Abstract
Ethylene-responsive factors (ERFs) are commonly considered to play an important role in pathogen defense responses. However, only few of ERF members have been characterized in Sea island cotton (Gossypium barbadense). Here, we reported a novel AP2/ERF transcription factors gene, named GbERFb which was cloned and identified from Sea island cotton by RACE. The expression of GbERFb was significantly induced by treatments with ethylene, Methyl jasmonate, salicylic acid, wounding, H2O2 and Verticillium dahliae (V. dahliae) infection. Bioinformatics analysis showed that GbERFb protein containing a conserved ERF DNA binding domain and a nuclear localization signal sequence, belonged to IXb subgroup of the ERF family. Further experiments demonstrated that GbERFb could bind the GCC box cis-acting element and interact with GbMAPKb (MAP kinase) directly in yeast. Over-expression of GbERFb in tobacco could increase the disease resistance to V. dahliae. The results suggest that the GbERFb, a new AP2/ERF transcription factor, could enhance the resistance to V. dahliae and be useful in improvement of crop resistance to pathogenes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cotton is one of the most important economic crop and occupies an important position in the world economy. There are a lot of biotic and abiotic stresses factors that caused reduction of cotton yield and quality. Among of them, Verticillium wilt of cotton caused by the soil-borne fungus Verticillium dahliae Kleb, is one of the major problem which was received extensive attention (Cai et al. 2012). Sea island cotton (Gossypium barbadense) is usually thought to be more tolerant to V. wilt than other cultivated species. Many biotic stress responsive genes from Sea island cotton were cloned and identified, such as GbERF1-like (Guo et al. 2016), GbHcm1 (Zhang et al. 2016), Gbve1 (Zhang et al. 2012), GbWRKY1 (Li et al. 2014). Increasing evidences indicated that the ethylene responsive factors (ERFs) were involved in the resistance to V. wilt (Meng et al. 2010; Guo et al. 2016).
The ERF, which is a major subfamily of AP2/ERF superfamily, was firstly founded in tobacco and regulated the expression of downstream PR genes (Ohme-Takagi 1995). ERF transcription factors are defined by highly conserved ERF DNA-binding domain (DBD), approximately 58–60 amino acids, which is able to bind the GCC box (AGCCGCC), a short cis-acting element existing in the promoters of many defense and hormones-inducible genes. Many of the ERF transcription factors have been identified in plant species, such as AtERFs (Lorenzo 2003; Onate-Sanchez et al. 2006; Vogel et al. 2014), GmERFs (Zhai et al. 2013; Hernandez-Garcia and Finer 2016), GbERFs (Zuo et al. 2007), OsERFs (Zhao et al. 2015) and TaERFs (Dong et al. 2010, 2012; Zhu et al. 2014). ERFs are commonly considered as excellent candidates for improving crop abiotic and biotic stress resistance. Previous studies showed that over-expression of some ERFs could improve tolerance of transgenic plants to pathogens. For example, TaERF1 from wheat (Triticum aestivum) could bind to GCC element in the promoter of defense- and stress-responsive genes, and activate these genes including pathogen response genes and then elevated in response to Rhizoctonia cerealis (R. Cerealis) stress (Zhu et al. 2014). Over-expression of the tomato ERF protein Pti5 increased pathogen-induced expression of GluB and Catalase and enhanced resistance to Pseudomonas syringae pv. in tomato (He et al. 2001). Arabidopsis ERF6 is able to constitutively activate defense-related genes PDF1.1 and PDF1.2, and confers to enhance the resistance to Botrytis cinerea (B. Cinerea). However, expression of ERF6 fused to the ERF-associated amphiphilic repression (EAR) motif, could strongly suppresses B. Cinerea induced defense genes expression, and consequently leading to reduction of resist to B. Cinerea (Meng et al. 2013). In cotton, over-expression of EREB1 could improve tolerance of susceptible cotton cultivars to V. dahliae (Meng et al. 2010). Over-expression of GbERF1-like could improve the disease resistance in both cotton and Arabidopsis thaliana against the V. dahliae, while down-regulation of GbERF1-like increased the susceptibility of cotton plants to V. dahliae. Further analysis revealed that GbERF1-like was involved in lignin synthesis (Guo et al. 2016).
Although many studies showed that ERFs played important roles in biotic stress, only few of ERF members have been characterized in Sea island cotton (Meng et al. 2010; Guo et al. 2016). In this study, we isolated a novel ERF gene from Sea island cotton, named GbERFb, which can be induced by exogenous hormones, V. dahliae infection and wounding. We examined its DNA-binding affinity and determined the interaction between GbERFb and GbMAPKb. We also analyzed the effect of overexpression of GbERFb in transgenic tobacco resistance to V. dahliae.
Materials and methods
Plant materials and treatments
The cotton variety (Gossypium barbadense cv. Pima90–53) and the fungal pathogen V. dahliae Linxi 2–1 were provided by Professor Zhiying Ma of Hebei Agricultural University, China.
The cotton seeds were germinated on wet cloth, and the resulting seedlings were transplanted into hydroponic cultures under greenhouse conditions at 25–28 °C with a 16/8 h light/dark cycle. In V. dahliae infection experiment, the seedlings were uprooted gently and their roots rinsed in distilled water three times, and then their roots were dipped in the conidia of V. dahliae suspension. Wounding experiments were consisted of gently rubbing the upper epidermis of the whole leaves with wet carborundum (mesh 600; Kishida Chemical Co., Osaka, Japan) for 30 s. The hormones and H2O2 treatments used 1.0 mM SA, 0.2 mM ET, 0.1 mM MeJA and 1% H2O2 100 ml spray to the leaves of cotton. The leaves of SA, ET, MeJA and wounding were harvested at different time intervals, including 0, 1, 3, 6, 12, 24 h. The leaves and roots of V. dahliae infection treatments were harvested at 0, 1, 3, 6, 12, 24 h and the leaves of 1% H2O2 were harvested at 0, 30 min, 1, 3, 6, 12 h.
Isolation of full-length cDNA
Total RNA was extracted of above treatments with RNA plant plus reagents (Tiangen, China). cDNA first-strand was synthesized from 2 μg total RNA using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen, China), following the manufacturer′s protocol. According to the sequences homology of AP2 DNA-binding domain (AAEIRD) and conserved domain (PPLSPLSP), the homologous primers for middle segments were designed (Supplemently Table 1) to amplify the fragment of GbERFb which was amplified by PCR. Using the Smart™ RACE cDNA amplification kit (TaKaRa, Japan), the 3′cDNA end were amplified by the aid of primer (Supplemently Table 1). Using the 5′/3′ RACE kit 2nd Generation (ROCHE, Switzerland), the 5′cDNA end were amplified by the aid of primer (Supplemently Table 1). All PCR products were purified, cloned into the pEASY-T3 vector and sequenced. Basing the nucleotide sequences of the 5′-/3′-RACE products, the full length cDNA primers (Supplemently Table 1), were used for the amplification of the full-length sequence of GbERFb.
Bioinformatic and phylogenetic analyses
Sequence homology analysis and sequence identities were performed using BLAST tools (http://www.ncbi.nlm.nih.gov/BLAST/). Conserved sequence was analyzed using conserved-domain search tool (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Tertiary structure was predicted using Scratch (http://www.ics.uci.edu/~baldig/scratch/). A phylogenetic analysis of the GbERFb was performed using MEGA 5 software. The GbERFb protein phosphorylation sites were predicted by NetPhos 2.0 Server (http://www.cbs.dtu.dk/cgi-bin/). nuclear localization signals (NLSs) were predicted using the motif_scan (http://myhits.isb-sib.ch/cgi-bin/motif_scan).
Quantitative real-time RT-PCR for GbERFb expression analysis
Total RNA extraction and cDNA synthesis were described above. Quantitative real-time RT-PCR was performed using TransStart Top Green qPCR SuperMix (TransGen, China). Transcription quantitative analysis of gene expression used Histone 3 as reference gene for normalization (Supplemently Table 1). The relative expression level of the target gene was calculated using the \(2^{{ - \Delta \Delta C_{\text{T}} }}\). There were three replicates for each sample.
Yeast one-hybrid assay
A yeast one-hybrid assay was used to analyze the GCC binding activity of GbERFb. Reporter vector containing the 3 × GCC and 3 × mGCC was prepared and fused into the EcoR I and Sal I sites of the pLacZi vector respectively. The vector pLacZi, pGCC-LacZi and pmGCC-LacZi were digested by Nco I, and integrated into the yeast strain YM4271 severally. The GbERFb was fused into the Kpn I and Not I sites of the activation domain of the pYES3 vector (Invitrogen, USA). All primers were shown in supplemently Table 1. The vector pYES3 and pGbERFb-YES3 were respectively transformed into all above YM4271 strain. The yeast clones were cultured on SD/-Ura/-Trp with 2% galactose plates for 3 days at 30 °C. The β-galactosidase colony-lift filter assay was used to screen the colonies with X-Gal.
Yeast two-hybrid assay
For screening the GbERFb interaction with GbMAPK, the GbMAPKb (GenBank KT033508) were cloned by homologous cloning from Gossypium barbadense cv. Pima90–53, which shared high homology with SIPK in tobacco (Seo et al. 2007). The GbMAPKb was cloned into the pGBKT7 vector with the GAL4 binding domain (BD). The GbERFb was cloned into the pGADT7 vector with the GAL4 activation domain (AD). All primers were shown in Supplemental Table 1. The pGADT7/pGBKT7, pGADT7/p GbMAPKb-GBKT7, pGbERFb-GADT7/pGBKT7, pGbERFb-GADT7/pGbMAPKb-GBKT7, were co-transformed into the AH109 yeast strain. Positive clones were plated onto selective SD medium (DDO: SD/-Leu/-Trp with X-a-gal, QDO: SD/-Ade/-His/-Leu/-Trp with X-a-gal).
Generation of transgenic GbERFb tobacco plant and V. dahliae infections
Construction of the plant expression vector pGbERFb-FGC5941 (the design primers see Supplemently Table 1) and the vector was transformed into tobacco (NC89) using Agrobacterium tumefaciens mediated transformation. V. dahliae was cultured on potato dextrose agar plates for about 3 weeks at 25 °C in dark. Spores were collected from the cultures and washed twice with sterile water. The spores were suspended by 1 ml sterile water (2×107 spores ml−1). Four or five weeks old tobacco leaves were micro-damaged and pointed with spores about 100 μl. Disease situation was observed after 7 days and untransformed plants as control.
Results
Cloning and sequence analysis of the GbERFb gene from G. barbadense
The full length cDNA of 903 bp was obtained and deposited in GenBank (KR902546). The ORF of GbERFb is contained 663 bp encoding 221 amino acid. The putative GbERFb protein sequence contained the basic amino acid regions (K151RKREEEEEERRKVVKKE) that predicted as nuclear localization signal (NLS). The phosphorylation sites analysis showed three conserved motif of predicted phosphorylation sites (LS30PPPTPQFHKQSTLS44QRRPS49IN, NS143TEFQPS149NK, PPLS205PLS208PF) (Fig. 1.). The predict tertiary structure of GbERFb showed the ERF domain which contained one α-helix and three β-sheets, the nuclear localization signal region and putative phosphorylation sites (Fig. 2). The predicted protein weight of GbERFb is 24.88 kD and protein theoretical PI is 9.30. The deduced amino acid sequence of GbERFb had a typic ERF DNA binding domain of 59 amino acids (77–135) which shared high amino acid identity with the other ERF conserved domain (Fig. 3). Sequence alignment showed that the protein sequence of GbERFb has a low identity with other homologous ERFs, such as AdERF13 (38.44%), AtERF5 (32.45%), AtERF6 (34.63%), GhERF1 (35.45%). Especially, GbERFb showed low sequence identity with other known cotton ERFs, such as GhERF1 (35.45%), GbERF (16.29%), GbERF2(29.24%). Base on the category of Toshitsugu Nakano (Nakano 2006) and Phylogenetic tree analysis, GbERFb and GhERF1 belong to subgroup IXb but GhERF1 has no CMIX-5 and CMIX-6 motif. ERF-IXa5 and EREB1 belong to IXa and GhERF12 belongs to IXc. Other cotton ERFs belong to Group VII. The results showed that GbERFb is a novel cotton ERF protein (Fig. 4).
The conserved ERF domain of GbERFb with those of other closely related ERF proteins including AdERF13 (ADJ67442), AtERF5 (NP_568679), DcERF1 (BAF75651), GmERF (AAQ10777), GmERF7 (AEQ55266), JERF1 (AAK95687), MtERF (XP_013467284), NtERF4 (NP_001312428), slERF5 (NP_001234512), TcERF2 (EOY22776). The three β-sheets and one α-helix of the ERF domain are indicated over the corresponding sequences
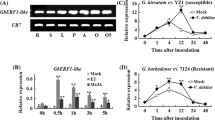
The expression patten of GbERFb induced by ET, MeJA, and SA
Many reports have been shown that ERFs participate in phytohormone metabolism in response to biotic stress. To explorer whether the GbERFb protein could be induced by hormone, the expression patterns of GbERFb was analyzed under ET, MeJA and SA treatment by qPCR. The results showed that GbERFb was up-regulated by foliar spraying of ET, MeJA and SA (Fig. 5a). In the treatment with ET, GbERFb expression showed no significanet change within 3 h, then increased rapidly and reached a peak at 6 h (~13-fold over the control), followed by a gradually decline. There was a similar expression pattern of GbERFb with the treatment of MeJA and SA, while their peaks was at 12 and 3 h respectively.
Expression patterns of GbERFb in Sea island cotton. a The effect of exogenous hormones treatments with ET, MeJA and SA in leaves, b the effect of exogenous H2O2 and wounding in leaves, c the effect of inoculating Verticillium dahliae in leaves and roots. Data are averages of three replicates by mean ± SD
Trancription of GbERFb in response of wounding, H2O2 and V. dahliae
The expression of GbERFb was analyzed under the wounding, H2O2 and inoculation with V. dahliae (Fig. 5b, c). After wounding treatment, the expression of GbERFb was up-regulated slightly about 3-fold at the 24 h in injured leaves and it was surmised when leaves were injured GbERFb take part in the response to wounding. The expression pattern of H2O2 treatment was up-regulated and the peak was at 3 h (~ninefold over the control). When inoculating the V. dahliae, the accumulation mRNA of GbERFb was increased both in leaves and roots. The variation tendency was the same in the leaves and the roots, and GbERFb peaked after 3 h about threefold over the control.
GbERFb specific interaction with the GCC box in vivo
To assess if GbERFb could specifically bind to the GCC box in vivo, yeast one-hybrid experiment was performed. The reporter and effector were constructed and transformed to the yeast YM4271, including pLacZi and pYES3, pLacZi and pYES3-GbERFb, p3 × GCC-LacZi and pYES3, p3 × GCC-LacZi and pYES3-GbERFb, p3 × mGCC-LacZi and pYES3, p3 × mGCC-LacZi and pYES3-GbERFb. The result showed that only the YM4271 contained the p3 × GCC-LacZi and pYES3-GbERFb plasmids could strongly active the expression of LacZ gene, which indicated that GbERFb could activate the expression of down-stream gene which its promoter contained the cis-element of the GCC box in yeast (Fig. 6).
Analysis of the GbERFb transcriptional activation activity with the GCC sequence by yeast one hybrid. a Diagram of the reporters, effectors construct used in the assays. PCYC1 the promoter of the minimal promoter of the yeast iso-1-cytochrome C gene, PGAL1 the promoter of galactose-inducible expression of gene, CYC1 terminater the terminater of the Cytochrome transcription gene. b Comparison of the transactivation activity of GbERFb and other control by lifted filter in various yeast cells. 1 pLacZi/pYES3, 2 pLacZi/pYES3-GbERFb, 3 p3×mGCC-LacZi/pYES3, 4 p3×mGCC-LacZi/pYES3-GbERFb, 5 p3×GCC-LacZi/pYES3, 6 p3×GCC-LacZi/pYES3-GbERFb
GbERFb likely interacted with GbMAPKb in yeast
Previous states showed the protein which contain the “PPLSPLSP” motif could be phosphorylated by tobacco WIPK/SIPK or homologous gene in other plant species (Seo et al. 2007; Meng et al. 2013; Ogata et al. 2015). To determine if GbERFb could be phosphorylated by MAPK, we first cloned the tobacco SIPK homologous gene GbMAPKb in G. barbadense (GenBank KT033508), and then tested the interaction between GbERFb and GbMAPKb by yeast two-hybrid assay. The GbMAPKb was fused to the GAL4 DNA binding domain of pGBKT7 vector as the bait, while the GbERFb was fused to the GAL4 activation domain of pGADT7 vector as the prey. The beit and the prey were co-introduced into the yeast strain AH109 and the interaction was quantified by X-α-gal. The result showed that all transformants could grow on DDO plates, but only transformed with pGbERFb-GADT7/pGbMAPKb-GBKT7 could be induced, while only transformed with pGbERFb-GADT7/pGbMAPKb-GBKT7 could grow on QDO plates and be induced (Fig. 7). These results indicated that GbERFb could interact with the GbMAPKb directly in yeast.
Interaction between the GbERFb and the candidate protein GbMAPKb by yeast two hybrid. The competent cell AH109 harbored the Gal4 DNA-binding domain (BD) and Gal4 activation domain (AD) fusion constructions. The pGbERFb-GADT7/pGbMAPKb-GBKT7 and other control were selected on SD medium lacking Leu and Trp (DDO) and interaction was selected on SD medium lacking Leu, Trp, His and Ade (QDO) for 5 days. All medium contained the X-a-gal
Over-expression GbERFb in tobacco enhances the fungus resistance to V. dahliae
To investigate the function of GbERFb in resistance to V. dahliae, we generated transgenic tobacco plants that over-expression of GbERFb using Agrobacterium tumefaciens-mediated transformation. Three transgenic tobacco lines were chosen for further analysis. The result showed that all three GbERFb over-expression lines were observed a significant reduction in the development of disease spots, while the control line showed about a third part necrotic lesions (Fig. 8). The statistics showed that over-expression of GbERFb could enhance disease resistance to V. dahliae in tobacco.
Discussion
AP2/ERF family, one of the largest transcription factor family in plant, is demonstrated to be concerned with biotic and abiotic stresses (Pre et al. 2008; Mizoi et al. 2012; Licausi et al. 2013; Shoji et al. 2013). In this study, a novel ERF transcription factor, named GbERFb was isolated and characterized from Sea island cotton. Bioinformatics analysis showed that GbERFb possessed a basic amino acid domain (K151RKREEEEEERRKVVKKE) that function as nuclear localization signal, which indicated GbERFb could target to the nucleus. Meanwhile, GbERFb has a conserved ERF DNA binding domain (59 amino acids) and yest one-hybrid experiment proved that GbERFb could be bind to GCC box cis-acting element in yeast. The results suggested that GbERFb could activate downstream genes expression by binding to the GCC box in promoter.
According to the amino acids difference, Sakuma et al. (2002) divided the A. thaliana ERF family into ERF subfamily and DREB subfamily. Afterwards, Nakano (2006) divided the A. thaliana ERF subfamily into 12 groups. Phylogenetic analysis showed that GbERFb belongs to subgroup IXb which possessed a conserved putative MAP kinase phosphorylation site with “PPLSPLSP”. It was reported that the genes in group IX have often been involved in response to pathogen Infection. For example, over-expression of A. thaliana ERF1 enhanced resistance to necrotrophic fungi (Berrocal-Lobo et al. 2002), constitutive expression of OsBIERF3 results in increased disease resistance in transgenic tobacco (Cao et al. 2005). Sequence analysis also revealed that GbERFb shares very low sequence similarity (<35.5%) with other published ERF proteins of cotton, including GbERF (accession number AAT77192), GbERF2(accession number AAT77191), GhERF1(accession number AAO59439), GhERF12(accession number AGZ87835), and EREB1(accession number ABN50365) and they belong to different group or subgroup. These results suggested that GbERFb encodes a novel member of the ERF family of cotton and may be involved in disease resistant regulation as a transcription factor.
ET, SA and MeJA as important plant signaling molecules had been showed to take part in defense response pathway (Reymond and Farmer 1998; Pieterse and van Loon 1999). Generally, these hormones signaling pathways regulate effectively against specific types, such as SA activating resistance against biotrophic pathogens, while JA playing a vital role in necrotrophic pathogens (Glazebrook et al. 2003). The cross-talk between ET/JA, and ET/SA signaling is thought to be fine-tune induced defenses in response to multiple attackers through the common factors of signal transduction pathways, such as ERFs (Leon-Reyes et al. 2010). Most of disease-related ERF genes shows a different expression patterns by hormone induced (Mazarei et al. 2007; Gao et al. 2008; Pre et al. 2008; Jin et al. 2016). The ERFs in tomato, Pti5 and Pti6 could only be induced by JA and ET, while Pti4 could be induced by both ET, JA and SA (Gu et al. 2000). In A. thaliana ERFs, ERF1, ERF14, ORA59 could be induced by JA and ET (Berrocal-Lobo et al. 2002; Pre et al. 2008), while ERF5 and ERF6 could be induced by ET, JA and SA (Moffat et al. 2012; Son et al. 2012; Sewelam et al. 2013). In this study, GbERFb could be not only strongly up-regulated by ET, SA and MeJA, but also induced by H2O2 and wounding. Recent studies suggested Reactive oxygen species (ROS) were closely associated with plant hormones, and play essential roles as highly specific signaling molecules under stress conditions (Mittler et al. 2011). In A. thaliana, ERF6 was strongly induced by H2O2, further studies suggested that ERF6-mediated oxidative stress signaling is intimately linked to pathogen defense signaling. Wounding is a common damage caused by biotic and abiotic stress, which could activate defense-related gene to prevent further damage. Some ERFs were also regulated by wounding, such as AaERF (Lu et al. 2013) and LeERF1/2/4 (Tournier et al. 2003). Our results showed that GbERFb was obviously up-regulated at 12 h post wounding. These results suggested GbERFb participate in a variety of signaling process and play a multiple role in response to diverse stresses.
Post-transcriptional regulation by phosphorylation is a significant regulation form in plant. Previous studies suggested that some ERFs could be phosphorylated by MAPKs, such as ERF6 were identified as the substrate of MAPK3/MAPK6 and play an important role in changing downstream ROS-responsive genes transcription and resistance to the fungal pathogen B. cinerea (Meng et al. 2013; Wang et al. 2013). However, ERFs of cotton phosphorylated by MAPKs has not been identified and the function of ERF mediated by MAPK still remain unclear. In our research, GbERFb could interact with the GbMAPKb which is homologous to SIPK gene in tobacco. It indicated that GbERFb could be phosphorylated by GbMAPKb and active the down-stream pathogen related gene expression.
To confirm the role of GbERFb in the resistance to V. dahliae, we generated the GbERFb over-expressing tobacco lines and tested the resistance to V. dahliae. The results showed that after 7 days inoculated with V. dahliae, the control lines gave rise to significant disease spot, while the transgenic lines did not appear the obvious disease spot. So we conclude that GbERFb is a positive regulator transcription factor of the resistance to V. dahliae in Sea island cotton by mediate complex various defense responses.
In conclusion, GbERFb was involved in hormones (ET, SA and MeJA), H2O2 and wounding signalling pathways. Yeast one-hybrid experiment confirmed that GbERFb have GCC box binding activity and yeast two-hybrid assay suggested that GbERFb could be phosphorylated by MAP kinase. Over-expression of GbERFb showed enhanced resistance to V. dahliae in the 35S::GbERFb transgenic tobacco. we conclude that GbERFb, putative substrate of MAP kinase, plays important roles in response in defense against fungal pathogens by regulating multiple signal transduction pathways. Clone and characterization of GbERFb will be helpful for further improving V. dahliae tolerance in cotton.
Abbreviations
- ERF:
-
Ethylene responsive factor
- AP2/ERF:
-
APETALA2/ethylene-responsive factor
- SA:
-
Salicylic acid
- MeJA:
-
Methyl jasmonate
- PR:
-
Pathogenesis related protein
- ORF:
-
Opening reading frame
- ROS:
-
Reactive oxygen species
References
Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29:23–32. doi:10.1046/j.1365-313x.2002.01191.x
Cai Y, Xiaohong H, Mo J, Sun Q, Yang J, Liu J (2012) Molecular research and genetic engineering of resistance to Verticillium wilt in cotton: a review. Afr J Biotechnol 8:7363–7372
Cao Y, Wu Y, Zheng Z, Song F (2005) Overexpression of the rice EREBP-like gene OsBIERF3 enhances disease resistance and salt tolerance in transgenic tobacco. Physiol Mol Plant Pathol 67:202–211. doi:10.1016/j.pmpp.2006.01.004
Dong N, Liu X, Lu Y, Du L, Xu H, Liu H, Xin Z, Zhang Z (2010) Overexpression of TaPIEP1, a pathogen-induced ERF gene of wheat, confers host-enhanced resistance to fungal pathogen Bipolaris sorokiniana. Funct Integr Genomics 10:215–226. doi:10.1007/s10142-009-0157-4
Dong W, Ai X, Xu F, Quan T, Liu S, Xia G (2012) Isolation and characterization of a bread wheat salinity responsive ERF transcription factor. Gene 511:38–45. doi:10.1016/j.gene.2012.09.039
Gao S, Zhang H, Tian Y, Li F, Zhang Z, Lu X, Chen X, Huang R (2008) Expression of TERF1 in rice regulates expression of stress-responsive genes and enhances tolerance to drought and high-salinity. Plant Cell Rep 27:1787–1795. doi:10.1007/s00299-008-0602-1
Glazebrook J, Chen W, Estes B, Chang H, Nawrath C, Metraux J, Zhu T, Katagiri F (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J 34:217–228. doi:10.1046/j.1365-313X.2003.01717.x
Gu YQ, Yang C, Thara VK, Zhou J, Martin GB (2000) Pti4 is induced by ethylene and salicylic acid, and its product is phosphorylated by the Pto kinase. Plant Cell 12:771–786. doi:10.1105/tpc.12.5.771
Guo W, Jin L, Miao Y, He X, Hu Q, Guo K, Zhu L, Zhang X (2016) An ethylene response-related factor, GbERF1-like, from Gossypium barbadense improves resistance to Verticillium dahliae via activating lignin synthesis. Plant Mol Biol 91:305–318. doi:10.1007/s11103-016-0467-6
He P, Warren RF, Zhao T, Shan L, Zhu L, Tang X, Zhou J (2001) Overexpression ofPti5 in tomato potentiates pathogen-induced defense gene expression and enhances disease resistance to Pseudomonas syringae pv. tomato. Mol Plant Microbe Interact 14:1453–1457. doi:10.1094/MPMI.2001.14.12.1453
Hernandez-Garcia CM, Finer JJ (2016) A novel cis-acting element in the GmERF3 promoter contributes to inducible gene expression in soybean and tobacco after wounding. Plant Cell Rep 35:303–316. doi:10.1007/s00299-015-1885-7
Jin J, Zhang H, Tan J, Yan M, Li D, Khan A, Gong Z (2016) A new ethylene-responsive factor CaPTI1 gene of pepper (Capsicum annuum L.) involved in the regulation of defense response to Phytophthora capsici. front. Plant Sci. doi:10.3389/fpls.2015.01217
Leon-Reyes A, Du Y, Koornneef A, Proietti S, Körbes AP, Memelink J, Pieterse CMJ, Ritsema T (2010) Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol Plant Microbe Interact 23:187–197. doi:10.1094/MPMI-23-2-0187
Li C, He X, Luo X, Xu L, Liu L, Min L, Jin L, Zhu L, Zhang X (2014) Cotton WRKY1 mediates the plant defense-to-development transition during infection of cotton by Verticillium dahliae by activating JASMONATE ZIM-DOMAIN1 expression. Plant Physiol 166:2179–2194. doi:10.1104/pp.114.246694
Licausi F, Ohme-Takagi M, Perata P (2013) APETALA2/ethylene responsive factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol 199:639–649. doi:10.1111/nph.12291
Lorenzo O (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell Online 15:165–178. doi:10.1105/tpc.007468
Lu X, Jiang W, Zhang L, Zhang F, Zhang F, Shen Q, Wang G, Tang K (2013) AaERF1 positively regulates the resistance to botrytis cinerea in Artemisia annua. PLoS ONE 8:e57657. doi:10.1371/journal.pone.0057657
Mazarei M, Elling AA, Maier TR, Puthoff DP, Baum TJ (2007) GmEREBP1 is a transcription factor activating defense genes in soybean and Arabidopsis. Mol Plant Microbe Interact 20:107–119. doi:10.1094/MPMI-20-2-0107
Meng X, Li F, Liu C, Zhang C, Wu Z, Chen Y (2010) Isolation and characterization of an ERF transcription factor gene from cotton (Gossypium barbadense L.). Plant Mol Biol Rep 28:176–183. doi:10.1007/s11105-009-0136-x
Meng X, Xu J, He Y, Yang KY, Mordorski B, Liu Y, Zhang S (2013) Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 25:1126–1142. doi:10.1105/tpc.112.109074
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309. doi:10.1016/j.tplants.2011.03.007
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta (BBA) Gene Regul Mech 1819:86–96. doi:10.1016/j.bbagrm.2011.08.004
Moffat CS, Ingle RA, Wathugala DL, Saunders NJ, Knight H, Knight MR (2012) ERF5 and ERF6 play redundant roles as positive regulators of JA/Et-mediated defense against botrytis cinerea in Arabidopsis. PLoS ONE 7:e35995. doi:10.1371/journal.pone.0035995
Nakano T (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432. doi:10.1104/pp.105.073783
Ogata T, Okada H, Kawaide H, Takahashi H, Seo S, Mitsuhara I, Matsushita Y (2015) Involvement of NtERF3 in the cell death signalling pathway mediated by SIPK/WIPK and WRKY1 in tobacco plants. Plant Biol 17:962–972. doi:10.1111/plb.12349
Ohme-Takagi M (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell Online 7:173–182. doi:10.1105/tpc.7.2.173
Onate-Sanchez L, Anderson JP, Young J, Singh KB (2006) AtERF14, a member of the ERF family of transcription factors, Plays a nonredundant role in plant defense. Plant Physiol 143:400–409. doi:10.1104/pp.106.086637
Pieterse CMJ, van Loon LC (1999) Salicylic acid-independent plant defence pathways. Trends Plant Sci 4:52–58. doi:10.1016/S1360-1385(98)01364-8
Pre M, Atallah M, Champion A, De Vos M, Pieterse CMJ, Memelink J (2008) The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol 147:1347–1357. doi:10.1104/pp.108.117523
Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1:404–411. doi:10.1016/S1369-5266(98)80264-1
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290:998–1009. doi:10.1006/bbrc.2001.6299
Seo S, Katou S, Seto H, Gomi K, Ohashi Y (2007) The mitogen-activated protein kinases WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants. Plant J 49:899–909. doi:10.1111/j.1365-313X.2006.03003.x
Sewelam N, Kazan K, Thomas-Hall SR, Kidd BN, Manners JM, Schenk PM (2013) Ethylene response factor 6 is a regulator of reactive oxygen species signaling in Arabidopsis. PLoS ONE 8:e70289. doi:10.1371/journal.pone.0070289
Shoji T, Mishima M, Hashimoto T (2013) Divergent DNA-binding specificities of a group of ETHYLENE RESPONSE FACTOR transcription factors involved in plant defense. Plant Physiol 162:977–990. doi:10.1104/pp.113.217455
Son GH, Wan J, Kim HJ, Nguyen XC, Chung WS, Hong JC, Stacey G (2012) Ethylene-responsive element-binding factor 5, ERF5, is involved in chitin-induced innate immunity response. Mol Plant Microbe Interact 25:48–60. doi:10.1094/MPMI-06-11-0165
Tournier B, Sanchez-Ballesta MT, Jones B, Pesquet E, Regad F, Latché A, Pech J, Bouzayen M (2003) New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett 550:149–154. doi:10.1016/S0014-5793(03)00757-9
Vogel MO, Moore M, Konig K, Pecher P, Alsharafa K, Lee J, Dietz KJ (2014) Fast retrograde signaling in response to high light involves metabolite export, MITOGEN-ACTIVATED PROTEIN KINASE6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell 26:1151–1165. doi:10.1105/tpc.113.121061
Wang P, Du Y, Zhao X, Miao Y, Song CP (2013) The MPK6-ERF6-ROS-responsive cis-acting Element7/GCC box complex modulates oxidative gene transcription and the oxidative response in Arabidopsis. Plant Physiol 161:1392–1408. doi:10.1104/pp.112.210724
Zhai Y, Wang Y, Li Y, Lei T, Yan F, Su L, Li X, Zhao Y, Sun X, Li J, Wang Q (2013) Isolation and molecular characterization of GmERF7, a soybean ethylene-response factor that increases salt stress tolerance in tobacco. Gene 513:174–183. doi:10.1016/j.gene.2012.10.018
Zhang B, Yang Y, Chen T, Yu W, Liu T, Li H, Fan X, Ren Y, Shen D, Liu L, Dou D, Chang Y (2012) Island cotton Gbve1 gene encoding a receptor-like protein confers resistance to both defoliating and non-defoliating isolates of Verticillium dahliae. PLoS ONE 7:e51091. doi:10.1371/journal.pone.0051091
Zhang Z, Zhao J, Ding L, Zou L, Li Y, Chen G, Zhang T (2016) Constitutive expression of a novel antimicrobial protein, Hcm1, confers resistance to both Verticillium and Fusarium wilts in cotton. Sci Rep Uk 6:20773. doi:10.1038/srep20773
Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, Zhou D (2015) The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell 27:2469–2483. doi:10.1105/tpc.15.00227
Zhu X, Qi L, Liu X, Cai S, Xu H, Huang R, Li J, Wei X, Zhang Z (2014) The wheat ethylene response factor transcription factor PATHOGEN-INDUCED ERF1 mediates host responses to both the necrotrophic pathogen Rhizoctonia cerealis and freezing stresses. Plant Physiol 164:1499–1514. doi:10.1104/pp.113.229575
Zuo K, Qin J, Zhao J, Ling H, Zhang L, Cao Y, Tang K (2007) Over-expression GbERF2 transcription factor in tobacco enhances brown spots disease resistance by activating expression of downstream genes. Gene 391:80–90. doi:10.1016/j.gene.2006.12.019
Acknowledgements
We would like to thank to Sha Tang (Chinese Academy of Agricultural Sciences) for critical review of this manuscript. This work was Sponsored by State Key Laboratory of Cotton Biology Open Fund (CB2015A15) and Natural Science Foundation of Hebei Province (C2014208093).
Author contributions
J. Liu, Y. Wang and H. Zhang conceived and designed the research. J. Liu, Y. Wang, G. Zhao carried out the experiments. J. Zhao, H. Du contributed the data analysis, J. Liu, Y. Wang, H. Zhang and X. He wrote the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Jianguang Liu and Yongqiang Wang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., Wang, Y., Zhao, G. et al. A novel Gossypium barbadense ERF transcription factor, GbERFb, regulation host response and resistance to Verticillium dahliae in tobacco. Physiol Mol Biol Plants 23, 125–134 (2017). https://doi.org/10.1007/s12298-016-0402-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-016-0402-y