = 0.021, p = 0.885; finger effect, F (2.8,138.6) = 11.73, p < 0.001; finger × group effect, F (2.8,138.6) = 1.162, p = 0.325). In both groups, most maxima were produced with the index finger.
Passive Fingers
Mean force and force amplitudes of each passive finger in relation to the active finger were assessed to evaluate the force enslaving effect (Table 4). Mixed-design ANOVAs were calculated for each active finger with passive finger as within-subject factor. The performance differed significantly between passive fingers (F (≥1.8,88.1) ≥ 5.14, p ≤ 0.010). Only the mean force of passive fingers with the middle finger as the active finger did not vary (finger effect, F (2,100) = 1.469, p = 0.235). In general, passive fingers adjacent to the respective active finger applied the highest mean force and relative force amplitude. If the index finger was active, ET patients showed significantly higher relative force amplitude compared to controls (group effect, F (1,49) = 5.631, p = 0.022). However, the difference looses significance after correction for multiple comparisons (Bonferroni). No further differences between groups were observed.
Correlation of Active and Passive Forces to Tremor and Ataxia Scores
The active fingers showed a significant correlation between CRST and mean force of the ring finger (R = 0.389, p = 0.050). The mean force of all fingers correlated to the postural tremor of the upper extremities sub-score of CRST (R ≥ 0.412, p ≤ 0.037). The mean force and relative force amplitude of several passive fingers correlated to ICARS and CRST and sub-scores “postural and intention tremor of upper extremities” and “posture and gait,” which indicate more severe impairment in more affected ET patients.
In conclusion, the individual active and passive fingers differed in mean forces and relative force amplitudes. Adjacent passive fingers showed the highest force enslaving effect, while more distant fingers were used less. The most dependent finger was the ring finger. Significant differences between groups were not observed except an increased enslaving effect in ET patients if the index finger was active. In more affected ET patients, the force enslaving effect was higher than in only slightly affected patients.
Discussion
The aim of the present study was to provide further support of cerebellar involvement in essential tremor (ET). Everyday hand and finger movements were investigated that are known to depend on intact cerebellar function. Overall, the observed deficits were mild. Patients with ET had deficits in the transport and hand-shaping component of a reach-to-grasp movement using a precision grip. Their hand transport time was prolonged, grip formation was slowed, and more local peaks in grip aperture were observed. More severely affected ET patients performed worse than less affected. They used a transport path that deviated more from a straight line and increased peak negative load more after grip initiation. Furthermore, they had more problems in predictive grip force control and control of passive finger forces in independent finger movements compared to less-affected ET patients. However, these parameters were not significantly different between ET patients and controls. In our patient population, tremor scores were relatively low. Abnormalities may become more obvious in more severely affected ET patients. Our findings are consistent with the current concept of cerebellar pathology in ET but need to be confirmed in a larger and more severely affected study population.
ET patients were primarily impaired in prehension kinematics. In accordance with findings in cerebellar patients, ET patients presented a prolonged hand transport time [35]. Cerebellar patients also show increased path curvatures in reaching movements [35, 38]. In the present study, straightness in ET patients was not significantly different from healthy subjects. Nevertheless, more severely impaired patients (higher score on CRST) exhibited more increased curvatures. Therefore, straightness in ET patients may be impaired in more advanced disease. However, given that straightness was correlated to postural tremor in the fast condition, we cannot exclude that the increased curvature might be a direct reflection of the postural tremor and is not necessarily an indicator of increasing cerebellar involvement.
For the hand-shaping component, the deficits demonstrated in the present ET study population were less distinct than those reported in previous cerebellar disease studies. We did, however, observe a prolonged grip formation time consistent with what has been previously described in cerebellar patients [35].
Our ET patients exhibited a normal time to peak grip force, which was consistent to another study in ET patients in a prehensile task [25]. Prolonged times to peak have been reported in patients with cerebellar degeneration [35, 39], but not in patients with cerebellar stroke [39]. Disease location within the cerebellum and severity of cerebellar impairment likely play a role.
The peak grip aperture did not differ between ET patients and controls, but ET patients presented more local peaks in grip aperture. Again, we cannot exclude that this may be caused at least in part by postural tremor. While an increased number of peaks is typical for cerebellar disorders [35, 40], peak grip aperture has been reported either normal [41] or enlarged [35, 40] in cerebellar patients. ET patients exhibited no difficulties in coordinating fingers and the opposite thumb while grasping the object. Their finger touch latency did not differ from controls; this contrasts with previous studies demonstrating prolonged finger touch latency in cerebellar patients [35, 40].
Grasping forces behaved differently in ET patients as compared to cerebellar patients. ET patients applied low grip forces in the precision grip task. We could not observe a relationship between the degree of tremor and/or ataxia and grip forces. Decreased grip forces in ET patients were reported earlier [25]. Most studies in cerebellar patients found increased forces [28, 39, 41]. However, others reported normal grip forces [39, 41]. It seems likely that the cause, location, and severity of cerebellar impairment influence grip force levels. Whereas patients with cerebellar infarctions apply low to normal grip forces [39, 41], these forces are increased in patients with more diffuse cerebellar degeneration [28, 35, 39, 42].
In the predictive grip force control experiment, ET patients who showed higher ataxia scores applied higher grip forces. This correlation with the clinical ataxia score, but not with the tremor score, may indicate cerebellar dysfunction. It seems possible that ET patients at the beginning of the disease apply lower grip forces, which increase as the disease progresses. Furthermore, increase of grip forces may become more obvious in more demanding tasks. Potentially, disease progression may imply increased involvement of the cerebellum.
Overall, predictive grip force control was preserved in ET patients. Yet, patients more profoundly affected performed worse. As outlined above, our patients were generally mildly affected. Disorders in predictive grip force control, which are typically observed in cerebellar patients [28], may be more prominent in more severely affected cerebellar patients.
Finally, independent finger movements and forces in ET patients were largely preserved. The force enslaving effect was increased if the index finger was active and also in more affected patients. This corroborates findings in cerebellar patients, which present only minor impairment in the control of force enslaving effects [29].
The mild abnormalities observed in the present study are consistent with cerebellar dysfunction but are not exclusive to cerebellar disorders. Prolonged movement times in grip formation and lifting of objects have also been observed in patients with Parkinson disease (PD) [43–45]. One of the main findings in PD is increased grip force [46, 47], though normal forces have been observed as well [45]. An elevated peak negative load force is also seen in PD patients [48]. We found a similarly increased peak negative load force in clinically more severely affected ET patients. Although the main pathology in PD is in the basal ganglia, the similar findings might be a result of PD dysfunction in the cerebello-thalamo-cortical pathway. Thus, the cerebellum might also be involved in PD (as a pathway) [49].
Conclusion
The observed mild deficits in hand and finger movement tasks are consistent with cerebellar dysfunction in ET. However, findings need to be confirmed in future studies examining more severely affected ET patients.
References
Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41.
Chandran V, Pal PK. Quality of life and its determinants in essential tremor. Parkinsonism Relat Disord. 2013;19:62–5.
Louis ED. Functional correlates of lower cognitive test scores in essential tremor. Mov Disord. 2010;25:481–5.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307.
Chandran V, Pal PK. Essential tremor: beyond the motor features. Parkinsonism Relat Disord. 2012;18:407–13.
Quattrone A, Cerasa A, Messina D, Nicoletti G, Hagberg GE, Lemieux L, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR Am J Neuroradiol. 2008;29:1692–7.
Nicoletti G, Manners D, Novellino F, Condino F, Malucelli E, Barbiroli B, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74:988–94.
Benito-León J, Alvarez-Linera J, Hernández-Tamames JA, Alonso-Navarro H, Jiménez-Jiménez FJ, Louis ED. Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. J Neurol Sci. 2009;287:138–42.
Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol. 1997;41:32–40.
Klein JC, Lorenz B, Kang JS, Baudrexel S, Seifried C, van de Loo S, et al. Diffusion tensor imaging of white matter involvement in essential tremor. Hum Brain Mapp. 2011;32:896–904.
Louis ED, Shungu DC, Mao X, Chan S, Jurewicz EC. Cerebellar metabolic symmetry in essential tremor studied with 1H magnetic resonance spectroscopic imaging: implications for disease pathology. Mov Disord. 2004;19:672–7.
Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord 28:1759–1761.
Louis ED. Essential tremor: a common disorder of Purkinje neurons? Neuroscientist. 2015 [Epub ahead of print]
Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–7.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Rajput A, et al. Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord. 2009;24:1600–5.
Louis ED, Benito-León J, Bermejo-Pareja F, Neurological Disorders in Central Spain (NEDICES) Study Group. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol. 2007;14:1138–46.
Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. 2001;124:2278–86.
Fernandez KM, Roemmich RT, Stegemöller EL, Amano S, Thompson A, Okun MS, et al. Gait initiation impairments in both essential tremor and Parkinson’s disease. Gait Posture. 2013;38:956–61.
Roemmich RT, Zeilman PR, Vaillancourt DE, Okun MS, Hass CJ. Gait variability magnitude but not structure is altered in essential tremor. J Biomech. 2013;46:2682–7.
Kronenbuerger M, Konczak J, Ziegler W, Buderath P, Frank B, Coenen VA, et al. Balance and motor speech impairment in essential tremor. Cerebellum. 2009;8:389–98.
Helmchen C, Hagenow A, Miesner J, Sprenger A, Rambold H, Wenzelburger R, et al. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126:1319–32.
Farkas Z, Szirmai I, Kamondi A. Impaired rhythm generation in essential tremor. Mov Disord. 2006;21:1196–9.
Avanzino L, Bove M, Tacchino A, Ruggeri P, Giannini A, Trompetto C, et al. Cerebellar involvement in timing accuracy of rhythmic finger movements in essential tremor. Eur J Neurosci. 2009;30:1971–9.
Kronenbuerger M, Gerwig M, Brol B, Block F, Timmann D. Eyeblink conditioning is impaired in subjects with essential tremor. Brain. 2007;130:1538–51.
Stani TM, Burchiel KJ, Hart MJ, Lenardo DP, Anderson VC. Effects of DBS on precision grip abnormalities in essential tremor. Exp Brain Res. 2010;201:331–8.
Anderson VC, Burchiel KJ, Hart MJ, Berk C, Lou JS. A randomized comparison of thalamic stimulation and lesion on self-paced finger movement in essential tremor. Neurosci Lett. 2009;462:166–70.
Glickstein M, Waller J, Baizer JS, Brown B, Timmann D. Cerebellum lesions and finger use. Cerebellum. 2005;4:189–97.
Brandauer B, Timmann D, Häusler A, Hermsdörfer J. Influences of load characteristics on impaired control of grip forces in patients with cerebellar damage. J Neurophysiol. 2010;103:698–708.
Brandauer B, Hermsdörfer J, Geissendörfer T, Schoch B, Gizewski ER, Timmann D. Impaired and preserved aspects of independent finger control in patients with cerebellar damage. J Neurophysiol. 2012;107:1080–93.
Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res. 2003;142:171–88.
Deuschl G, Bain P, Brin M. Consensus statement of the movement disorder society on tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13 Suppl 3:2–23.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113.
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–11.
Fahn S, Tolosa E, Marín C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Baltimore: Williams & Wilkins; 1993. p. 225–34.
Brandauer B, Hermsdörfer J, Beck A, Aurich V, Gizewski ER, Marquardt C, et al. Impairments of prehension kinematics and grasping forces in patients with cerebellar degeneration and the relationship to cerebellar atrophy. Clin Neurophysiol. 2008;119:2528–37.
Marquardt C, Hermsdörfer J, Mai N. 3DA—three-dimensional motion analysis. Operating manual. München: MedCom; 1994.
Marquardt C, Mai N. A computational procedure for movement analysis in handwriting. J Neurosci Methods. 1994;52:39–45.
Rand MK, Shimansky Y, Stelmach GE, Bracha V, Bloedel JR. Effects of accuracy constraints on reach-to-grasp movements in cerebellar patients. Exp Brain Res. 2000;135:179–88.
Fellows SJ, Ernst J, Schwarz M, Töpper R, Noth J. Precision grip deficits in cerebellar disorders in man. Clin Neurophysiol. 2001;112:1793–802.
Zackowski KM, Thach Jr WT, Bastian AJ. Cerebellar subjects show impaired coupling of reach and grasp movements. Exp Brain Res. 2002;146:511–22.
Küper M, Brandauer B, Thürling M, Schoch B, Gizewski ER, Timmann D, et al. Impaired prehension is associated with lesions of the superior and inferior hand representation within the human cerebellum. J Neurophysiol. 2011;105:2018–29.
Rost K, Nowak DA, Timmann D, Hermsdörfer J. Preserved and impaired aspects of predictive grip force control in cerebellar patients. Clin Neurophysiol. 2005;116:1405–14.
Artieda J, Pastor MA, Lacruz F, Obeso JA. Temporal discrimination is abnormal in Parkinson’s disease. Brain. 1992;115(Pt 1):199–210.
Nowak DA, Hermsdörfer J. Objective evaluation of manual performance deficits in neurological movement disorders. Brain Res Rev. 2006;51:108–24.
Benice TS, Lou JS, Eaton R, Nutt J. Hand coordination as a quantitative measure of motor abnormality and therapeutic response in Parkinson’s disease. Clin Neurophysiol. 2007;118:1776–84.
Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson’s disease. Brain. 1998;121:1771–84.
Fellows SJ, Noth J. Grip force abnormalities in de novo Parkinson’s disease. Mov Disord. 2004;19:560–5.
Wenzelburger R, Kopper F, Zhang BR, Witt K, Hamel W, Weinert D, et al. Subthalamic nucleus stimulation for Parkinson’s disease preferentially improves akinesia of proximal arm movements compared to finger movements. Mov Disord. 2003;18:1162–9.
Hallett M. Tremor: pathophysiology. Parkinsonism Relat Disord. 2014;20 Suppl 1:118–22.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solbach, K., Mumm, M., Brandauer, B. et al. Prehension Kinematics, Grasping Forces, and Independent Finger Control in Mildly Affected Patients with Essential Tremor. Cerebellum 15, 498–508 (2016). https://doi.org/10.1007/s12311-015-0717-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0717-1