Abstract
Cadmium (Cd) in soil–plant system can abridge plant growth by initiating alterations in root zones. Hydroponics and rhizoboxes are useful techniques to monitor plant responses against various natural and/or induced metal stresses. However, soil based studies are considered more appropriate in order to devise efficient food safety and remediation strategies. The present research evaluated the Cd-mediated variations in elemental dynamics of rhizospheric soil together with in planta ionomics and morpho-physio-biochemical traits of two differentially Cd responsive maize cultivars. Cd-sensitive (31P41) and Cd-tolerant (3062) cultivars were grown in pots filled with 0, 20, 40, 60 and 80 µg/kg CdCl2 supplemented soil. The results depicted that the maize cultivars significantly influenced the elemental dynamics of rhizosphere as well as in planta mineral accumulation under applied Cd stress. The uptake and translocation of N, P, K, Ca, Mg, Zn and Fe from rhizosphere and root cell sap was significantly higher in Cd stressed cv. 3062 as compared to cv. 31P41. In sensitive cultivar (31P41), Cd toxicity resulted in significantly prominent reduction of biomass, leaf area, chlorophyll, carotenoids, protein contents as well as catalase activity in comparison to tolerant one (3062). Analysis of tolerance indexes (TIs) validated that cv. 3062 exhibited advantageous growth and efficient Cd tolerance due to elevated proline, phenolics and activity of antioxidative machinery as compared to cv. 31P41. The cv. 3062 exhibited 54% and 37% less Cd bio-concentration (BCF) and translocation factors (TF), respectively in comparison to cv. 31P41 under highest Cd stress regime. Lower BCF and TF designated a higher Cd stabilization by tolerant cultivar (3062) in rhizospheric zone and its potential use in future remediation plans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is one of the most important staple cereal crop worldwide and is the third largest cultivated cereal in Pakistan. Heavy metal accumulation in agricultural soil has increased to a large extent due to industrialization which in turn cause health and environmental problems on global scale (Zhao et al. 2019). Cadmium (Cd) is naturally occurring toxic trace metal which is found at a concentration of 0.1–0.5 ppm in earth’s crust. Cadmium deposition in the agricultural soil from natural and anthropogenic activities not only leads to soil poisoning but also affects food quality (Ran et al. 2020).
Cadmium is non-essential for plants and humans but is a metal of high concern with regard to accumulation in food chain owing to its higher uptake rate by roots and acropetal transport (Cai et al. 2020). In plants, Cd-induced phytotoxicity extends at morphological, physiological and molecular levels which lead to stunted plant growth by disrupting the essential metabolic processes and by decimating the membrane integrity (Khanna et al. 2019). Cadmium stress initiates mechanisms of leaf necrosis as well as chlorosis and obstructs photosynthetic apparatus by reduced uptake of mineral nutrients and denaturation of photosynthetic enzymes, thereby leads to less plant biomass (Khan et al. 2019). Procreation of reactive oxygen species (ROS) i.e. hydroxyl radicle, hydrogen peroxide etc. was triggered by Cd stress which can dismantle essential biomolecules such as nucleic acids, lipids and proteins (Singh et al. 2019).
Being a divalent cation, Cd also competes for its transport with different nutrients at absorption site which in turn decreases the uptake of vital nutrients from soil i.e. Zn, Mn, Ca, Mg and Fe (Zhang et al. 2020). It is well recognized that soil–plant interface play an important role for the bioavailability of Cd to plant tissues (Qin et al. 2020). Plants hold strong ability to modify metal absorption in the rhizosphere through root exudates (Tao et al. 2020).
Plant roots uptake Cd through transporters of mineral nutrients such as Fe2+, Zn2+, Ca2+ and Mg2+ (Gallego et al. 2012). Uptake of essential nutrients from rhizosphere is influenced by Cd which triggers severe nutrient deficiency stress in plants (Qin et al. 2020). Antagonistic interaction between Cd and essential nutrient accumulation differ with plant genotype and Cd concentration in soil solution (Abbas et al. 2020).
Maize can tolerate certain levels of Cd without showing toxicity symptoms and can be cultivated on Cd polluted soils (Yang et al. 2014). However, metal tolerance capabilities vary amongst and within plant species based upon biomass accumulation (Muszyńska et al. 2017). Plants have established diverse intrinsic and extrinsic mechanisms to cope with heavy metal toxicity. These extrinsic strategies include complexation of metal ions with low molecular weight organic acids (LMWOAs), sequestration into root cell wall, organic acid based enhanced nutrient uptake in roots, rhizospheric microbial interaction, rhizospheric acidification/basification responses, precipitation and redox reactions (Han et al. 2018). Subsequently, variations in responses of maize cultivars may exist against Cd stress including the restriction of metal’s translocation from roots to shoot, cellular antioxidant enzymes based detoxification and sequestration, phytochelatins dependent metallic complexation and vacuolar compartmentalization which might play an important role in phytomanagement of metal contaminated soil for food safety (Yang et al. 2014). Furthermore, metallophytes may also own various categories of metal transporters that are involved in complex interaction between Cd accumulation and nutrients homeostasis in soil/plant tissue which impart Cd tolerance by increasing accumulation of essential ions.
The Cd deposition ranges from 0.02 to 184 mg/kg (normal soil) to 1.14–24.34 mg/kg (mining areas of Sindh) in Pakistan (Waseem et al. 2014). Furthermore, in urban and industrial wastewater of Faisalabad, Lahore and Karachi regions in Pakistan, Cd concentration ranges from 0.18 to 5.35 mg/l which is higher than the permitted value (0.10 mg/l) given by NEQS-Pak (Mahmood and Malik 2014). The increased levels of Cd in biotic systems across Pakistan threaten the plant and human health. The selection and cultivation of Cd tolerant crops is practicable and cost-effective approach to minimize the influx of Cd into human food chain. Therefore, research and field trials are required for the identification of traits associated with metal tolerance capacity of commonly grown maize cultivars. To be effective, a plant needs to be tested for heavy metal tolerance both in solution and soil culture. Exploration of plant system is very complex in soil as uptake of pollutants by a plant is influenced by soil physical and chemical properties as well as soil microbiota (Armas et al. 2015). Less variations in Cd uptake was reported in soil system as compared to hydroponics in Arundo donax L. (Sabeen et al. 2013). It is, therefore, pertinent to examine the maize cultivars in soil system (natural system) to analyze the growth responses of differentially Cd responsive genotypes under Cd stress as our previous studies were performed in hydroponics and rhizoboxes (artificial systems). The genotype 3062 revealed better accumulation of biomass, photosynthetic pigments, antioxidant activities, nutrients uptake in roots and shoots, release of organic acids together with less production of MDA and H2O2 as compared to 31P41 growing under Cd stress in hydroponics and rhizoboxes (Tanwir et al. 2013; Javed et al. 2017).
The present study was, therefore, designed for selection of low Cd accumulating maize cultivars with high biomass production to gain better insights into Cd tolerance mechanisms in soil system. This is the first study which evaluated the rhizospheric mineral contents before and after cultivation of Cd tolerant (cv. 3062) and Cd susceptible (cv. 31P41) maize cultivars. Furthermore, the correlations were established among Cd retention ability, mineral ion uptake and physio-biochemical attributes of both maize cultivars for their ultimate use in sustainable food production and phytomanagement in Cd polluted soil. Current study was aimed to find possible alterations in ion dynamics in the rhizospheric soil of two differentially Cd responsive maize cultivars as well as Cd impact on nutrient uptake and growth. The study objectives were to monitor (a) the ion dynamics in the rhizospheric soil of two Z. mays cultivars under Cd treatments (b) Cd-mediated fluctuations in rhizospheric system and their possible correlation with in planta ionomics and physio-biochemical attributes of maize cultivars (c) cultivar based rhizospheric alterations in soil system and their possible role in food safety aspects under Cd polluted environment. The research outcomes would ultimately be beneficial to devise strategies for growing maize in Cd polluted areas as well as effective remediation tools to combat Cd pollution.
Materials and methods
Experimental soil
Soil (upper layer 0–20 cm) was obtained from a botanical garden located at Government College University, Faisalabad (31°24/N, 73°04/E), a city in northeastern Punjab, Pakistan. The soil was mixed thoroughly, dried in air, ground and passed through a 10 mm sieve before using in the experiment. Subsequently, the representative soil samples were air dried, ground, and passed through a sieve with pore size of 2 mm. Thereafter, soil physico-chemical properties were assessed by the methods of Alloway (1995) and presented in Table 1.
Experimental layout for pot experiment
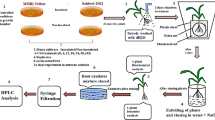
The seeds of two maize cultivars 31P41 (Cd-sensitive) and 3062 (Cd-tolerant) were provided by Pioneer Seeds, Sahiwal, Pakistan. Prior to seed sowing, experimental soil was spiked with different CdCl2 concentrations (0, 20, 40, 60 and 80 µg/kg) and was kept in polythene bags for two weeks for metal stabilization. Forty plastic pots, filled with 10 kg of dry Cd spiked soil, were used to grow maize seeds in a greenhouse with 14/10 h mean day/night length and 25/15 °C ± 2 day/night temperature. The average daytime relative humidity was maintained to 70% during the course of the experiment. Initially, eight maize seeds/pot were sown which after emergence were thinned to five plants. Plants were maintained for fifty days and irrigated with half-strength/0.5 Hoagland’s solution (Arnon and Hoagland 1940) by applying 500 ml of nutrient solution to each pot after every ten days interval to fulfill water and nutrient requirements. Each treatment was maintained in four replicates which were managed in a completely randomized design (CRD). Plants were subsequently harvested to analyze the morpho-physio-biochemical parameters and mineral element composition. Representative soil samples were collected from each pot after harvesting the plants and assessed for physico-chemical properties by the methods of Alloway (1995) as presented in Table 1.
Plant ion analysis
Maize plants were harvested after 50 days and roots were washed twice with distilled water, given a quick dip in 20 mM EDTA and then, washed again with distilled water in order to remove adsorbed metallic cations from plant surfaces. The washed samples were then oven dried for 24 h at 105 °C. The oven-dried roots and shoots (0.5 g each) were separately added into the digestion flasks containing 8 ml of digestion mixture [HNO3: HClO4 (7:3 v/v)] and left overnight for digestion. Next day, these flasks were heated on hot plate until the fumes color change to white. After cooling, dH2O was poured into each flask and filtered through Whatman filter paper grade 1 and diluted up to 50 ml with redistilled water. The digested plant samples were analyzed by using atomic absorption spectrophotometer (Varian FAAS-240, Triad Scientific, NJ, USA) for determination of root and shoot Cd, Ca, Mg, Zn, Fe and K contents. Interactions with the sample matrix were eliminated by using standards addition method. The digested material was analyzed for P and N contents spectrophotometrically by using Barton’s reagent and method of Bremner and Keeney (1965) respectively.
Bio-concentration (BCF) and translocation factors (TF) and tolerance indexes (TIs)
BCF and TF of Cd accumulation for maize were calculated by using the follow formulae;
- BCF=:
-
Cadmium concentration in shoot (μg kg−1)/Cadmium concentration in soil (μg kg−1).
- TF=:
-
Cadmium concentration in shoot (μg kg−1)/Cadmium concentration in root (μg kg−1).
A given plant species with lower BCF and TF values depicts its inability to extract large amount of metals from soil and limited metal translocation to areal parts respectively.
TIs were calculated to visualize the impact of Cd stress on studied morpho-physiological parameters as follows;
- TI (%) :
-
Parameters of Cd treated plants/respective parameters of control plants × 100.
Analysis of plant growth and photosynthetic pigments
Leaf area meter (L12000, L1-COR, USA) was used to measure the leaf area of individual maize plants. Chlorophyll pigments were measured by mixing frozen leaf tissue (0.25 g) in 10 ml acetone (80%). After 1 h, the mixture was centrifuged at 5000 g for 15 min at 4 °C. The supernatant was utilized for chlorophyll a, b and total chlorophyll estimation by measuring absorbance at 650 nm and 665 nm respectively through spectrophotometer (Minocha et al. 2009). For carotenoid quantification, supernatant was added in 1 M NaOH (5 ml) together with 15 ml dimethyl ether. The absorbance was measured at 450 nm for the estimation of carotenoid contents (Alba et al. 2005).
Determination of MDA and H 2 O 2
The frozen plant tissue (0.1 g) was homogenized in 2 ml 0.1% TCA (trichloro acetic acid) and 4 ml thiobarbituric acid (5%) in TCA (20%) for the estimation of MDA. The optical density was noted at 440, 532 and 600 nm wavelength by using spectrophotometer (Hitachi U-2910, Tokyo, Japan) (Heath and Packer 1968).
The frozen plant tissue was extracted in (5%) TCA, the 0.1 ml plant extract was added in 1 ml of phosphate buffer (5 mM) and 2 ml of KI (1 M). The absorbance of reaction mixture for H2O2 was measured at 390 nm (Velikova et al. 2000).
Determination of antioxidant activities
Superoxide dismutase (SOD) activity was quantified by homogenizing 0.5 g plant tissue with 3 ml sodium carbonate buffer and centrifuged at 12,000 rpm for 15 min. 70 µl supernatant was added in 500 µl NBT (24 mM), 100 µl EDTA (0.1 mM), 100 µl NH2OH.HCl (1 mM), µl Triton X-100 (0.03%) and 1.630 ml sodium carbonate buffer maintained at pH 10.2. The absorbance of reaction mixture was detected at 560 nm for quantification of SOD activity (Gong et al. 2005).
Peroxidase (POD) activity was determined by mixing of 0.5 g frozen plant tissue in 5 ml of 50 mM KH2PO4 maintained at pH 7. This mixture was centrifuged for 15 min at 12,000 rpm. The supernatant (100 µl) was added in 50 µl guaiacol solution, 30 µl H2O2 and 3 ml phosphate buffer. The absorbance of reaction mixture was measured at 436 nm for determining POD activity (Cakmark et al. 1993).
Catalase concentration was measured by using 70 µl aliquot prepared for measuring POD activity and adding 930 µl of H2O2 (15 mM), vortexed and finally mixing 1.500 ml phosphate buffer. The absorbance for catalase activity of reaction samples were measured at 240 nm (Cakmark et al. 1993).
Determination of total phenolic and proline contents
For quantification of total phenolic contents, extract of the frozen fresh plant material (1 g) was prepared in 50% methanol (5 ml). The plant extract (50 µl) was taken in a test tube, added 1 ml water, Folin–Ciocalteau reagent (500 μl) and after 5 min added 2% Na2CO3 (2.5 ml). After fifty minutes of incubation at room temperature, absorbance was recorded at 710 nm. The calibration curve was performed with gallic acid and total phenolic concentration was expressed as gallic acid equivalents (Julkunen-Tiitto 1985).
For proline estimation, plant extract was prepared by homogenizing (0.1 g) frozen plant material in 5 ml sulphosalicylic acid (3%). Plant extract was mixed with 1 ml glacial acetic acid, acidic ninhydrin and 4 ml toluene and the absorbance was measured through spectrophotometer at 521 nm (Bates et al. 1973).
Total soluble protein
Plant (0.25 g) extract was prepared in 5 ml phosphate buffer, mixed with 2 ml Bradford reagent and placed in a water bath for approximately 30 min. The reaction mixture was used for absorbance measurement through spectrophotometer at 595 nm for the determination of total soluble protein (Bradford 1976).
Statistical analysis
Before statistical analysis, data normalization was carried out by using the inverse or logarithmic transformation whenever necessary. The statistical analysis of the data was executed by analysis of variance (ANOVA) and least significant difference method (Fisher’s LSD) was used to compare the means at p value of ≤0.05 level. Pearson’s correlation analysis and principle component analysis (PCA) biplots were prepared with XLSTAT software version 2016.1.
Results
Plant and rhizospheric ion dynamics
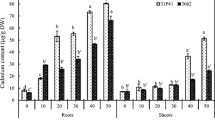
In the present study, we anticipated Z. mays cultivars in soil system exhibiting different potential for Cd accumulation and root to shoot translocation as detected in earlier hydroponic experiments. Acquired results provided evidence of variation in Cd uptake and root to shoot translocation among 3062 and 31P41 cultivars suggesting that the former cultivar exhibited significantly less Cd uptake and root to shoot translocation capabilities in comparison to later in soil system. Plant root and shoot Cd contents were significantly higher in cv. 31P41 which exhibited 23% higher uptake from root and 52% higher root to shoot translocation as compared to cv. 3062, respectively under 80 µg/kg Cd treatment (Fig. 1).
Acquisition of Zn was different in the roots and shoots of both cultivars under varying levels of Cd. Roots of cv. 3062 contained higher Zn as compared to cv. 31P41 at 80 μg/kg Cd level. Moreover, root to shoot translocation of Zn was slightly higher as compared to its control and significantly increased in cv. 3062 than cv. 31P41 at 80 µg/kg Cd level (Fig. 1).
Data trend for the mentioned section is given in Fig. 1 where cv. 31P41 is absorbing more Fe but root to shoot translocation is lower as compared to cv. 3062 at 80 μg/kg Cd levels.
Potassium retention capacity of both cultivars under various Cd levels depicted maximum decline under 80 µg/kg Cd. Interestingly, cv. 3062 exhibited improved K uptake at 20 µg/kg Cd as compared to cv. 31P41. However in comparison to cv. 3062, plants of cv. 31P41 responded with 61.30% decline in root K and 82.95% decline in shoot K uptake under maximum applied Cd stress of 80 µg/kg (Fig. 1).
Calcium acquisition exhibited maximum reduction at 80 µg/kg Cd treatment where higher reduction in roots (60.78%) and shoots (75.25%) was observed in cv. 31P41 as compared to cv. 3062 (Fig. 2). Our results validated that under increasing Cd levels intermediate levels of Ca content was recorded in cv. 3062 than cv. 31P41 at varying Cd levels and the effect of Cd was less in cv. 3062.
The root and shoot Mg content was decreased in both cultivars under various applied Cd treatments. The root Mg content of cv. 3062 showed slightly higher value (14.85 mg/kg DW) than cv. 31P41 under applied Cd stress whereas Mg content exhibited lower root to shoot translocation at 80 µg/kg Cd stress in both the cultivars but reduction was more pronounced in cv. 31P41 as compared with cv. 3062 (Fig. 2).
Results revealed that N uptake efficiency of cv. 3062 was higher as compared to cv. 31P41 where 86.70% decreased root N and 89.32% shoot N content was recorded in cv. 31P41 under 80 µg/kg Cd as compared to cv. 3062 (Fig. 2). Furthermore, the roots of cv. 3062 showed slightly higher levels of root P content at 20 and 40 µg/kg Cd. However, root and shoot P contents were significantly decreased in cv. 31P41 (64, 80% respectively) as compared to cv. 3062 under 80 µg/kg Cd stress (Fig. 2).
Elemental dynamics in rhizospheric soil of both the cultivars altered significantly but exhibited cultivar and applied Cd stress specificity. Based on soil analysis before and after maize cultivation, we anticipated that Cd retention in soil cultivated with cv. 3062 (78.84 µg/kg) was found higher as compared to soil where cv. 31P41 was maintained at 80 µg/kg Cd stress, indicative of higher Cd uptake and acropetal translocation in the later cultivar (Table 1). Furthermore, we compared soil mineral nutrient contents before and after maize cultivation under various applied Cd regimes and found decreased levels of essential nutrient (Ca, N, K, Mg, P, Fe and Zn) in rhizospheric soil samples of cv. 3062 which contributed significantly in growth and development of cv. 3062 as compared to cv. 31P41 where increased root and shoot Cd levels were recorded which antagonistically affected essential nutrient uptake from rhizospheric soil (Table 1).
Bioconcentration (BCF) and translocation factors (TF)
Applied Cd stress in maize cultivars (3062, 31P41) influenced the uptake and translocation of Cd in term of BCF and TF (Table 1). The maximum increase in BCF (2.44) and TF (0.63) in cv. 31P41 was recorded, respectively at 60 and 80 µg kg−1 applied Cd. The cultivar 31P41 exhibited gradual increase in BCF value up to 60 µg kg−1 and then slight decrease at 80 µg kg−1 while TF value was continuously increased up to 80 µg kg−1 applied Cd. On the other hand, BCF values of 1.59 and 1.13 were obtained in cv. 3062 at Cd treatment levels of 20 and 80 µg kg−1, respectively, indicating 54 and 35% lower values than exhibited by cv. 31P41. However, TF values continuously decreased up to 60 µg kg−1 Cd stress and slightly increased at 80 µg kg−1 in both the cultivars.
Effect of Cd on plant biomass and photsynthetic pigments
Increase in biomass was significantly higher in cv.3062 than cv.31P41 under applied Cd regimes (Table 2). It was found that cv. 3062 retained 55.55 and 24.41% higher shoot dry and fresh weights as compared to cv. 31P41 at 80 µg/kg Cd stress. Furthermore, root dry and fresh weights in cv. 3062 depicted a better growth of 53.22 and 25.84% in comparison to cv. 31P41 at 80 µg/kg CdCl2. In both Z. mays cultivars, photosynthetic attributes were significantly decreased under all applied Cd stress levels, however cv. 3062 showed better levels of Chl. a, Chl. b, carotenoids and total chlorophyll in contrast to cv. 31P41 at maximum Cd stress level of 80 µg/kg.
Tolerance index
The tolerance index (TI) revealed a variation in both maize cultivars under applied Cd regimes where cv. 3062 depicted higher values (84, 85%) for shoot dry and fresh biomass in comparison to cv. 31P41 which exhibited TIs (44, 64%) under 80 µg/kg Cd stress. The root dry and fresh weight of cv. 3062 showed TIs of 64, 58% in contrast to cv. 31P41 where 31, 44% TIs was observed at 80 µg/kg Cd level. Similarly TIs of 50, 52, 54, 50% were recorded in leaf area, Chl. b, Chl. a, carotenoids and total chlorophylls of cv. 3062 in comparison to cv. 31P41 under maximum Cd stress. Together these results about TIs demonstrated that cv. 3062 maintained higher biomass, photosynthetic traits and showed different mode of growth tolerance as indicator of Cd tolerance.
Cadmium effect on MDA and H 2 O 2 content
Increase in soil Cd treatment levels significantly enhanced the production of MDA in both maize cultivars (Table 3). The production of MDA was higher by 21.39% in 3062 as compared to 31P41 cultivar. Surprisingly, non-significant variations were recorded for H2O2 content of both cultivars at all applied Cd stress conditions (Table 2).
Antioxidant enzyme activity
We analyzed that varying Cd treatment levels significantly increased peroxidase and superoxide dismutase activity in studied maize cultivars (Table 3). Maize plants exhibited 18.42, 27.95% higher POD and SOD levels respectively in comparison to cv. 31P41 at 80 μg/kg Cd stress. In addition, cv. 3062 recorded 158 and 130% better tolerance index in comparison to cv. 31P41 at higher Cd regimes.
Total phenolics and proline content
It was found that total phenolic and proline contents of maize cultivars were not significantly different under different applied Cd stress levels (Table 3). However cv. 3062 showed 13.15% elevated level of proline contents as compared to cv. 31P41.
Effect of Cd on total soluble protein and catalase activity
Total soluble proteins and catalase activity were significantly reduced with increasing level of applied Cd treatments (Table 3). However, this decrease was cultivar dependent and cv. 31P41 exhibited more reduction (0.32 µmol/g FW) in protein and a 1.02 mg−1 Protein catalase activity was recorded under 80 µg kg−1 Cd level as compared to cv. 3062. The cv. 3062 showed lower values by 64% and 46% of tolerance index of protein and catalase activity in comparison to cv. 31P41.
Pearson’s correlations and principal component analysis
Principal component analysis biplots for applied Cd treatments (T1–T5) and plant morpho-physio-biochemical attributes of maize cultivars (31P41 and 3062) was executed to summarize the correlations among significantly important variables which exhibit smaller set of linear combinations (Fig. 3). For cv. 31P41 and 3062, biplots depicted variations of 94.94% (Fig. 3a) and 86.33% (Fig. 3b) respectively. The variables present very close to each other and in the same quadrant were correlated positively. Correlations among the studied parameters were represented by red dots while correlations among the Cd treatments were represented by blue dots. Furthermore, correlation analysis indicated that significant positive and/or negative correlations exist among growth, physiological, biochemical attributes and root minerals uptake in maize cultivars (Table 4). Growth attributes like shoot and root fresh and dry weights, leaf area, chl a, chl b, total chlorophyll and carotenoid contents were positively correlated with root sodium, potassium, magnesium, phosphorus, zinc. However a significant negative correlation was observed between the growth traits and root Cd contents. For example, plant total chlorophyll contents were positively correlated with root nitrogen (0.638***), root potassium (0.812***), root magnesium (0.805***), root phosphorus (0.775***) and root zinc (0.511***) contents. However, significant negative correlation exist between Cd content in roots (-0.918***) and total chlorophyll contents.
Pearson correlation biplots for significantly important morpho-physio-biochemical attributes of 31P41 (a) and 3062 (b) grown under different CdCl2 treatments. Red dots represents correlations among the studied parameters while the blue ones among the applied Cd treatments. Cat = catalase, LA = leaf area, P FW = plant fresh weight, P DW = plant dry weight, Car = carotenoid, Tot. Chl = total cholorophyll, MDA = malondialdehyde, T S Pro = total soluble protein, Prol = proline, K P = plant potassium contents, Zn P = plant zinc contents, P P = plant phosphorus contents, Mg P = plant magnesium contents, N P = plant nitrogen contents, Cd P = plant calcium contents.
Discussion
Plant and rhizospheric ionomics of maize under Cd treatment
In earlier studies, the tolerant (cv. 3062) and sensitive (cv. 31P41) Z. mays cultivars responded to Cd toxicity by triggering rhizospheric basification both in nutrient solutions and mucilage surrounding their roots (Javed et al. 2017). The current work, therefore, investigated the impact of Cd-mediated fluctuations in soil system of the same two maize cultivars as well as associated correlations with rhizospheric plant ion dynamics and plant morpho-physio-biochemical attributes. When soil was analyzed after plant harvesting, it was observed that Cd stress differentially trigger variations in soil ionic concentrations which were cultivar specific (Table 1). Tolerant cultivar (cv. 3062) maintained higher concentration of Cd in soil when analyzed after plant harvesting as compared to sensitive one (cv. 31P41) at all applied Cd treatment levels. Furthermore, lower BCF and TF values for cv. 3062 also indicated that the cultivar is not able to extract large amount of Cd from soil colloids and resist acropetal metal translocation. The soil contents for Ca, N, K, Mg, P and Zn were found to be lowered in the rhizosphere of cv. 3062 than cv. 31P41 after plant harvesting under different Cd levels. These results were according to our hypothesis that Cd tolerant cultivar (cv. 3062) has some mechanism, which are absent in cv. 31P41, to prevent Cd from entering the plant roots which in turn helps plants to maintain better nutrient acquisition. It was perceived that cv. 3062 influenced the soil Cd availability owing to secretion of LMWOAs which in turn modulated the rhizospheric pH and confer Cd tolerance with concomitant enhanced nutrient acquisition under applied Cd stress as compared to cv. 31P41 in soil system. Secretion of root exudates influenced Cd bioavailability and toxicity by changing rhizospheric pH, redox potential and the activity of soil microbe for chelating Cd. Previous studies reported that roots of some plants such as wheat excrete organic acids such malic acid, oxalic acid and citric acid which can chelate with Cd to prevent its entry into root system (Nazar et al. 2012). It was reported that release of organic acids under Cd stress would generate cadmium-phosphate complex unavailable to plants and enhance nutrient acquisition (Akhtar et al. 2019).
Root and shoot ion uptake and translocation of maize cultivars under Cd stress
The roots and shoots Cd contents of maize cultivars were significantly increased with applied Cd treatment levels while the corresponding Cd contents were decreased in soil after plant harvesting predominantly in cv. 31P41. Soil pH is one of the dominant factors which influence Cd bioavailability to plants and soil acidification of one unit may increase HAc-extractable Cd by about 50%. Higher Cd uptake by both cultivars observed in the present study is also likely due to the Cd-triggered rhizospheric acidification recorded at higher Cd treatment levels (Javed et al. 2017). Results of the current work revealed that Cd localization in maize roots exceeded significantly than the shoots in soil system (Fig. 1) which validates the findings of earlier studies (Tanwir et al. 2015). (Redjala et al. 2009) reported that higher levels of Cd treatment causes higher Cd deposition in apoplasts of maize root cells which in turn resulted in increased plant Cd retention (Sterckeman et al. 2011). Higher Cd retention capability to the cell wall of maize hybrids was likely to be mediated by negative charges and free electron pairs of oxygen (O) atoms of OH- groups, the ring O atoms, the bridging O atoms of monosaccharides as well as COOH- groups of glucuronic/galacturonic acids (Vatehová et al. 2016). It was envisioned that Cd bound to root cell walls initiated reorganization of pectins by the release of low methyl esterified pectin units which leads to further deposition of Cd ions in apoplasts of studied cultivars due to increased surface area (Douchiche et al. 2007; Javed et al. 2014). In root tissues of Z. mays, cell wall lignification and endodermal suberization after Cd exposure restricted Cd loading to xylem and exhibited higher metal retention in the roots (Lux et al. 2011), as was recorded in the current study. Furthermore, the differential root to shoot Cd localization of studied cultivars (Fig. 1) might be linked with cultivar’s genetic variations as well as variations in Cd localization capabilities. Lower Cd translocation to shoots might also result from metal complex formation with sulphur ligands in root cells which restrict Cd accumulation to above ground parts of maize cultivars (Tanwir et al. 2015).
Cadmium treatments decreased the Zn absorption by maize root predominantly in sensitive cultivar (Fig. 1). The reduction in root Zn contents might be due to Cd-induced decrease in Zn acquisition and/or its leakage to the surroundings rhizosphere (Qin et al. 2020). Interestingly, at highest Cd treatment (80 µg/kg), the root Zn uptake increased in cv. 3062 which possibly reflect the existence of a metal tolerance mechanism in Cd tolerant cultivar.
Applied Cd stress resulted in reduced Fe retention in maize roots and the effect was prominent in the roots of Cd tolerant cultivar (Fig. 1) which was opposite to our earlier findings (Tanwir et al. 2015; Javed et al. 2017). The Fe transporter IRTI was reported to be down regulated by Cd stress in T. aestivum which revealed that Fe uptake is inhibited by Cd ions (Greger et al. 2016).
Cadmium stress impaired K accumulation in Z. mays particularly in the roots and shoots of 31P41 (Fig. 1) which corroborates our earlier results (Tanwir et al. 2015; Javed et al. 2017). Reduction in Cd-mediated K accumulation could inhibit the biosynthesis of plant carotenoids and chlorophyll pigments (Kurtyka et al. 2008). Cd stress at lower treatment concentration has also been reported to inhibit K accumulation in roots of sugar beet by reduced activity of PM-ATPase enzyme (Lindberg and Wingstrand 1985). The reduction in root K contents might result from Cd-mediated reduction in K accumulation and/or K leakage to rhizosphere (Zhang et al. 2020).
Calcium accumulation of cv. 3062 and cv. 31P41 were significantly affected by different Cd treatment levels and the effect was cultivar specific (Fig. 2). Since, Cd ions utilized the Ca transport channels for influx into plant tissues (Zhang et al. 2020) therefore, it is likely that Cd antagonistic effect on Ca uptake originates from cationic competition at uptake sites either for cellular influx or for its acropetal transportation which in turn influenced the plant metabolic processes (Perfus-Barbeoch et al. 2002).
With increasing applied Cd levels, Mg accumulation in studied maize cultivars was decreased significantly and the reduction effect was prominent in the sensitive cultivar (Fig. 2). The relatively higher Mg contents in Z. mays roots than shoots might result from a minor reduction in Mg acropetal transport because both Cd and Mg ions compete with each other for ZIP transporters (Wang et al. 2007).
Nitrogen is very essential for plant growth promotion and plays an important role towards production of nucleic acid, hormone, protein, amino acid, chlorophylls and enzyme activity (Shi et al. 2017). It is likely that Cd-induced N and P deficiency reduces maize photosynthetic rate and growth as recorded in the present study. Reduction in phosphorus and nitrogen uptake was recorded in maize cultivars under Cd stress as reported in earlier studies (Bui et al. 2018; Rqfique et al. 2019). Phosphorus is an essential element for the growth and its deficiency hampers the photosynthetic system and promotes ROS production (Fig. 2) (Veronica et al. 2017).
Plants responded to Cd stress primarily by modifying their internal architecture, nutrient mobility and solubility patterns which in turn influenced Cd accumulation capability (Javed et al. 2017). Different plant genotypes differ in their ability to uptake essential nutrient and Cd accumulation can be estimated by calculating the efficiency of essential nutrients; therefore, plants with higher nutrient uptake capacity could minimize Cd uptake (Anjum et al. 2015). In the present study, cv. 3062 showed improved nutrient retention efficiency (Ca, Zn, K, Mg, N, P) as compared to cv. 31P41 by modulating rhizospheric activities and decreased Cd uptake from soil. Such an ability of cv. 3062 (Cd tolerant cultivar) can be associated with plants capacity to perform several processes which reduce Cd bioavailability by adsorption on soil particles, precipitation as phosphates or hydroxides promoted by soil basification (Javed et al. 2017; Hussain et al. 2013).
Impact of Cd stress on lipid peroxidation and antioxidants of maize cultivars
In both the studied maize cultivars, H2O2 contents increased under Cd stress which corroborates the findings of (Anjum et al. 2016). Higher in planta H2O2 concentration inhibits the processing of Calvin cycle by initiating oxidative stress which significantly reduced the plant’s photosynthetic activities and growth (Anjum et al. 2015). Furthermore, elevated plant malondialdehyde content under Cd stress depicts that the metal stress initiated the oxidative stress, damage to cellular membranes and increased ions leakage as reported in Solanum (Hajaji et al. 2012).
The antioxidative machinery safeguard plants against oxidative damages by scavenging of ROS under metal stress (Ali and Ashraf 2011). Catalase contents both in tolerant and sensitive cultivars were decreased significantly, in the present study, with increasing Cd treatment levels which validate the findings of previous investigations (Farid et al. 2013; Sun et al. 2013). Under Cd stress, reduced CAT activity might be linked with inhibition of enzymatic activity or with increasing lipid peroxidation levels which mediates Cd stress in plants (Qin et al. 2020). However, POD and SOD activity of the studied cultivars was increased significantly with applied Cd treatments where the increment was cultivar specific. Our findings validate the results of earlier studies which reported increasing activities of SOD and POD in Z. mays under Cd toxicity and coined it as a metal stress tolerance response (Anjum et al. 2016; Shah et al. 2020). Moreover, the maintenance of higher CAT, SOD and POD activities in tolerant maize cultivar (3062) as compared to the sensitive one (31P41) indicates the involvement of these antioxidants in minimizing Cd-induced damages under Cd stress. Finally, both the cultivars retained higher content of lipid peroxidation and antioxidant system, but cv. 3062 depicted better lipid peroxidation levels and antioxidant contents as deciphered from tolerance index which might represent a strategy to cope with Cd toxicity in this cultivar.
Influence of Cd stress on biomass and physio-biochemical attributes of maize cultivars
Reduction in plant biomass under Cd stress was found to be cultivar specific and might be associated with inherent Cd tolerance of studied cultivars. Increasing Cd treatment levels significantly decreased plant fresh and dry biomass predominantly in sensitive cultivar (cv. 31P41) and are likely due to reduction in root water absorption (Sun et al. 2013) as was obvious from wilting of leaves. The greater Cd absorption and translocation in plants through phloem vessels impaired the transpiration rate by stomatal closure (Chaffei et al. 2004) and can be a reason for abridged shoot fresh biomass of studied Z. mays cultivars. Applied Cd stress significantly reduced the leaf area and biosynthesis of photosynthetic pigments in maize cultivars (Table 2) which in turn reduced the plant’s photosynthetic rate. Reduction in photosynthetic rate might be owing to lower CO2 availability due to stomatal closure under metal stress (Singh et al. 2019). Impaired chlorophyll contents under Cd stress have already been reported by (Shah et al. 2020). Reduced biosynthesis of chlorophyll and carotenoids under Cd stress might be due to ROS formation, reduced nutrient uptake by roots or due to elevated level of degrading enzyme chlorophyllase (Qin et al. 2020). In the present study, marked reduction in dry biomass as compared to fresh biomass in cv. 31P41 pointed out that Cd stress exerted more negative impact on photosynthesis of susceptible cultivar than its water relation attributes. Comparative studies of plant biomass and pigments in both the cultivars showed that cv. 3062 had an advantageous growth under Cd stress with greater influence on rhizospheric processes and Cd uptake from soil. Better nutrient retention probably caused an increase in plant biomass and helped cv. 3062 to preserve photosynthetic enzymes and pigments against Cd stress. Our study corroborates the findings of (Ekmekçi et al. 2008) who reported that the tolerant cultivar 32D99 maintains higher biomass under Cd stress as compared to sensitive maize cultivar 3223.
Effect of Cd on protein, proline and phenolics contents of maize cultivars
Cd stress significantly reduced protein contents of studied maize cultivars, an effect probably due to the denaturation of plant proteins, as metal stress can trigger the mechanisms responsible for degradation of protein in Z. mays. Reduced protein contents in Z. mays plants may modulate anti-oxidative enzyme activities which cope with Cd-induced ROS production and the biosynthesis of phytochelatins for Cd compartmentalization within the cells (Hossain et al. 2012). Although differential in tolerant and sensitive cultivars, proline and phenolic contents were significantly increased with Cd treatment levels, which corroborate metal resistance properties and ultimately an adaptation mechanism of cv. 3062 to Cd stressed environment (Javed et al. 2017). Higher biosynthesis of phenolic contents following Cd treatments were envisioned to be connected with strengthening of the cell wall, interaction with toxic metals and neutralization of oxidative stress in cv. 3062 (Tanwir et al. 2013). Proline accumulation under metal stress is one of the first signals which are regarded as an indication of stress sensing in Z. mays (Hussain et al. 2013). Increased proline contents may result from Cd mediated production of ROS as the proline contents have an active role to destroy the free radicals and ROS species (Shah et al. 2020). Proline contents has been reported to increase in Cymbopogon flexuosus when grown in Cd polluted soils as compared to the uncontaminated ones (Handique and Handique 2009). Elevated level of proline contents were recorded in metal tolerant macrophytes in comparison to sensitive ones which was linked with plant’s inherent metal stress tolerance (Maggio et al. 2002) as depicted by the results of the present study. Our results are in agreement with the previous results (Ekmekçi et al. 2008) who reported that 32D99 maize cultivars showed improved tolerance with enhanced levels of antioxidative machinery and osmolytes under Cd stress.
Conclusion
Cadmium stress negatively affects plant’s growth, photosynthetic activity as well as associated physio-biochemical metabolic pathways in both maize cultivars (3062 and 31P41). Increasing Cd levels severely reduced the intake and transfer of ions from soil and root system because it competes with mineral ions for its transport from rhizosphere to root, then from roots to shoot by occupying their transport channels. Both the maize cultivars have differential tendency to bear the damaging impacts of Cd toxicity and ability to acclimatize in Cd polluted environment. Based on analysis of tolerance indexes (TIs), it was anticipated that cv. 3062 achieved systematically higher growth in soil system, which was facilitated by the activities of antioxidant machinery and enhanced uptake of nutrients (Ca, Zn, K, Mg, N and P) under Cd stress in soil system. Results validated our previous studies suggesting that cv. 3062 exhibited pH modulations and organic acid exudation in the surrounding root mucilage assuring plant survival in Cd polluted niche due to increased nutrient uptake, pigment expression, biomass production and antioxidant levels than cv. 31P41. Research outcomes are important with regard to understanding the shifts in rhizospheric mineral nutrient dynamics of Cd sensitive and tolerant maize cultivars and may provide efficient approaches to reduce Cd retention in edible plant parts and/or phytoremediation of Cd polluted soil colloids. Future studies by involving Cd specific fluorescent dyes as well as inhibitors for different channels together with ultra-structural modification in root architecture and leave segments should be executed to further dissect the possible Cd defense mechanisms of studied maize cultivars.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
Abbas S, Javed MT, Shahid M, Hussain I, Haider MZ, Chaudhary HJ, Maqsood A (2020) Acinetobacter sp. SG-5 inoculation alleviates cadmium toxicity in differentially Cd tolerant maize cultivars as deciphered by improved physio-biochemical attributes, antioxidants and nutrient physiology. Plant Physiol Biochem 155:815–827
Akhtar MJ, Ali Q, Javid R, Asghar HN, Ahmad I, Iqbal MZ, Khaliq A (2019) Organic and inorganic amendments immobilized cadmium and improved maize growth and yield in Cd-contaminated soil. Int J Agric Biol 22(6):1497–1506
Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Giovannoni JJ (2005) Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17(11):2954–2965
Ali Q, Ashraf M (2011) Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defense mechanism. J Agron Crop Sci 197:258–271
Alloway BJ (1995) Heavy metals in soil. Blackie and Son Glasgow.
Anjum SA, Tanveer M, Hussain S (2016) Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ Sci Pollut Res 23:11864–11875
Anjum SA, Tanveer M, Hussain S, Bao M, Wang L, Khan I, Shahzad B (2015) Cadmium toxicity in maize (Zea mays L): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res 22(21):17022–17030
Armas T, Pinto AP, de Varennes A, Mourato MP, Martins LL, Gonçalves MLS, Mota AM (2015) Comparison of cadmium induced oxidative stress in Brassica juncea in soil and hydroponic cultures. Plant Soil 388(1–2):297–305
Arnon DI, Hoagland DR (1940) Crop production in artificial culture solution, sand and soil with special reference to factors influencing yield and absorption of inorganic nutrient. Soil Sci 50:463–483
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Bremner JM, Keeney DR (1965) Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal Chim Acta 32:485–495
Bui TTX, Lu M, Vu DD, Dinh HN, Ullah N, Rahman SU, Zhang Y (2018) Physiological responses of Toxicodendron vernicifluum (Stokes) FA Barkley to cadmium stress under sufficient-and deficient-nitrogen conditions. Trees 32(5):1457–1471
Cai Y, Zhang S, Cai K, Huang F, Pan B, Wang W (2020) Cd accumulation, biomass and yield of rice are varied with silicon application at different growth phases under high concentration cadmium-contaminated soil. Chemosphere 242:125–128
Cakmark I, Strbac D, Marchener H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J Exp Bot 44(1):127–132
Chaffei C, Pageau K, Suzuki A, Gouia H, Ghorbel MH, Masclaux-Daubresse C (2004) Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant Cell Physiol 45(11):1681–1693
Douchiche O, Rihouey C, Schaumann A, Driouich A, Morvan C (2007) Cadmium-induced alterations of the structural features of pectins in flax hypocotyl. Planta 225:1301–1312
Ekmekçi Y, Tanyolac D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165(6):600–611
Farid M, Ali S, Shakoor MB, Bharwana SA, Rizvi H, Ehsan S, Hannan F (2013) EDTA assisted phytoremediation of cadmium, lead and zinc. Int J Agron Plant Prod 4(11):2833–2846
Gadd GM, Griffith AJ (1978) Microorganisms and heavy metal toxicity. Microb Ecol 4:303–317
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Benavides REP, MP, (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169(2):313–321
Greger M, Kabir AH, Landberg T, Maity PJ, Lindberg S (2016) Silicate reduces cadmium uptake into cells of wheat. Environ Pollut 211:90–97
Hajaji AN, Gouia H, Carrayol H, Chaffei CH (2012) Ammonium alleviates redox state in solanum seedlings under cadmium stress conditions. J Environ Anal Toxicol 2:116–120
Han Y, Zhang L, J, Gu J Zhao, J Fu, (2018) Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. Cultivated in Pb mine tailings. Int Biodeterior 128:15–21
Handique GK, Handique AK (2009) Proline accumulation in lemongrass (Cymbopogon flexuosus Stapf) due to heavy metal stress. J Environ Biol l30: 299–302
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophy 125(1):189–198
Hossain MA, Piyatida P, Jaime A, Silva TD, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 872–875.
Hussain I, Akhtar S, Ashraf MA, Rasheed R, Siddiqi EH, Ibrahim M (2013). Response of maize seedlings to cadmium application after different time intervals. ISRN Agron, 1–9.
Javed MT, Akram MS, Tanwir K, Chaudhary HJ, Ali Q, Stoltz E, Lindberg S (2017) Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol Environ Safe 141:216–225
Javed MT, Lindberg S, Greger M (2014) Cellular proton dynamics in Elodea canadensis leaves induced by cadmium. Plant Physiol Biochem 77:15–22
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217
Khan MY, Prakash V, Yadav V, Chauhan DK, Prasad SM, Ramawat N, Sharma S (2019) Regulation of cadmium toxicity in roots of tomato by indole acetic acid with special emphasis on reactive oxygen species production and their scavenging. Plant Physiol Biochem 142:193–201
Khanna K, Jamwal VL, Kohli SK, Gandhi SG, Ohri P, Bhardwaj R, Ahmad P (2019) Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 217:463–474
Kurtyka R, Małkowski E, Kita A, Karcz W (2008) Effect of calcium and cadmium on growth and accumulation of cadmium calcium potassium and sodium in maize seedlings. Pol J Environ Stud, 17(1).
Lindberg S, Wingstrand G (1985) Mechanism for Cd2+ inhibition of (K+ + Mg2+) ATPase ac-tivity and K+ (86Rb+) uptake in roots of sugarbeet (Beta vulgaris). Physiol Plant 63:181–185
Lux A, Martinka M, Vaculik M, White PJ (2011) Root responses to cadmium in the rhizo-sphere: a review. J Exp Bot 62:21–37
Maggio A, Miyazaki S, Veronese P, Fujita T, Ibeas JI, Damsz B, Narasimhan ML, Hasegawa PM, Joly RJ, Bressan RA (2002) Does proline accumulation play an active role in stress induced growth reduction. Plant J 31:699–712
Mahmood A, Malik RN (2014) Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab J Chem 7:91–99
Minocha R, Martinez G, Lyons B, Long S (2009) Development of a standardized method-ology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can J Forest Res 39(4):849–861
Muszyńska E (2017) HanusFajerska E (2017) In vitro multiplication of Dianthus carthusianorum calamine ecotype with the aim to revegetate and stabilize polluted wastes. Plant Cell Tiss Org 128(3):631–640
Nazar R, Iqbal N, Masoo A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. AJPS 3:1476–1489
Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32:539–548
Qin S, Liu H, Nie Z, Rengel Z, Gao W, Li C, Zhao P (2020) Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: a review. Pedosphere 30(2):168–180
Ran J, Zheng W, Wang H, Wang H, Li Q (2020) Indole-3-acetic acid promotes cadmium (Cd) accumulation in a Cd hyperaccumulator and a non-hyperaccumulator by different physiological responses. Ecotoxicol Environ Safe 191:110213
Redjala T, Sterckeman T, Morel JL (2009) Cadmium uptake by roots: contribution of apo-plast and of high- and low-affinity membrane transport systems. Environ Exp Bot 67:235–242
Sabeen M, Mahmood Q, Irshad M, Fareed I, Khan A, Ullah F, Tabassum S (2013) Cadmium phytoremediation by Arundo donax L. from contaminated soil and water. Biomed Res Int, 324830.
Shah AA, Ahmed S, Ali A, Yasin NA (2020) 2-Hydroxymelatonin mitigates cadmium stress in Cucumis sativus seedlings: Modulation of antioxidant enzymes and polyam-ines. Chemosphere 243:125308
Shen M, Schneider H, Xu R, Cao G, Zhang H, Li T, Zhao Z (2020) Dark septate endo-phyte enhances maize cadmium (Cd) tolerance by the remodeled host cell walls and the altered Cd subcellular distribution. Environ Exp Bot 172:104000
Shi H, Ma W, Song J, Lu M, Rahman SU, Bui TTX, Zhang Y (2017) Physiological and transcriptional responses of Catalpa bungei to drought stress under sufficient-and de-ficient-nitrogen conditions. Tree Physiol 37(11):1457–1468
Singh S, Singh VP, Prasad SM, Sharma S, Ramawat N, Dubey NK, Chauhan DK (2019) Interactive Effect of Silicon (Si) and Salicylic Acid (SA) in Maize Seedlings and Their Mechanisms of Cadmium (Cd) Toxicity Alleviation. J Plant Growth Regul 38(4):1587–1597
Sterckeman T, Redjala T, Morel JL (2011) Influence of exposure solution composition and of plant cadmium content on root cadmium short term uptake. Environ Exp Bot 74:131–139
Sun J, Wang R, Zhang X, Yu Y, Zhao R, Li Z, Chen S (2013) Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol Biochem 65:67–74
Tanwir K, Akram SA, Masood S, Chaudhary HJ, Lindberg S, Javed MT (2015) Cadmium-induced rhizospheric pH dynamics modulated nutrient acquisition and physiological attributes of maize (Zea mays L). Environ Sci Pollut Res 22(12):9193–9203
Tao Q, Zhao J, Li J, Liu Y, Luo J, Yuan S, Huang H (2020) Unique root exudate tartar-ic acid enhanced cadmium mobilization and uptake in Cd-hyperaccumulator Sedum al-fredii. J Hazard Mater 383:121177
Vatehová A, Malovíková K, Kollárová D, Kučerová D, Lišková, (2016) Impact of cadmi-um stress on two maize hybrids. Plant Physiol Biochem 108:90–98
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66
Veronica N, Subrahmanyam D, Kiran TV, Yugandhar P, Bhadana VP, Padm V, Voleti SR (2017) Influence of low phosphorus concentration on leaf photosynthetic characteristics and antioxidant response of rice genotypes. Photosynthetica 55(2):285–293
Wang M, Zou J, Duan X, Jiang W, Liu D (2007) Cadmium accumulation and its effects on metal uptake in maize (Zea mays L.). Bioresour Tech 98:82–88
Waseem A, Arshad J, Iqbal F, Sajjad A, Mehmood Z, Murtaza G (2014) Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed Res Int 2014:813206. https://doi.org/10.1155/2014/813206,29pages
Yang Y, Nan Z, Zhao Z (2014) Bioaccumulation and translocation of cadmium in wheat (Triticum aestivum L) and maize (Zea mays L) from the polluted oasis soil of Northwestern China. Chem Speciat Bioavailab 26(1):43–51
Zhang C, He Q, Wang M, Gao X, Chen J, Shen C (2020) Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotoxicol Environ Safe 190:110090
Zhao T, Zhang K, Chen J, Shi X, Li X, Ma Y, Xu S (2019) Changes in heavy metal mobility and availability in contaminated wet-land soil remediated using lignin-based poly (acrylic acid). J Hazard Mater 368:459–467
Acknowledgments
Provision of financial resources by Higher Education Commission (HEC) of Pakistan (Grant No: 20-4243/NRPU/R&D/HEC/14/885) is highly appreciated.
Author information
Authors and Affiliations
Contributions
Conceptualization, Muhammad Tariq Javed, Muhammad Sohail Akram, Shafaqat Ali, Data curation, Saghir Abbas, Muhammad Tariq Javed and Muhammad Sohail Akram; Formal analysis, Saghir Abbas, Qasim Ali, Naeem Iqbal, Muhammad Sohail Akram and Kashif Tanwir; Funding acquisition, Muhammad Tariq Javed Investigation, Saghir Abbas, Muhammad Sohail Akram and Hassan Javed Chaudhary; Methodology, Saghir Abbas, Muhammad Tariq Javed, Qasim Ali, Muhammad Sohail Akram and Kashif Tanwir; Project administration, Shafaqat Ali; Resources, Muhammad Tariq Javed, Shafaqat Ali, Naeem Iqbal, Hassan Javed Chaudhary; Software, Shafaqat Ali, Hassan Javed Chaudhary; Supervision, Muhammad Tariq Javed; Validation, Qasim Ali and Kashif Tanwir, Naeem Iqbal; Visualization, Saghir Abbas, Qasim Ali and Kashif Tanwir; Writing—original draft, Saghir Abbas, Muhammad Tariq Javed, Shafaqat Ali, Writing—review & editing, Saghir Abbas, Muhammad Tariq Javed, Kashif Tanwir, Naeem Iqbal, Muhammad Sohail Akram. The presented data is the part of MPhil research of Mr. Saghir Abbas.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbas, S., Javed, M.T., Ali, Q. et al. Elucidating Cd-mediated distinct rhizospheric and in planta ionomic and physio-biochemical responses of two contrasting Zea mays L. cultivars. Physiol Mol Biol Plants 27, 297–312 (2021). https://doi.org/10.1007/s12298-021-00936-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-00936-0