Abstract
The objective was to test the effects of PGR on canola (Brassica napus L.) biochemistry including oil yield under drought stress. A two-year (Y1 and Y2) split plot field experiment on the basis of a randomized complete block design with three replications was conducted. The main factor was, drought stress levels, including irrigation after a reduction of 40 (D1), 60 (D2) and 80% (D3) of field capacity (FC) moisture, and the sub-factor was PGR including control (S1), soil application of humic acid (S2), foliar applications of amino acid (S3), fulvic acid (S4) or seaweed extract (S5), and the combination of all PGR (S6). Although drought stress significantly decreased plant chlorophyll contents (a, b and total), oil percentage and oil yield, PGR significantly increased them. The D3 treatment, compared with control, decreased crop oil yield by 48.67 and 35.29% in the first and second year, respectively. However, treatment Y2D3S6 significantly increased oil percentage (43.10%) compared with control (40.97%). The PGR increased seed oil yield, in D3, by a maximum of 254 kg ha-1. The PGR numerically (p ≤ 0.0886) increased proline to 6.14 mg g-1 LFW (Y1D3S6) compared with control (4.79 mg g-1 LFW). The PGR also significantly increased sugar content to 17.05 mg g-1 LFW, significantly different from the control (12.95 mg g-1 LFW). In conclusion, the tested PGR can improve the biochemical properties (quality) including oil yield of canola in drought stress conditions, which is of economic and health significance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food production may be limited for the world’s growing population in the next years. Accordingly, investigating the factors, which affect growth and yield of agricultural crops, especially in stress conditions, is one of the most important aspects of crop production (Fitton et al. 2019; Chmielewska et al. 2020). However, one important approach is to use sustainable methods, which may enhance plant growth and quality under different conditions including stress. Such methods are environmentally and economically recommendable as they reduce the use of chemical fertilization (Miransari 2011; Miransari and Mackenzie 2015). The use of biostimulants or plant growth regulators (PGR) including polysaccharides, vitamins, plant hormones, amino and organic acids is among such methods (Supraja et al. 2020; Bakhshian et al. 2022).
The oil seed plant, canola (Brassica napus L.), is an annual, long-day and cold-loving plant. It is one of the most important oil plants that is cultivated in different parts of the world due to its: (1) high production potential, (2) wide range of adaptation to climatic conditions, (3) high percentage and quality of the oil, and (4) relative tolerance to drought stress (Batool et al., 2022).
The plant is one of the most important industrial crop plants with valuable fatty acids and proteins containing amino acids required by the human body. The plant seed has 40–49% oil and 35–39% protein (Flakelar et al. 2015). Although improved cultivars of canola have a high yield potential, their growth and yield production decreases in stress conditions. Accordingly, more research is essential to illustrate the mechanisms controlling the plant growth under stress (Zhu et al. 2016).
The development of environmentally compatible agriculture in arid and semi-arid regions facing stresses such as drought is of great importance. Drought stress reduces plant growth, impairs nutrient uptake and damages plant physiological traits. Different methods can be used to alter plant physiological and biochemical properties leading to drought stress tolerance. Proper plant nutrition and new technologies are among such methods and play an important role in achieving sustainable agriculture under drought stress conditions (Ilyas et al. 2020; Zamani et al. 2020).
One of the promising approaches to overcome drought stress is the utilization of plant growth regulators (PGR) including substances other than chemical fertilizers, which can stimulate plant growth under different conditions including stress (Hosseini et al. 2020; Tahaei et al. 2022; Azizi et al. 2023). The PGR are metabolic enhancers that can be used to increase the effectiveness of common mineral fertilizers. In recent years, the use of plant growth stimulants in arid and semi-arid regions has increased due to their potential to reduce the overuse of chemical fertilizers and improve plant nutrient uptake (Bulgari et al. 2019).
The PGRs include different stimulants such as amino acids, seaweed extract, fulvic and humic acid. By chelating plant essential elements, such PGRs increase soil fertility, nutrient uptake and crop production. Research has indicated that amino acids increase plant tolerance to environmental stresses by adjusting ion transport and regulating stomatal opening and closure. Due to having vitamins, amino acids and growth hormones of cytokinin and auxin, and seaweed extract can also favorably affect plant growth (Battacharyya et al. 2015; Drobek et al. 2019; Mirbolook et al. 2021).
With respect to the above-mentioned details, and because there is little data, to our knowledge on the use of PGR affecting canola physiology in drought stress conditions, the present study was performed. The objective was to investigate the soil and foliar application of different PGR including organic acids and seaweed extracts on the biochemical properties of canola including canola oil in drought stress conditions.
Materials and methods
Experimental design
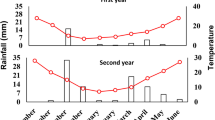
A split plot experiment on the basis of a randomized complete block design with three replications was conducted in 2018–2019 and 2019–2020 in Darab city, Fars province, Iran (54°34׳ E, 28°61׳ N, 1074 m above the sea level). Monthly rainfall and average minimum and maximum monthly temperatures during the experiment at Darab Synoptic Meteorological Station are presented in Table 1.
The experimental treatments consisted of drought treatments (main plots) including irrigation after depletion of 40 (D1), 60 (D2) and 80% (D3) of field capacity (FC) moisture, and plant growth regulators (PGR) (subplots) at a rate of 0.005 (5 kg per 1000 L water) including: (A) control (S1, without PGR), (B) soil application of humic acid (S2) in two different stages (second irrigation and the end of rosette phase), (C) foliar application of amino acids (S3), fulvic acid (S4), and seaweed extract (S5) at two different growth stages (the end of rosette phase and the beginning of flowering), and (D) the combination of PGR (S6) at the same time. The PGR were all obtained from a commercial source certified by the Iranian Soil and Water Research Institute. Before conducting the experiment, soil (0–30 cm) physicochemical properties were determined using the standard methods (Miransari et al. 2008, Table 2).
The field, before planting, was prepared at the FC moisture by plowing and disking. According to the experimental design, 54 plots of 2 × 6 m with a margin of 2 m were established. The field was fertilized according to soil analysis and farmers’ practices in the region. Accordingly, 100 kg ha-1 of triple superphosphate, potassium sulfate and urea were mixed with the soil before planting in both years. Supplementary amounts of urea (100 kg ha-1) at the end of rosette phase and at the start of flowering were used in each year. The suitable cultivar of the region (RGS003) was disinfected with Captan fungicide, and was planted in November 2018 and 2019 at the rate of 5 kg ha-1 by a seed planter (with a 5–7 cm distance on the rows spaced at 25 cm). Weed control was done by hand. The plants were harvested in May 2019 and 2020.
Drought treatments
All plots were equally irrigated after planting. The plots were treated with the irrigation treatments after the complete emergence of seedlings at the V2-V3 growth stage. Soil moisture was measured by the weighing method through repeated and daily soil sampling in the middle of each plot. The amount of water (Table 3) for irrigating each plot was calculated by considering the FC moisture, the plot area, and the depth of root development (Eq. 1).
In which θfc and θpwp are soil moisture at FC and permanent wilting point, respectively, t is the percentage of soil moisture depletion, ρ is soil bulk density, D is the depth of root development, A is plot area, and Ea is irrigation water efficiency. The number of irrigations, the volume of irrigation water and rainfall and the total volume of water used during the experiment are presented in Table 3.
Measurements
Chlorophyll contents
Chlorophyll a, b, and total chlorophyll were measured using mature leaves. Leaf samples were extracted by acetone and light absorption was measured using Vis 2100 spectrophotometer at 645 and 663 nm (Arnon 1949). Finally, chlorophyll values were calculated using Eqs. 2, 3 and 4. In the following equations, V is the sample volume, OD is the absorption rate and W is the wet weight of the sample.
Seed oil
The extraction and measurement of seed oil were done by grinding the grain sample. The powdered seeds, at 10 g, were wrapped in a filter paper, and placed in the Soxhlet device. Each sample was treated with 200 mL of n-hexan solvent and the device was switched on, and after four hours, the extracted oil sample was measured and the percentage of seed oil was reported (Mohammadpour et al. 2019). Finally, by multiplying oil percentage in the grain yield, the oil yield was calculated.
Proline content
The proline content was measured using 0.5 g of fresh leaf sample, which was mixed well with 10 ml of sulfosalicylic acid in a mortar and was then filtered. Two milliliters of the filtered solution was mixed with 2 mL of ninhydrin acid (a mixture of 1.25 g of ninhydrin in 30 ml of glacial sulfuric acid and 20 ml of phosphoric acid 6 M) and the sample was heated in a hot water bath at 100 °C for one hour. The sample was cooled down, mixed with 4 mL of toluene, and shaken for 15–20 min. Finally, the absorbance was read using a Vis 2100 spectrophotometer at the wavelength of 625 nm (Bates et al. 1973).
Soluble sugar
Soluble sugars, were measured by mixing 0.2 g of fresh leaf sample with 5 ml of 95% ethanol and then with 5 ml of 70% alcohol. The solution was centrifuged at 3500 g for 10 min, and 0.1 ml of it was treated with 3 ml of fresh anthrone, and the sample was placed in a hot water bath for 10 min. Finally, the sample was cooled down, and the absorbance was read at the wavelength of 625 nm using the Vis 2100 spectrophotometer (Nelson 1944).
Statistical analysis
Analysis of variance for different traits was performed using SAS statistical software version 9.3 (SAS Institute, USA). Mean comparison was also carried out using least significant different (LSD) test at P ≤ 0.05. The correlation of the measured parameters was determined using Pearson’s correlation method. The graphs were plotted by SAS Proc Plot.
Results
Analysis of variance
According to Table 4, the experimental treatments including year, drought and PGR significantly affected plant Chla, b and total. Although oil percentage was just significantly affected by drought stress, the oil yield was significantly affected by year, drought, PGR and the interaction of year and drought. Drought and the interaction of drought and year significantly affected plant proline content, and soluble sugar was just significantly affected by PGR (Table 4).
Chlorophyll contents
Plant chlorophyll contents including Chla (ranging from 1.11 to 2.95 mg g-1 LFW), b (ranging from 0.67 to 0.75 mg g-1 LFW) and total (ranging from 1.96 to 4.69 mg g-1 LFW) were significantly decreased by drought stress (Fig. 1). However, the use of PGR, specially S5 and S6 significantly increased plant Chla content compared with control (Fig. 2). The least and the highest Chla contents were resulted by Y1D3S4 (1.11 mg g-1 LFW), and Y2D1S2 (2.95 mg g-1 LFW) respectively. However, treatment Y2D3S6 increased Chla to 2.57 mg g-1 LFW significantly higher than the control (1.37 mg g-1 LFW) treatment (Table 5; Fig. 3).
Although drought stress significantly decreased plant Chlb (Fig. 1), the PGR treatments, especially S6 significantly enhanced Chlb related to the control (Fig. 2). Treatment Y2D3S1 (0.67 mg g-1 LFW) resulted in the least Chlb and treatments Y2D1S6 (1.75 mg g-1 LFW), Y2D1S3 (1.69 mg g-1 LFW), and Y2D2S6 (1.67 mg g-1 LFW) resulted in the highest Chlb content. Interestingly, treatment S3 enhanced Chlb content to 1.55 mg g-1 LFW, significantly different from the control treatment (0.67 mg g-1 LFW). The least and the highest Chlab values were resulted by treatments Y1D3S1 (1.96 mg g-1 LFW), and Y2D1S6 (4.69 mg g-1 LFW), respectively. Treatment Y2D3S6 significantly increased Chlab to 4.13 mg g-1 LFW compared with the control treatment (2.04 mg g-1 LFW) (Table 5; Fig. 3).
Seed oil
Drought stress at the highest level (D3) significantly decreased plant oil percentage (ranging from 40.60 to 45.20%) and yield (ranging from 702.67 to 1696.67 kg ha-1), compared with the control treatment (Fig. 1). However, the PGR treatments, especially S6, were able to significantly enhance seed oil percentage (Fig. 2). Treatment Y1D3S4 (40.60%) resulted in the least, and treatments Y1D1S3 (45.33%), Y2D1S3 (45.20%), and Y2D2S6 (44.73%) resulted in the highest oil percentage. Interestingly, treatment Y2D3S6 significantly increased oil percentage (43.10%) compared with control (40.97%) (Table 5; Fig. 4).
Although drought stress resulted in a significant reduction of seed oil yield (Fig. 1), the use of PGR, especially S6, significantly increased seed oil yield (Fig. 2). The least and the highest oil yields were related to treatments Y1D3S1 (702.67 kg ha-1), and Y2D1S6 (1696.67 kg ha-1) and Y1D1S6 (1675.67 kg ha-1), respectively. Interestingly, in the D3 treatments the use of S6 significantly increased oil yield, from 702.67 to 956.67 kg ha-1 in the first year, and from 895.67 to 1115.33 kg ha-1 in the second year (Table 5; Fig. 4).
Proline and soluble sugar
Drought stress significantly increased plant proline content (Fig. 1), and the use of PGR did not significantly affect proline (p ≤ 0.0886) (Fig. 2). The least and the highest proline contents were resulted by treatments Y1D1S1 (4.49 mg g-1 LFW), and Y1D3S6 (6.14 mg g-1 LFW), respectively. The use of PGR numerically increased proline to 6.14 mg g-1 LFW (Y1D3S6) compared with control (4.79 mg g-1 LFW) in the first year and to 5.39 mg g-1 LFW related to the control (4.75 mg g-1 LFW) in the second year (Table 5; Fig. 4).
Although plant sugar content was not significantly affected by drought stress (Fig. 1), the PGR treatments, especially S6, significantly increased sugar (Fig. 2). The sugar content was the least by treatment Y1D1S4 (12.27 mg g-1 LFW) and it was the highest by treatments Y1D3S6 (17.05 mg g-1 LFW), Y2D2S6 (16.99 mg g-1 LFW) and Y1D1S6 (16.96 mg g-1 LFW) (Table 5; Fig. 4). The use of PGR significantly increased sugar content to 17.05 mg g-1 LFW, significantly different from control (12.95 mg g-1 LFW) in the first year and to 16.04 mg g-1 LFW (Y2D3S6) compared with control (14.79 mg g-1 LFW) in the second year (Table 5; Fig. 4).
Correlation coefficients
Correlation coefficients indicated the measured parameters were significantly and positively correlated. Accordingly, while chlorophyll contents were significantly and positively correlated with seed oil percentage and seed oil yield, just Chla was positivity and significantly correlated with sugar content. Plant proline content was significantly and positively correlated with seed oil percentage and negatively and significantly with seed oil yield (Table 6).
Discussion
According to the results, different PGR treatments positively affected the biochemical properties of canola including chlorophyll contents, seed oil percentage and yield, and proline and sugar contents in drought stress conditions. The most effective treatment was the combination of all the tested PGR including humic acid, amino acid, fulvic acid, and seaweed extract (Layek et al. 2018).
The results indicated canola plants used different mechanisms to alleviate drought stress, the most important of which is osmotic regulation resulting increasing proline and sugar contents. The significant differences between the first and the second year significantly affected plant chlorophyll contents and oil yield. The interaction of drought and year was also significant in plant proline content and soluble sugar. Such differences may be a result of the climatic conditions in the two years, especially the higher rainfall in the second year (Table 1).
In drought stress conditions, the plant needs to increase compatible solutes, including proline and soluble sugars to maintain its regular functioning. If the availability and uptake of nutrients sufficiently increase in drought stress conditions, for example by using PGR, the production of compatible solutes also increases (Askarnejad et al. 2021; Tahaei et al. 2022; Azizi et al. 2023).
The accumulation of soluble sugars in drought stress conditions may improve plant growth and biochemical properties by the following mechanisms: (1) the regulation of cell volume, (2) reducing free radicals damage, (3) stability of enzymatic functioning, and (4) maintaining the structure of cellular membrane (Rezayian et al. 2018; Du et al. 2020; Raman et al. 2020).
Du et al. (2020) investigated the effects of drought stress on sugar metabolism in soybean (Glycine max L.) seedlings and indicated the following as the main reasons for the accumulation of soluble sugars: (1) increased carbohydrate metabolism, and (2) the expression of different genes including GmBAM1, GmAMY3, GmC-INV, GmSPS, and GmAMY3. The conclusion was that the soybean plants tolerated the stress by altering the allocation, transport, and metabolism of sugar. Accumulation of proline in the plant resulting from the activity of the related enzymes can also regulate osmotic regulation under environmental stresses (Ghaffari et al. 2019). Drought stress can reduce the activity of proline oxidase as a proline-degrading enzyme (Jiang and Asami 2018; Lee et al. 2019).
PGR may also affect plant growth and development by increasing the production of plant hormones such as auxin, cytokinin and gibberellin (Supraja et al. 2020). The authors investigated the effects of foliar algal extracts (20–100%) including 40.90% carbohydrates and 26.18% proteins acting as precursors of plant growth, on seed germination and seedling growth in tomato plants. They found the extract significantly increased seed germination and seedling growth.
Bijanzadeh et al. (2021) investigated the effects of humic acid (1.0 mM) and jasmonic acid (50 µM) on the biochemical properties of triticale. They found that the use of PGR significantly increased plant chlorophyll a (19.9%), b (21%), and proline content in drought stress conditions. The higher uptake of K+ resulted in higher plant chlorophyll contents. The conclusion was that the use of the tested PGR increased plant tolerance under drought stress by increasing proline content and the activities of antioxidant enzymes.
Decreased oil yield in drought stress conditions can be attributed to the effect of water stress on the reduction of grain yield (reduced production of photosynthates) and the capacity of grains for oil accumulation (Sabbahi et al. 2023). Drought stress also reduces the oil content by affecting the granulation stage and the length of the grain-filling period. However, the use of PGR increased the percentage and yield of canola oil, which can be due to increased photosynthesis, grain and oil yield, resulting by higher nutrient uptake (Jahani et al. 2021; Khaleghnezhad et al. 2021).
Due to the reduced transfer of assimilates in drought stress conditions, plant oil percentage and yield significantly decreased. However, the single or combined use of PGR significantly affected chlorophyll contents and biochemical properties (essential oil, proline and sugar contents) (Safian et al., 2022) in drought stressed canola.
Conclusion
Although drought stress significantly decreased the biochemical properties of canola including chlorophyll contents, oil seed percentage and oil seed yield, it increased proline content and did not affect plant sugar content. However, the use of PGR significantly increased plant biochemical properties even under severe drought stress. According to the results, the tested PGR, specially the combination of amino, humic and fulvic acids with seaweed extract significantly alleviated the unfavorable effects of drought stress on the biochemical properties of canola under field conditions. The mechanisms, which may contribute to the enhanced biochemical properties of canola in drought stress conditions, have been presented. The single and the combined use of the tested PGRs are recommendable for canola production in drought stress conditions as there were not any antagonistic effects when the combination of the PGRs were also tested.
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Askarnejad MR, Soleymani A, Javanmard HR (2021) Barley (Hordeum vulgare L.) physiology including nutrient uptake affected by plant growth regulators under field drought conditions. J Plant Nutr 44:2201–2217
Azizi MH, Soleymani A, Javanmard HA (2023) Wheat (Triticum aestivum L.) biochemical and nutritional properties affected by plant growth regulators under field drought conditions. Cereal Research Communications in Press, UK
Bakhshian M, Naderi MR, Javanmard HR, Bahreininejad B (2022) The growth of summer savory (Satureja hortensis) affected by fertilization and plant growth regulators in temperature stress. Biocatal Agric Biotechnol 43:102371
Bates LS, Waldren RP, Tear ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Batool M, El-Badri AM, Hassan MU, Haiyun Y, Chunyun W, Zhenkun Y, Jie K, Wang B, Zhou G (2022) Drought stress in Brassica napus: effects, tolerance mechanisms, and management strategies. J Plant Growth Regul
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hort 196:39–48
Bijanzadeh E, Emam Y, Pessarakli M (2021) Biochemical responses of water-stressed triticale (X Triticosecale wittmack) to humic acid and jasmonic acid. J Plant Nutr 44:252–269
Bulgari R, Franzoni G, Ferrante A (2019) Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 9:306
Chmielewska A, Kozłowska M, Rachwał D, Wnukowski P, Amarowicz R, Nebesny E, Rosicka-Kaczmarek J (2020) Canola/rapeseed protein–nutritional value, functionality and food application: a review. Crit Rev Food Sci Nutr 61:3836–3856
Drobek M, Frąc M, Cybulska J (2019) Plant biostimulants: importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—a review. Agronomy 9:335
Du Y, Zhao Q, Chen L, Yao X, Zhang W, Zhang B, Xie F (2020) Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem 146:1–12
Fitton N, Alexander P, Arnell N, Bajzelj B, Calvin K, Doelman J, Gerber J, Havlik P, Hasegawa T, Herrero M (2019) The vulnerabilities of agricultural land and food production to future water scarcity. Glob Environ Change 58:101944
Flakelar CL, Luckett DJ, Howitt JA, Dorana G, Prenzler PD (2015) Canola (Brassica napus) oil from Australian cultivars shows promising levels of tocopherols and carotenoids, along with good oxidative stability. J Food Compos Anal 42:179–186
Ghaffari H, Tadayon MR, Nadeem M, Cheema M, Razmjoo J (2019) Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol Plant 41:23
Hosseini P, Mohsenifar K, Rajaie M, Babaeinezhad T (2020) Improvement and regeneration of canola seeds (Brassica napus) with growth promoting compounds under different irrigation intervals. Iran J Seed Sci Res (Abstract English) 7:463–475
Ilyas M, Nisar M, Khan N, Hazrat A, Khan AH, Hayat K, Fahad S, Khan A, Ullah A (2020) Drought tolerance strategies in plants: a mechanistic approach. J Plant Growth Regul 40:926–944
Jahani F, Tohidi-Moghadam HR, Larijani HR, Ghooshchi F, Oveysi M (2021) Influence of zinc and salicylic acid foliar application on total chlorophyll, phenolic components, yield and essential oil composition of peppermint (Mentha piperita L.) under drought stress condition. Arab J Geosci 14:1–12
Jiang K, Asami T (2018) Chemical regulators of plant hormones and their applications in basic research and agriculture. Biosci Biotechnol Biochem 82:1265–1300
Khaleghnezhad V, Yousefi AR, Tavakoli A, Farajmand B, Mastinu A (2021) Concentrations-dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum moldavica L. under drought stress. Planta 253:1–18
Layek J, Das A, Idapuganti RG, Sarkar D, Ghosh A, Zodape ST, Lal R, Yadav GS, Panwar AS, Ngachan S (2018) Seaweed extract as organic bio-stimulant improves productivity and quality of rice in eastern Himalayas. J Appl Phycol 30:547–558
Lee BR, Islam MT, Park SH, Jung HI, Bae DW, Kim TH (2019) Characterization of salicylic acid-mediated modulation of the drought stress responses: reactive oxygen species, proline, and redox state in Brassica napus. Environ Exp Bot 157:1–10
Miransari M (2011) Soil microbes and plant fertilization. Appl Microbiol Biotechnol 92:875–885
Miransari M, Bahrami HA, Rejali F, Malakouti MJ (2008) Using arbuscular mycorrhiza to alleviate the stress of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol Biochem 40:1197–1206
Miransari M, Mackenzie AF (2015) Development of soil N testing for wheat production using soil residual mineral N. J Plant Nutr 38:1995–2005
Mirbolook A, Rasouli-Sadaghiani M, Sepehr E, Lakzian A, Hakimi M (2021) Synthesized zn (II)-amino acid and-chitosan chelates to increase Zn uptake by bean (Phaseolus vulgaris) plants. J Plant Growth Regul 40:831–847
Mohammadpour H, Sadrameli SM, Eslami F, Asoodeh A (2019) Optimization of ultrasound-assisted extraction of Moringa Peregrina oil with response surface methodology and comparison with Soxhlet method. Ind Crops Prod 131:106–116
Nelson N (1944) A photometric adaptation of the smoggy method for the determination of sugars. J Biol Chem 153:375–380
Raman H, Raman R, Mathews K, Diffey S, Salisbury P (2020) QTL mapping reveals genomic regions for yield based on an incremental tolerance index to drought stress and related agronomic traits in canola. Crop Pasture Sci 71:562–577
Rezayian M, Niknam V, Ebrahimzadeh H (2018) Differential responses of phenolic compounds of Brassica napus under drought stress. Iran J Plant Physiol 8:2417–2425
Sabbahi R, Azzaoui K, Rhazi L, Ayerdi-Gotor A, Aussenac T, Depeint F, Taleb M, Hammouti B (2023) Factors affecting the quality of canola grains and their implications for grain-based foods. Foods 12:2219
Safian N, Naderi MR, Torabi M, Soleymani A, Salemi HR (2022) Corn (Zea mays L.) and sorghum (Sorghum bicolor (L.) Moench) yield and nutritional quality affected by drought stress. Biocatal Agric Biotechnol 45:102486
Supraja K, Behera B, Balasubramanian P (2020) Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind Crops Prod 151:112453
Tahaei SA, Nasri M, Soleymani A, Ghooshchi F, Oveysi M (2022) Plant growth regulators affecting corn (Zea mays L.) physiology and rab17 expression under drought conditions. Biocatal Agric Biotechnol 41:102288
Zamani S, Naderi MR, Soleymani A, Nasiri BM (2020) Sunflower (Helianthus annuus L.) biochemical properties and seed components affected by potassium fertilization under drought conditions. Ecotoxicol Environ Saf 190:110017
Zhu M, Monroe JG, Suhail Y, Villiers F, Mullen J, Pater D, Hauser F, Jeon BW, Bader JS, Kwak JM (2016) Molecular and systems approaches towards drought tolerant canola crops. New Phytol 210:1169–1189
Acknowledgements
We would like to thank the Fars Agricultural and Natural Resources Research and Education Center, AREEO, Shiraz, Iran and the Islamic Azad University of Ahvaz, Iran, for their sincere assistance in conducting this research.
The authors would also like to thank very much the international publisher, AbtinBerkeh Scientific Ltd. Company (https://AbtinBerkeh.com), including AbtinBerkeh Academy (https://academy.abtinberkeh.com) Isfahan, Iran, for editing the manuscript and revising it according to the journal format.
Funding
There was not any funding for the present research.
Author information
Authors and Affiliations
Contributions
PH conducted the experiments, collected and analysed data, KM supervised the research, MR and TB co-supervised the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they do not have any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, P., Mohsenifar, K., Rajaie, M. et al. Plant growth regulators affecting canola (Brasica Napus L.) biochemistry including oil yield under drought stress. Physiol Mol Biol Plants 29, 1663–1674 (2023). https://doi.org/10.1007/s12298-023-01399-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-023-01399-1