Abstract
Isolated central nervous system post-transplant lymphoproliferative disorder (ICNS-PTLD) in pediatric patients is a distinctly rare entity without accepted diagnostic criteria or treatment recommendations. We present a case of an 8-year-old male with a prior history of a kidney transplant who developed and ICNS-PTLD. We highlight the pathobiology and diagnostic features with a brief review of the literature on these rare cases. There is a complex interplay between CD30, Epstein Barr Virus and MYC as part of lymphocyte transformation leading to PTLD. In the appropriate clinical setting, CD30 and EBV positivity along with normal MYC expression are highly predictive of CNS-PTLD over a Primary CNS lymphoma. ICNS-PTLD has only been rarely reported in children. The faithful diagnosis is necessary for prognostication and to accrue data for treatment recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-transplant lymphoproliferative disorders (PTLD) are neoplasms that arise in the setting of iatrogenic immunosuppression after a heterologous transplant of solid or hematopoietic organs. Their clinical setting defines them, and they are designated into categories based on histologic, immunophenotypic, and genetic findings from early lymphoproliferative lesions to frank lymphomas. The overall incidence is small, occurring in less than 1.5% transplants. In decreasing order, multiviscera, small bowel, heart, kidney, and bone marrow transplants are most common transplants to precede PTLDs. The biggest risk factor is if the recipient is immune-naïve to Epstein Barr Virus (EBV). This single factor alone increases the risk 10- to 75-fold, although EBV is detected in only 66–86% of cases.
PTLDs can involve nearly any organ and the CNS shows involvement in 3–15% of patients. Isolated PTLD in the central nervous system (CNS) (ICNS-PTLD) is distinctly rare. There are several case series, but the rarity precludes a meaningful estimation of incidence. These studies do observe trends. The brain is more frequently secondarily involved. The more commonly transplanted organ is the kidney, and there is a high incidence of EBV detection. Additionally, there is some evidence to suggest that azathioprine-based immunosuppressives are more likely than cyclosporine-based medications to be associated with CNS involvement [1, 2].
The reports of pediatric ICNS-PTLD are even fewer, mostly reported as single case reports. The distinction between isolated and systemic PTLD may be of prognostic significance as one large study reported a 29% survival rate in ICNS-PTLD and no survival in systemic cases [3]. In this paper, we present the case of an 8-year-old male with ICNS-PTLD, and we highlight the pathobiology and the diagnostic features of this very rare entity. Currently, there is no consensus on the diagnosis or treatment of ICNS-PTLD in children, but appropriate and faithful diagnosis is paramount to accruing data for treatment recommendations and determining prognosis.
Materials and methods
The clinical records were reviewed for pertinent clinical, radiological, and treatment data. Immunohistochemistry (IHC), in situ hybridization (ISH), and fluorescent in situ hybridization (FISH) were performed on formalin-fixed paraffin embedded tissue in CLIA-certified clinical laboratories. All controls were appropriate. IHC was performed on an Leica automatic stainer using the following antibodies CD5 (4C7, 1:100, Leica Biosystems, Buffalo Grove, IL), CD45 (2B11 + PD7/26, 1:400, Agilent/Dako, Santa Clara, CA), CD20 (L26, 1:250, Agilent/Dako, Santa Clara, CA), PAX5 (BC/24, 1:40, Biocare Medical, Pacheco, CA), CD30 (Ber-H2, 1:20, Agilent/Dako, Santa Clara, CA), MUM1 (BC5, 1:100, Biocare Medical, Pacheco, CA), CD10 (56C6, 1:20, CellMarque), BCL6 (PG-B6p, 1:50, Agilent/Dako, Santa Clara, CA), MYC (Y69, 1:100, Biocare; performed at ProPath Laboratories, Dallas, TX). Epstein Barr Virus ISH (Novocastra™ ISH probes, Leica Biosystems, Buffalo Grove, IL). Fluorescent in situ hybridization (FISH) was performed using the following probes from Abbott Molecular, Des Plaines, IL: Vysis LSI BCL6 (ABR) (3q27) dual color break apart rearrangement probe, Vysis LSI MYC (8q24) dual color break apart rearrangement probe, Vysis LSI IGH/BCL2 (14q32.3/18q21.3) dual color, dual fusion translocation probe. More than 100 nuclei were examined per probe set.
Clinical history
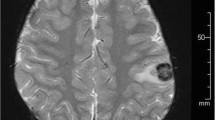
The patient, an 8 year-old male, underwent a living related renal transplant for end stage kidney disease secondary to nephronophthisis/medullary cystic kidney disease. Approximately 3 years later, the patient presented to the Emergency Department with leg pain progressing to leg twitching and eventually left-sided weakness. He was found to be acutely hypertensive, in addition to having decreased grip strength on the left, decreased plantar flexion strength, and an inability to hold leg elevated. Magnetic resonance imaging was performed, which indicated a 2.3 × 2.0 cm peripherally enhancing intraparenchymal mass centered within the right frontal lobe with extensive surrounding vasogenic edema. Excisional biopsy was consistent with monomorphic B-cell PTLD.
Pathological examination
Examination of the histologic sections revealed an infiltration of large cells into brain matter. Focal areas showed vascular cuffing. The infiltrating cells showed increased nuclear size with markedly irregular contours and occasionally prominent, multiple nucleoli. The infiltrating malignant cells were immunoreactive for CD45, CD20, PAX-5, CD30, and MUM1 and negative for MYC, CD10, BCL6, and CD5. The EBER-ISH was also positive. (Fig. 1).
Histologic and immunophenotypic findings of the intraparenchymal brain mass. a representative low magnification image of the atypical infiltrate shows atypical discohesive cells arranged in a perivascular pattern. b A representative high magnification image of the atypical infiltrate shows large atypical discohesive cells with irregular nuclei and focally prominent nucleoli. c CD20 immunohistochemical stain confirms B cell lineage of the atypical cells. d CD30 immunohistochemical stain highlights over 90% of the atypical cells. e Epstein-Bar Virus (EBV)-encoded RNA in situ hybridization highlights EBV infected nuclei. f MYC immunohistochemical staining is negative
Our initial evaluation on the morphology and immunophenotype was most compatible with a CD30 positive diffuse large B-cell lymphoma. On further workup, the patient’s cerebrospinal fluid sample was found to be negative for malignant cells on cytologic examination and a bilateral bone marrow biopsies demonstrated hypocellular bone marrow with no morphologic or flow cytometric evidence of B-cell non-Hodgkin lymphoma. Further imaging failed to reveal systemic disease, confirming the diagnosis of ICNS-PTLD.
Discussion
From a pathobiologic perspective, PTLD is attributed to a clearly defined etiology. EBV-transformed lymphocytes proliferate as a result of impaired T cell immunosurveillance secondary to post-transplant immunosuppressive therapy, particularly tacrolimus and OKT3 that disable T cell functions. However, according to the World Health Organization definition, PTLD is defined diagnostically as “any lymphoid or plasmacytoid proliferation that develops in immunosuppressed recipients of solid organ, bone marrow, or stem cell allografts,” and EBV positivity is not required for the diagnosis of PTLD [4]. This EBV negative population may be attributed to other virally-induced transformation of lymphocytes or variable penetrance of EBV molecular markers. Nevertheless, based on a multiple center study, 83% are histologically monomorphic and 94% of primary CNS PTLD are EBV positive [1]. However, CNS-PTLD appears with a relatively lower EBV load compared with PTLD involving loci outside CNS [5].
When the CNS is the only site involved by PTLD, as the case we report here, it is critical to be differentiated from primary CNS lymphoma (PCNSL) or other CNS lesions associated with immunosuppression. Although it is usually obvious to consider PTLD when a clear clinical history of post-transplant immunosuppressive therapy is present, it is desirable to define a molecular or pathological feature to confirm the diagnosis. Both CNS PTLD and lymphoma may exhibit transformed immunoblast or centroblast-like large cells, and perivascular distribution. Although CNS PTLD is more likely associated with sheet-like growth, architectural patterns are frequently distorted in fragmented CNS biopsies [4].
According to a recently published study that compares clinicopathologic features of 11 CNS PTLD cases with 17 cases of PCNSL, the statistically significant factors are EBV ISH (100% positive in CNS PTLD vs 0% in PCNSL), CD30 (100% in CNS PTLD vs 0% in PCNSL) and MYC (13% positive CNS PTLD vs 80% positive in PCNSL) [6]. Therefore, the cardinal immunophenotypic feature for CNS PTLD is positive EBV and CD30 combined with normal level of MYC protein expression. The case presented in this article is immunoreactive for EBV and CD30, negative for MYC, and parallel fluorescent in situ hybridization testing confirmed normal MYC (not rearranged). It is important to note that while the CD30 is negative in PCNSL, CD30 can be positive in a subset of DLBCL [6].
The tight association between EBV and CD30 is not surprising as EBV infection substantially enhances CD30 expression [7]. The viral induction of CD30 is not limited to EBV but also seen in hepatitis B, C and HIV [8, 9]. CD30 is a member of the TNF receptor family and biologically functions as signal transduction molecule inducing NF-kb expression and subsequently inhibits cell apoptosis. The pro-proliferative and anti-apoptotic role of CD30 may explain the association with poor outcome in DLBCLs that are positive for CD30 [10].
Positive EBV associated with negative MYC protein expression appears to be a relatively unique feature in CNS PTLD, although a subset can exhibit EBV and MYC upregulation [6]. For example, EBV is associated with upregulated MYC activity in endemic Burkitt lymphoma [11]. Experimental studies demonstrated that LMP1, a component of EBV, activates multiple signaling pathways and enhances expression of BCL-2 and c-MYC [12]. However, a revisit of Burkitt lymphoma suggested that the association of EBV and MYC upregulation is not due to the direct upregulation of c-MYC by EBV. Instead, c-MYC activates both proliferative and apoptotic machinery and the cellular outcome of proliferation is determined by the relative weight of these two opposite sets of machinery [13]. EBV suppresses the apoptotic machinery (e.g., Bim) downstream to MYC and shifts the balance towards proliferation [11]; therefore, the association of EBV and MYC is context dependent. We speculate that in CNS PTLD, MYC positivity is not a defining marker and probably does not significantly contribute to cell proliferation.
In summary, our case and an analysis of literature provide a pathologic perspective suggesting that CNS PTLD can be reliably diagnosed and differentiated from primary CNS lymphoma based on unique set of immunophenotypic markers in addition to clinical history. Additionally, the faithful diagnosis of ICNS-PTLD is important for treatment decisions and prognostication.
References
Evens AM, Choquet S, Kroll-Desrosiers AR, Jagadeesh D, Smith SM, Morschhauser F, Leblond V, Roy R, Barton B, Gordon LI, Gandhi MK, Dierickx D, Schiff D, Habermann TM, Trappe R (2013) Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transp: Off J Am Soc Transp Am Soc Transp Surg 13(6):1512–1522. https://doi.org/10.1111/ajt.12211
Cavaliere R, Petroni G, Lopes MB, Schiff D, International Primary Central Nervous System Lymphoma Collaborative G (2010) Primary central nervous system post-transplantation lymphoproliferative disorder: an international primary central nervous system lymphoma collaborative group report. Cancer 116(4):863–870. https://doi.org/10.1002/cncr.24834
Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P, First MR, Woodle ES (2005) Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc 37(2):954–955. https://doi.org/10.1016/j.transproceed.2004.12.130
Swerdlow SH CE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Verdiman J.W. (Eds): (2008) WHO classification of tumours of haematopoietic and lymphoid tissue. IARC, Lyon
Gheorghe G, Radu O, Milanovich S, Hamilton RL, Jaffe R, Southern JF, Ozolek JA (2013) athology of central nervous system posttransplant lymphoproliferative disorders: lessons from pediatric autopsies. Ped Dev Pathol: Off J Soc Ped Pathol Paed Pathol Soc 16(2):67–73. https://doi.org/10.2350/12-01-1148-OA.1
Sundin A, Grzywacz BJ, Yohe S, Linden MA, Courville EL (2017) B-cell posttransplant lymphoproliferative disorder isolated to the central nervous system is Epstein-Barr virus positive and lacks p53 and Myc expression by immunohistochemistry. Hum Pathol 61:140–147. https://doi.org/10.1016/j.humpath.2016.12.007
Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, Martelli MF, Stein H (1995) CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 85(1):1–14
Pizzolo G, Vinante F, Nadali G, Krampera M, Morosato L, Chilosi M, Raiteri R, Sinicco A (1997) High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol 108(2):251–253. https://doi.org/10.1046/j.1365-2249.1997.d01-1005.x
Younes A, Aggarwall BB (2003) Clinical implications of the tumor necrosis factor family in benign and malignant hematologic disorders. Cancer 98(3):458–467. https://doi.org/10.1002/cncr.11524
Maes B, Anastasopoulou A, Kluin-Nelemans JC, Teodorovic I, Achten R, Carbone A, De Wolf-Peeters C, Group EL (2001) Among diffuse large B-cell lymphomas, T-cell-rich/histiocyte-rich BCL and CD30+ anaplastic B-cell subtypes exhibit distinct clinical features. Ann Oncol: Off J Eur Soc Med Oncol 12(6):853–858. https://doi.org/10.1023/A:1011195708834
Allday MJ (2009) How does Epstein-Barr virus (EBV) complement the activation of Myc in the pathogenesis of Burkitt’s lymphoma? Semin Cancer Biol 19(6):366–376. https://doi.org/10.1016/j.semcancer.2009.07.007
Gires O, Kohlhuber F, Kilger E, Baumann M, Kieser A, Kaiser C, Zeidler R, Scheffer B, Ueffing M, Hammerschmidt W (1999) Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J 18(11):3064–3073. https://doi.org/10.1093/emboj/18.11.3064
McMahon SB (2014) MYC and the control of apoptosis. Cold Spring Harbor Perspect Med 4(7):a014407. https://doi.org/10.1101/cshperspect.a014407
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Han, P.C., Brewer, K.S., Olar, A. et al. Isolated central nervous system post-transplant lymphoproliferative disorder in a pediatric patient: a case report with pathobiological perspective. J Hematopathol 11, 9–12 (2018). https://doi.org/10.1007/s12308-017-0314-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-017-0314-y