Abstract
Purpose
A popular choice for lateral epicondylitis (LE), corticosteroid injections have been associated with prominent side effects, which has led to the conception of modalities like platelet-rich plasma (PRP). This randomised trial aimed to evaluate and compare the 6-week, 3-month and 1-year outcomes with PRP and corticosteroid injections in LE. We hypothesised that PRP would prove more effective in relieving pain and improving function.
Methods
At the sports medicine unit of our tertiary care teaching centre, 80 patients with LE were randomised into either receiving PRP (group A) or corticosteroids (group B) injections. Pre-injection visual analogue scale (VAS), disabilities of the arm, shoulder and hand (DASH) score, Mayo elbow performance score (MEPS) and grip strength score (GSS) were recorded. Common extensor origins were identified and infiltrated with 3 ml of either PRP or corticosteroid (triamcinolone in 2% xylocaine) using a peppering technique. Follow-up scores and extent of pain relief were recorded and compared.
Results
At 6 weeks, there were greater improvements in group B versus A in mean VAS (13.8 vs. 44.5; p < 0.001), DASH (64.2 vs. 53.3; p < 0.001), MEPS (88.0 vs. 74.5; p = 0.004) and GSS (89.3 vs. 73.4; p = 0.039). These scores showed a reversed pattern at 3 months when group A outcomes superseded group B (VAS p = 0.002; DASH p < 0.001; MEPS p = 0.002; GSS p = 0.045). At 1-year follow-up, group A continued to enjoy better pain relief and function (VAS p = 0.024; DASH p < 0.001; MEPS p = 0.009; GSS p = 0.028).
Conclusions
Albeit corticosteroid injections show good short-term results at 6 weeks, patients receiving PRP injections fare better at 3 and 12 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The commonest cause of upper limb pain, lateral epicondylitis (LE), annually affects 4–7 patients per 1000, mostly between 45 and 54 years [1]. Significant associations include dominant limbs (90%), sporting activities (“tennis elbow”), increasing age, body mass index (> 25), low social support, shoulder and wrist conditions, corticosteroid therapy and smoking [2]. Both inflammatory and degenerative (angiofibroblastic degeneration—tendinosis) pathologies have been implicated at the wrist-extensor origins (most commonly, the extensor carpi radialis brevis (ECRB) origin) [3]. A pragmatic clinical approach most often clinches the diagnosis which usually responds to conservative therapy (including local injections) [4].
Among injections, corticosteroids (CS) have been most widely espoused (60% studies), followed by botulinum toxin, autologous blood and platelet-rich plasma (PRP). Notwithstanding this, a consensus on the optimal injection is lacking. Questionable efficacy (beyond 8 weeks), post-injection tendon ruptures, joint pain, skin changes, subdermal atrophy, facial flushing and hyperglycaemia associated with CS injections have raised a few eyebrows [5,6,7]. The anti-inflammatory action of CS in suppressing a supposedly non-inflammatory process has also come into question [3].
Of proposed alternatives, autologous blood has been bedeviled with pain, reaction, and practical inconvenience of injecting 10–15 ml blood locally. A more plausible alternative, PRP, can augment the reparative process by delivering high concentrations (3–4 times) of the bioactive component of whole blood, viz. platelet through small (2–3 ml) infiltrations [5, 8,9,10,11]. Despite success, PRP use has been limited (12% of all elbow injections) by a paucity of randomised studies. Even fewer have evaluated and compared 1-year results of PRP and CS injections in LE [5, 12].
With the above in mind, this study aimed to measure the efficacy of PRP in LE and to compare the results with local CS injections in a randomised manner. It was hypothesised that PRP injections would prove more effective than CS injections in improving pain and function at 1-year follow-up.
Methods
With a prior institutional ethics committee approval (vide letter no. EC/06/16/1024), this prospective study assessing outcomes of LE managed by PRP (group A) and CS (group B) injections was conducted from July 2016 to June 2017 at the sports medicine unit of our tertiary care teaching centre. The randomised trial was conducted in accordance with the design and principles of the Consolidated Standards of Reporting Trials (CONSORT).
Trial design
A randomised (equal 1:1 randomisation), parallel-group, controlled design, which is the gold standard for evaluating the comparative efficacy of > 1 therapeutic interventions, was adopted. No changes were made to any of the methods throughout the trial.
Participants
Eighty patients aged 18–55 with diagnosed lateral epicondylitis, unresponsive to conservative therapy for > 3 months, were randomly recruited in each group (CS and PRP) after obtaining informed consents. Differential diagnoses of elbow pain (cervical radiculopathy and osteochondritis dissecans) and systemic conditions (rheumatoid disorders and diabetes) were excluded.
Study settings and details of interventions
All injections were performed as office outpatient procedures under strict aseptic conditions by a single senior fellowship-trained surgeon well versed in shoulder and elbow sports medicine surgery.
Pre-injection preparation
Patients were kept anti-inflammatory-analgesic-free for 2 weeks (to allow for relative washout of the drugs), and pre-injection scores were noted (Fig. 1).
PRP preparation
Out of 20 ml whole venous blood, 18 ml was transferred into 4 red-capped plain tubes (labelled 1, 2, 3 and 4) with 4.5 ml each, and 2 ml was used for cell counts. Two-spin centrifugation was adopted with the first at 160 g for 12 min at room temperature [17]. The sample segregated into three layers—upper plasma, middle buffy coat and lower red cell layer. Supernatant plasma and buffy coat from each tube (total 10–12 ml) were pipetted onto another set of red-capped plain tubes labelled A and B (5–6 ml per tube) under laminar flow. A second spin at 460 g for 18 min was then provided at room temperature. Platelet pellets were collected at the bottom of each tube. Around 3 ml of platelet-poor plasma was discarded, and roughly 2 ml of plasma with platelet pellets was thoroughly mixed in each tube. The final PRP thus obtained was around 4 ml and was transferred from both tubes into one plain tube labelled “PRP”, stored for 15 min at room temperature, and subjected to a platelet count (Fig. 2).
Injection technique
On a 90° flexed elbow and pronated forearm (passively stretched ECRB allowed clearer identification), the common extensor origin was identified, painted and draped. Bony landmarks (lateral epicondyle, supracondylar ridge, olecranon and radial head) were palpated; a 22G needle was introduced along the supracondylar ridge (proximal to lateral epicondyle) and gently advanced into the undersurface of the ECRB and the common extensor tendon using a peppering technique: single skin penetration and 10–20 tendon penetrations (without emerging from the skin). Repetitive puncturing of degenerating tissues, first described in 1964 for LE, initiates bleeding into tissues and hastens healing [18]. 3 ml of PRP and 40 mg triamcinolone with 2% xylocaine were injected in groups A and B (controls), respectively (Fig. 2).
Post-injection and rehabilitation protocol and follow-up
Sterile dressings were removed 2 days later. Discharged after a brief 30-min rest, all patients followed standardised rehabilitation (limb rest—3 days, need-based cold fomentation and oral paracetamol). Additional requirements were noted on subsequent office visits. Gentle range of movement (ROM) and isotonic exercises were prescribed after a week. Resistive training of wrist extensors using TheraBand (THERABAND Akron, OH) and rotator cuff and periscapular muscle exercises were started at 3 weeks. Follow-up clinical scores were recorded and compared at preoperative 6-week, 3-month, and 12-month visits (details below). Complete relief from initial symptoms was enquired for and responses were recorded.
Primary and secondary outcomes
A change in visual analogue scale (VAS) before and after the injections was recorded and was the primary outcome of the present study. As secondary outcomes, the disabilities of the arm, shoulder and hand (DASH) score, Mayo elbow performance score (MEPS) and grip strength score (GSS) obtained on a hydraulic hand dynamometer (BASELINE, NY, USA) were recorded onto a data collection form [14, 15] (Fig. 1). In addition, the serum and PRP samples of group A before and after PRP preparation, respectively, were subjected to platelet counts. Also, requirements of pain-relief medications and the feeling of “complete relief from pain” were measured in both groups following the injections.
Measuring GSS
Using the Southampton protocol for grip strength measurement, the patient’s midpronated forearm was positioned on an armchair (wrist neutral and overhanging) [16]. The participant was made to squeeze the hydraulic hand dynamometer (BASELINE, NY, USA) for as long and as tightly as possible or until the maximum reading (in lbs) had appeared. The patient was asked to relax. Two further readings were similarly obtained at 10-min intervals, and mean values were computed as the GSS (Fig. 1).
Sample size calculation
Mean VAS, the primary outcome variable, for groups A (1.6) and B (2.8) (from published literature), was employed to calculate the sample size [13]. Initially calculated at 66 (power 90%, α = 0.05, s ~ 1.5), assuming a 20% dropout rate, the total sample size was finally set as 80 (40 per group).
Randomisation sequence generation, allocation concealment, implementation and blinding
A computer-generated random sequence was utilised, and letters “A” (PRP) and “B” (CS) placed in identical, opaque, sealed and stapled envelopes by an independent researcher (not involved with the care of the patients) to minimise selection bias. The allocation sequence was concealed from the surgeon, and the envelopes were only opened at the time of allocation of intervention. To prevent subversion of the allocation sequence, the details of the patients (name, date of birth and hospital number), once allocated, were recorded on each envelope and its containing card and stored in a safe isolated locker for later correlation and verification. The need for patient identification during blood withdrawal for PRP injections made the study a non-blinded one.
Statistics
The primary endpoint was a change in the VAS at 6-week, 3-month and 1-year intervals. Secondary endpoints included the changes in DASH, MEPS and GSS measured at similar intervals. The Statistical Package for the Social Sciences (SPSS v.20) was utilised, and continuous variables were compared using the Student’s t test. Nominal, categorical data between the groups were analysed with the Chi-square test or Fisher’s exact test as appropriate. Non-normally distributed continuous variables were compared using the Mann–Whitney U test (p < 0.05 considered significant).
Results
Participant flow, losses, recruitment
All 80 patients deemed eligible for the study between July 2016 and June 2017 were randomly allocated into groups A and B of equal size (n = 40 each) based on the allocation procedure described. There were no losses to follow-up (Fig. 3).
Baseline data
Among 46 ladies and 34 men (p = 0.11), the overall mean age was 40.8 years. Both groups were comparable in terms of age, involved sides, hand dominance, duration of symptoms and occupation. No dropouts made all patients available for follow-up (Table 1).
Outcomes
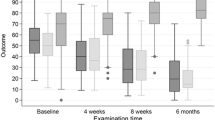
Mean pre-injection (0-week) VAS for both groups was similar (p = 0.285) (Table 2). Mean platelet concentrations in the whole blood and the prepared PRP in group A patients were 193.1 × 103/µL and 823.3 × 103/µL, respectively, with an approximate 4.3-fold increase in the concentration following PRP preparation. Cold compress and/or paracetamol tablets were needed for 80% group A patients (~ 3 days) for pain versus 25% in group B (p = 0.001). At 6 weeks, greater (p < 0.001) improvement in mean VAS was seen in group B (13.75) versus group A (44.5), which, however, reversed at 3 months (4 in group A versus 22.75 in group B; p = 0.002) (Table 2, Fig. 4).
Similar improvements were observed in mean DASH scores with comparative baseline values (p = 0.417), greater improvements in group B at 6 weeks (p < 0.001) but group A better at 3 months post-injection (p < 0.001). Likewise, data for mean MEPS and GSS, along with VAS and DASH scores, demonstrated identical patterns on follow-up (Table 2, Fig. 4). “Complete relief of pain” at 6 weeks in patients of groups A and B was seen in 5% and 55%, respectively (p = 0.001). The values, however, reversed at 3 months and were, respectively, 65% and 30% (p = 0.027). At 3 months, the number of recurrences in groups A and B was 2 (5%) and 10 (25%), respectively (p = 0.012). No major adverse effects were reported in any patient.
At 1-year follow-up, the trend of significantly better performance and scores with PRP injections continued to prevail (VAS p = 0.024; DASH p < 0.001; MEPS p = 0.009; GSS p = 0.028) (Table 2). When asked about complete pain relief, 85% and 20% patients of groups A and B, respectively, answered “yes” (p < 0.001).
Discussion
Findings of the present study indicate significant improvement in pain (VAS, DASH, MEPS) and function (DASH, MEPS, GSS) following injection therapy for LE. The hypothesis of superior pain relief and functional improvement with PRP injections compared to CS injections at 1-year follow-ups proved true. The effectiveness, in all aspects, was observed to be more rapid in onset with CS injections, while PRP injections had a slower, yet more well sustained impact.
Mean ages of 42.4 and 39.6 years in groups A and B, respectively, were comparable with previously published literature (mean age = 43) [9, Table 3]. More women (57.5% statistically insignificant) were possibly seen due to local societal framework wherein most household chores (washing and wringing clothes, cutting, chopping and peeling vegetables, carrying heavy, grocery-laden bags) have been conventionally and predominantly performed by women [19]. Such activities naturally predispose to repetitive elbow stresses, microtrauma, both relevant for the initiation and propagation of LE.
Popular among 71% fellowship-trained upper limb surgeons, steroid elbow injections are being presently interrogated after reports incriminating them in delayed healing have surfaced. A questionable impact of anti-inflammatory properties in an essentially non-inflammatory pathology has also impelled scientists to discover newer and safer modalities [5, 7, 8]. Recently, attention has been garnered by a relatively underutilised eligible surrogate, PRP [5]. Among its 4 varieties [viz. pure and leucocyte PRP and pure and leucocyte platelet-rich fibrin (PRF)], L-PRP is presently most suited for LE [10]. Teeming with essential growth factors [platelet-derived growth factor, transforming growth factor-β, insulin-like growth factor and epidermal growth factor], activated platelets from PRP reinforce and intensify repair. An ensuing chain reaction effectuates cellular proliferation, recruitment, differentiation, angiogenesis and collagen-1 synthesis that augments tensile strengths of traumatised tendons [11].
A Polish randomised study elucidated prolonged pain relief and better function with PRP vis-a-vis steroids for LE at 1 year after intervention [20, Table 3]. Gossen et al. also observed sustained improvements 2 years after PRP injections [21]. Our findings are in agreement with the above as PRP recipients displayed gratifying outcomes at 1 year. The above-mentioned papers, however, did not evaluate an essential component of hand function, the grip strength, as an outcome measure. This has a pivotal role in hand functioning and is potentially impaired in LE and ECRB tendinopathies. Poor handgrip strength can (with slower force development and electromechanical delays) stall reaction times, imperil recurrence and engender substandard quality of life [22, 23]. In the present study, reliable improvements in GSS of group A patients translated into a substantial role of PRP in ameliorating the important hand function in LE patients.
Yadav et al. recently reported better VAS, QuickDASH and grip strength in 60 LE patients randomised between receiving PRP and CS injections, which is in line with our results. Nonetheless, by including another, elbow-specific score (namely the MEPS) in the present study (accounting for elbow pain, motion stability and function), it was possible to more convincingly demonstrate the implications of PRP and CS injections [13, Table 3]. Also, we differed from the above study as peppering was employed, which enables wider penetration and delivery of active products of PRP and reparative apparatus [18]. Perhaps contributory to successful outcomes observed in the present study, routine peppering should be performed for extra-articular injections in chronic tendinopathies [21].
In 90 LE patients randomly allocated to PRP, CS and saline injections, Seetharamaiah and colleagues reported significantly better outcomes and fewer complications at 6 months with PRP. The authors did acknowledge the lack of sample size calculation from their methodology to be a limitation along with only VAS and facial pain scale (FPS) being used to record results. In contrast, patients from the present study demonstrated additional improvements in function with PRP that could be illustrated with betterment of 4 different scores at 1 year after injection [7, Table 3]. In the above study, although the authors did record serum and PRP–platelet levels, the values and eventually utilised concentrations were not mentioned in the results. Our observed 4.3-fold (mean) increase in platelet concentration in PRP post-preparation (from 193.1 × 103/µL to 823.3 × 103/µL) was within the recommended range of optimum levels for use in promoting healing [24]. This is one singularly important parameter (that we found to be associated with good outcomes) has been overlooked by many in published literature.
Most authors in their studies, in fact, have looked at one or two scoring systems as the outcome measure (for instance, the DASH score), which despite exhaustive evaluation of functional abilities, does not take into account quality of pain relief, grip strength, ROM and stability that are accounted for by the VAS, MEPS and GSS [20, 25, 26, Table 3]. By including these in the present study, the initial (6 weeks) success of CS injections and eventual (12 months) superiority of PRP injections were more convincingly determined (Table 3).
Gautam et al. addressed certain deficiencies in methodology of existing literature by incorporating patient-reported measures such as the VAS, DASH, modified Mayo score and hand grip strength. The authors, however, randomised a relatively small sample (15 for each group), followed up patients for 6 months only and have again not mentioned details about grip strength measurement. In the same study, it took 6 months for PRP to overtake CS in terms of improvement in clinical score. Platelet concentrations at injection have neither been measured nor mentioned in their text [27, Table 3]. This is a cardinal, often underplayed determinant of successful outcomes in PRP recipients. Concentrations between 500 × 103/µL and 1000 × 103/µL most positively influence tendon healing through cellular proliferation, migration, collagen and matrix metalloproteinase production as per experimental and clinical studies [24]. While lower concentrations can have a suboptimal impact, excessively high platelet levels negatively and paradoxically influence cell growth and prove counterproductive [28]. Such a discrepancy could have been behind the delayed response observed by Gautam et al. In contrast, group A patients of the present study received measured amounts of PRP injections (mean concentration 823.3 × 103/µL) which was perhaps the reason for better VAS, DASH, MEPS and GSS scores of group A versus B at 3 months and 12 months versus 6 months in the aforementioned paper [27].
The strengths of the present study include a prospective, randomised controlled design, single-surgeon technique (reducing performance bias) and consideration of a diverse variety of primary and secondary outcome variables. Among a few limitations, a lack of blinding could have induced observer bias when evaluating outcomes. Blinding was not feasible due to the need for confirmatory verification of blood products in group A patients. Also, sealed envelopes could have potentially resulted in subversion of the allocated sequences through randomisation. This was, however, prevented through meticulous and confidential record-keeping. The risk of confounding factors present between both groups was addressed, at least partially, by randomisation and having discrete inclusion and exclusion criteria and similar demographic variables among both groups. Further reduction of these factors could have been best addressed by matching or performing injections in either arm of same patients (bilateral cases).
The present study has attempted to update existing practice of elbow surgery through a design commensurate with high level of evidence (randomised controlled trial). Although ultrasonographic measurements of tendon thickness were not done for financial constraints, persuasive 3-month and 1-year clinical outcomes across 4 scores with PRP injections serve as strong clinical proof of their impact on LE. CS injections, on the other hand, show encouraging results early on, while their efficacy fades beyond few weeks of injecting. Further trials with larger numbers can possibly reiterate these findings and consolidate the role of PRP in the current practice.
References
Hamilton PG (1986) The prevalence of humeral epicondylitis: a survey in general practice. J R Coll Gen Pract 36(291):464–465
Descatha A, Dale AM, Jaegers L, Herquelot E, Evanoff B (2013) Self-reported physical exposure association with medial and lateral epicondylitis incidence in a large longitudinal study. Occup Environ Med 70(9):670–673. https://doi.org/10.1136/oemed-2012-101341
Kraushaar BS, Nirschl RP (1999) Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am 81(2):259–278
Struijs PA, Kerkhoffs GM, Assendelft WJ, Van Dijk CN (2004) Conservative treatment of lateral epicondylitis: brace versus physical therapy or a combination of both-a randomized clinical trial. Am J Sports Med 32(2):462–469. https://doi.org/10.1177/0095399703258714
Krogh TP, Bartels EM, Ellingsen T, Stengaard-Pedersen K, Buchbinder R, Fredberg U, Bliddal H, Christensen R (2013) Comparative effectiveness of injection therapies in lateral epicondylitis: a systematic review and network meta-analysis of randomized controlled trials. Am J Sports Med 41(6):1435–1446. https://doi.org/10.1177/0363546512458237
Brinks A, Koes BW, Volkers AC, Verhaar JA, Bierma-Zeinstra SM (2010) Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord 13(11):206. https://doi.org/10.1186/1471-2474-11-206
Seetharamaiah VB, Gantaguru A, Basavarajanna S (2017) A comparative study to evaluate the efficacy of platelet-rich plasma and triamcinolone to treat tennis elbow. Indian J Orthop 51(3):304–311. https://doi.org/10.4103/ortho.IJOrtho_181_16
Niedermeier SR, Crouser N, Speeckaert A, Goyal KS (2018) A survey of fellowship-trained upper extremity surgeons on treatment of lateral epicondylitis. Hand (N Y) 1:1558944718770212. https://doi.org/10.1177/1558944718770212
Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J (2016) Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol 17(2):101–112. https://doi.org/10.1007/s10195-015-0376-5
Dhurat R, Sukesh M (2014) Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg 7(4):189–197. https://doi.org/10.4103/0974-2077.150734
Mishra A, Pavelko T (2006) Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med 34(11):1774–1778. https://doi.org/10.1177/0363546506288850
Kaux JF, Crielaard JM (2013) Platelet-rich plasma application in the management of chronic tendinopathies. Acta Orthop Belg 79(1):10–15
Tamimi FM, Montalvo S, Tresguerres I, Blanco Jerez L (2007) A comparative study of 2 methods for obtaining platelet-rich plasma. J Oral Maxillofac Surg 65(6):1084–1093. https://doi.org/10.1016/j.joms.2006.09.012
Okçu G, Erkan S, Sentürk M, Ozalp RT, Yercan HS (2012) Evaluation of injection techniques in the treatment of lateral epicondylitis: a prospective randomized clinical trial. Acta Orthop Traumatol Turc 46(1):26–29
Hudak PL, Amadio PC, Bombardier C (1996) Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med 29(6):602–608
Morrey BF, An KN, Chao EYS (1993) Functional evaluation of the elbow. In: Morrey BF (ed) The elbow and its disorders, 2nd edn. WB Saunders Co, Philadelphia, pp 1993–1995
Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40(4):423–429. https://doi.org/10.1093/ageing/afr051
Yadav R, Kothari SY, Borah D (2015) Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res 9(7):RC05-7. https://doi.org/10.7860/jcdr/2015/14087.6213
Mishra TK (1988) Make them equal partners in development. Yojana 32(9):18–20
Lebiedziński R, Synder M, Buchcic P, Polguj M, Grzegorzewski A, Sibiński M (2015) A randomized study of autologous conditioned plasma and steroid injections in the treatment of lateral epicondylitis. Int Orthop 39(11):2199–2203. https://doi.org/10.1007/s00264-015-2861-0
Gosens T, Peerbooms JC, van Laar W, den Oudsten BL (2011) Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med 39(6):1200–1208. https://doi.org/10.1177/0363546510397173
Chourasia AO, Buhr KA, Rabago DP, Kijowski R, Irwin CB, Sesto ME (2012) Effect of lateral epicondylosis on grip force development. J Hand Ther 25(1):27–37. https://doi.org/10.1016/j.jht.2011.09.003
Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C (2006) Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing 35(4):409–415
Giusti I, D’Ascenzo S, Mancò A, Di Stefano G, Di Francesco M, Rughetti A, Dal Mas A, Properzi G, Calvisi V, Dolo V (2014) Platelet concentration in plateletrich plasma affects tenocyte behavior in vitro. Biomed Res Int. https://doi.org/10.1155/2014/630870
Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T (2010) Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med 38(2):255–262. https://doi.org/10.1177/0363546509355445
Khaliq A, Khan I, Inam M, Saeed M, Khan H, Iqbal MJ (2015) Effectiveness of platelets rich plasma versus corticosteroids in lateral epicondylitis. J Pak Med Assoc 65(11 Suppl 3):S100–S104
Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S (2015) Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong) 23(1):1–5. https://doi.org/10.1177/230949901502300101
Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE (2004) Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 34(4):665–671. https://doi.org/10.1016/j.bone.2003.12.010
Acknowledgements
The authors would like to acknowledge and appreciate the contributions of Ms. Parul Takkar, our statistician, towards the statistical computation of our data and its analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prateek Kumar Gupta, Ashis Acharya, Vishesh Khanna, Sirshendu Roy, Kamini Khillan and Senthil Nathan Sambandam declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, P.K., Acharya, A., Khanna, V. et al. PRP versus steroids in a deadlock for efficacy: long-term stability versus short-term intensity—results from a randomised trial. Musculoskelet Surg 104, 285–294 (2020). https://doi.org/10.1007/s12306-019-00619-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-019-00619-w