Abstract
Although most of the babies are born healthy and appear normal, a few babies exhibit abnormal medical conditions. Newborn screening for inborn errors of metabolism is an established panel of tests that assist in the timely recognition of treatable disorders. 8007 Neonates born in a well known hospital from August 2019 to August 2021 were screened for the following five tests: Thyroid stimulating Hormone, 17-hydroxy progesterone (17-OHP), Total Galactose(GAL), Glucose 6 Phosphate Dehydrogenase (G6PD) and Biotinidase (BTD). Dried blood spots (DBS) were processed for the above tests using Enzyme-linked immunosorbent assay (ELISA), colorimetric, and dissociation-enhanced lanthenide-fluroscent immunoassay (DELFIA) techniques. DBS with abnormal results were retested for confirmation. Affected infants were recalled for venous blood collection for confirmation. We found 4 newborns with Hypothyroidism (CH 1: 2002), 4 with congenital adrenal hyperplasia (CAH 1:2002), 9 with G6PD deficiency (1:900), one with galactose-phosphate-uridyl transferase deficiency (1: 8000) and one with biotinidase deficiencyduring the study period. Parents of G6PD deficient babies were counseled. Congenital Hypothyroidism (CH) and Congenital Adrenal Hyperplasiababies were treated and followed up to find the response. The outcome of the screening result shall prevent the family and society in turn from facing severe and unbearable consequences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early detection of metabolic and genetic disorders by screening newborn babies has been an integral part of neonatal care. Timely treatment or intervention can help prevent intellectual defects and life-threatening illnesses [1]. Newborn screening in India began in the early 2000s, initially targeting conditions like congenital hypothyroidism and phenylketonuria (PKU). The Indian Council of Medical Research (ICMR) and state governments, Kerala, Goa and Chandigarh, have since expanded programmes to include other metabolic disorders. The National Health Mission supports these efforts, aiming to reduce infant mortality and morbidity. Policies emphasize early detection and treatment to improve outcomes [2]. Despite progress, challenges like limited coverage, infrastructure, and awareness persist. Recent initiatives focus on expanding the scope and reach of screening, ensuring equitable access to all newborns across the country [3, 4].Through early identification, newborn screening tests (NBST) provides an opportunity for treatment before organ damage or death occurs, leading to a significant reduction in morbidity and mortality [5].
It has been more than 50 years since NBST started gaining importance after testing for phenylketonuria was successfully accomplished [6, 7]. There has been increasing awareness of newborn screening in India for the last 10–15 years, with a rise in the availability of screening methods. Pune city is situated in western part India, 200 km away from Mumbai, with a population of 7.5 million. There is no mandatory NBST program. It is encouraging that some institutes do perform NBST, but it differs from region to region. Pediatricians from our hospital recommended five parameters, as per Wilson and Junger criteria [8], the abnormal results of which can be treated, managed, and monitored, with the view that (a) the abnormal condition can have a health problem or frequency (b) the availability of acceptable (reliable and simple) test performance; (c) the disorder not manifesting at birth; (d) treatment can prevent morbidity and mortality; (e) a delay in diagnosis can cause irreversible damage and; (f) the treatment cost can be affordable enmasse. The parameters are TSH, 17-OHP, Gal, G6PD, and BTD.
There is a lot of inconsistency in the availability of newborn screening facilities in our country. Nevertheless, we have not yet reached the stage where it becomes a national program and each baby undergoes screening. When a defect gets identified at an early-stage, treatment can be initiated, and it has been observed that such babies turn out to have attained normalcy.
When we survey the reported data on newborn screening in India, we see that 1:250 babies are affected each year [9]. By making newborn screening a national program, it would be helpful to reduce the burden of treatment and rehabilitation. There are around 0.15 million live births in Pune per year (30,000 in rural areas and 70,000 in urban areas). It is very encouraging that the Indian government has initiated UMMID (Unique Methods of Management and Treatment of Inherited Disorders) and assigned five NIDAN (National Inherited Diseases and Administration) kendras supported by the Department of Biotechnology.
The Clinical Biochemistry department of Deenanath Mangeshkar Hospital and Research Centre (DMH), Pune, Maharashtra, situated in the western part of India, undertook the responsibility of carrying out newborn screening tests for the above mentioned five tests. Phenylketonuria, is very rare in Maharashtra, India. Phenylketonuria being almost absent in Pune, was not included in the screening program.
Methods
First Phase: ELISA and Colorimetric Technique for Newborn Screening
DMH entered the Newborn Screening program in 2018. The Dried Blood Spot (DBS) spotted on the provided filter papers were outsourced for the measurement of TSH, 17-OHP, and G6PD.The obtained results were delivered to the referring clinicians. We had no confidence in the transport conditions or quality control status.
Therefore, in 2019, the Department of Clinical Biochemistry standardized the ELISA and colorimetric techniques using the kits obtained from Synergy Diagnostics for the measurement of TSH, 17-OHP, G6PD, GAL, and biotinidase in the DBS spotted on Whatman (903) filter paper. DBS were measured for the above analytes from August 2019 to August 2021. 4598 neonates were screened during the period. The first 500 blood spots collected on days 2 or 3 of birth were used to obtain the hospital-based reference range.
The criteria of age and health of mothers, gender distribution, normal distribution of data, and ethical compliance were adhered to derive a reference range.
Education: All mothers enrolled at our hospital for delivery were given the newborn screen brochure mentioning the tests and their clinical significance. Parents were educated about the benefits of the tests, the cost involved, and the required attention in case of any abnormal condition found, at the time of registration and during the third trimester visit. All the nurses and the residents were trained to collect heel-prick blood and spot it on the provided Whatman filter paper attached to the request form, dully fill it in, and allow it to dry. The request form included information on the mother’s name with age, mobile number, father’s name with age, gestational age, child’s date of birth and weight, mode of delivery and date of blood collection, and contact number. The billing section collected the amount at the time of discharge.
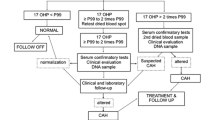
TSH and 17-OHP were processed by enzyme immunoassay, and GAL, G6PD, and BTD were processed by enzymatic -color -based reactions [10,11,12,13,14]. DBS samples of 4620 babies born from August 2019 to August 2021 were analyzed. 32 parents did not permit to pricking the babies for NBST. The DBS were processed in two batches per week. The methods involved (a) punching the blood spots using a puncher, enabling to punch out a spot of 3.2 mm in diameter; (b) eluting the analytes using the elution buffer provided in the kit; and (c) keeping controls at regular intervals for observing intra-assay variation (Flow charts 1–5). Controls were processed in each batch to determine the inter-assay-variation. The samples with above/lower cut-off limits and the samples lying in the indeterminate range were retested (in single) on the retained spots for confirmation. If it was still abnormal, the patient was recalled telephonically for repeat testing on blood or serum. The cut-offs for identifying the disorders were as follows: CH > TSH 20 mU/L whole blood (WB), CAH 17-OHP > 90 ng/ml, galactosemia GAL > 11 ng/ml, G6PD deficiency G6PD < 2 U/g HB, and BTD deficiency BTD < 30 µmol/dL, respectively [14]. The indeterminate levels were CH 11–19 mU/L WB, CAH 60–90 ng/mL, galactosemia 5–10 ng/mL, G6PD 2–8 U/g Hb and BTD deficiency 20 -30 µmol/dL.
Flow Chart 1: Workflow for Thyroid Stimulating Hormone TSH
FlowChart 2: Workflow for 17 Hydroxy-Progesterone (17-OHP)
Flow Chart 3: Workflow for Glucose 6 Phosphate Dehydrogenase (G6PD)
Flow Chart 4: Total Galactose
Flow Chart 5: Workflow for Biotinidase
Abnormal levels were repeated on retained spots. If still found abnormal, the analytes were repeated on recollected blood samples; higher than cut-off TSH (serum T4 and TSH), higher than cut-off 17-OHP (serum 17-OHP & electrolytes and synacthen stimulation test), lower than cut-off G6PD (whole blood quantitative blood G6PD), higher than cut-off BTD (serum BTD), and higher than cut-off galactose (serum total galactose/Galactose uridyl phosphate transferase). Confirmed abnormal results were informed to the clinician, who in turn took the necessary actions.
Second Phase: DELFIA Technique for Newborn Screening
While measuring the analytes in the DBS using ELISA/colorimetric tests using Synergy kits, it was observed that the technique was consuming 5–6 h with the requirement of two or more technicians for every batch of 48 spots. Hence, we requested that the hospital management permit us to establish partial automation for the NBST program. The hospital granted permission to install Perkin Elmer (PE) system in July 2021. The analyzer included a dried blood spot puncher (Panthera-puncher 9, PE, Turku, Finland), a fluorescence reader (Victor 2D Fluorometer, PE) with multi -data processing software, a plate washer (DELFIA washer-disk remover, PE), a shaker (DELFIA plate shaker, PE), an incubator (Tri NEST incubator shaker, PE), and a − 80 °C freezer. PE provided the installation, operation, training, and evaluation certificates.
DELFIA kits were used for measuring all five analytes.Punching each blood spot and transferring it to the microwells was made easier. The addition of reagents to the wells was manual; washing the reacted wells was automatic. The developed fluorescence was measured, a calibration curve was plotted, and the test fluorescence was extrapolated, and the results were displayed automatically. The process was simple and consumed significantly less time (4–5 h), requiring only one technician as compared to the first -phase technique.In a typical sandwich-type DELFIA assay, samples or the eluates containing TSH were pipetted into antibody -coated microtitration plates. A reagent containing a second anti-hTSH antibody conjugated with a europium chelate label was also pipetted. After a short room temperature incubation on the shaker, a sandwich formed with hTSH molecules trapped and marked with the europium label. Separation of the unbound tracer was performed in a simple wash stage. An enhancement solution was then added to each well. This dissociated the europium and formed a new, highly fluorescent chelate, which was measured using the VICTOR Fluorometer. Fluorescence was proportional to the presence of the hTSH in the sample.The principle was similar to other assays (kit inserts were referred).
From September 2021 to December 2022, DBS of 3430 neonates delivered in the hospital were processed for measuring all five analytes. 21 parents did not permit us to process the DBS spots. The methods involved punching the blood spots of 3.2 mm in diameter using an auto puncher, transferring them to the microwells, adding eluting buffer, transferring the eluate to reaction wells, and processing them for fluorescence immunoassay. Controls were run at three different places for assessing intra-assay variation (C.V. % for TSH-3.4,17-OHP-4.5, GAL-5.8, G6PD-6.5,BTD-3.8). Controls were processed in each batch for determining the percentage inter-assay-variation (TSH-3.8,17-OHP-4.8, GAL-6.5, G6PD-6.8,BTD-5.4). (Tables 1 and 2).The first fifty DBS were processed by both ELISA and colorimetric (Synergy Diagnostics) and DELFIA (Perkin Elmer) techniques to evaluate the deviation between the methods. There was good agreement between the two methods. Hence, the same cut-offs were used for following the infants. If the value for any analyte was higher, above the cutoff, the assay was repeated from retained DBS as mentioned in the first phase. If it was still abnormal, the patient was recalled telephonically for repeat testing on blood or serum as we did during the first phase.
Statistical analysis: A two group parametric comparison study was used to compare the differences between male and female children.
Results
Inter-and intra assay variation was within acceptable limits (Table 2), indicating the validity of the results. Reported cut-offs were used to determine whether to repeat the retained spots or call the abnormal neonate for further work-up. However, we also determined the reference range for classifying the newborn babies delivered in DMH (Table 3). Mother’s weight, prevalence of CH, birth weight pattern, and gestation are similar in Nepal and Pune [15]. Table 4. Other available data do not mention gestation and birth weight. We have observed that both ELISA, colorimetric, and DELFIA methods were comparable and able to identify abnormal status efficiently (Tables 5 and 6). There was no gender difference in any of the parameters used for screening newborns for inborn errors of metabolism (Table 7). Four babies were found to have confirmed CH (higher TSH in DBS, lower serum free T4, and high TSH). They were treated, followed and found to have attained normalcy. Six of the neonates were found to have high 17-OHP in DBS. Four of them were found to have higher serum 17-OHP. They were treated by the pediatrician and are being followed by measuring serum 17-OHP. Eleven babies (all male children) were found to have G6PD deficiency on DBS. Two of them did not confirm to have deficiency when whole blood G6PD was measured. One of the neonates had higher galactose (22 mg/dL) on DBS. Galactose-uridyl phosphate transferase was also low. The treating pediatrician examined the neonate and is following the child. One neonate had lower than cutoff BTD level but could not be confirmed on a serum biotin assay (Table 8). A correlation analysis suggested the relation between birth weight and the results of the screening tests. TSH and 17-OHP were significantly related to birth weight (Table 8).The Table 9 shows the reported studies by various scientists in India with regard to the prevalence status.
2148 neonates were delivered through cesarean Section 13% of the neonates were preterm deliveries. 14% of the neonates weighing less than 2500 g required NICU admission, while 9% of the neonates were above 2500 g in birth weight. 72% of the neonates weighed more than 2500 g at birth; 1.5% were weighing more than 2500 g though delivered pre-term (Table 8).
Discussion
The current health scene in India demands the introduction of newborn screening on a mandatory basis. We constituted a platform by strengthening the infrastructure and manpower to carry out newborn screening tests (NBST) in Deenanath Mangeshkar Hospital & Research Centre, Pune, India. We introduced screening, congenital hypothyroidism, congenital adrenal hyperplasia, galactosemia, G6PD deficiency, and BTD deficiency. We also established an infrastructure to confirm the diagnosis observed by NBST using advanced techniques. By now, a cadre of metabolic physicians has been built up to treat these identified children with inborn errors of metabolism. In short, we have undertaken a public health program aiming to prevent undesired health outcomes through early detection, diagnosis, and treatment.
We initiated the program by, (i) educating the parents regarding the purpose and benefits of NBST so that they can permit their babies to undergo heel pricking (ii) training to collect heel prick spots, submitting to the laboratory, performing the assay and storage of filter paper spots, (iii) assessment of the results and preparation of the report (iv) delivering the report to the clinician with interpretations (v) following up the neonates, if found positive for endocrine metabolic disorder, recall to repeat the test (vi) collect venous blood (vii) monitoring the program for improvement and proposing for higher technology to the hospital management which could offer support to the society. We found no gender difference in any of the screening tests.
At Sir Ganga Ram Hospital, situated in the northern part of India, NBST has been screening for four disorders since 2006: hypothyroidism, congenital adrenal hyperplasia, G6PD, and BTD deficiency. Among 16,832 newborns screened up to August 2014, the incidence of CH was 1:1870, of CAH 1:4208, and for G6PD deficiency, 1:263 in males, and 1:1402 in females [16]. In the absence of screening, the most common indication for G6PD requisition is hemolytic jaundice. It is, therefore, recommended that G6PD testing be done after an acute hemolytic episode has subsided. Bhutani and colleagues insisted on the inclusion of G6PD deficiency in the NBST program [17]. Kapil and colleagues [16] found an incidence of CH 1:22 (almost 5%) in Himachal Pradesh, which is an iodine deficient area. In most of the studies, the incidence of CH is higher in India than in the West. The incidence of CAH is higher in the southern states than in the northern states.
Preventing these disorders is a better choice than letting these disabilities develop and then trying to treat them.
The Indian Council of Medical Research (ICMR) initiated a Task Force on Inborn Metabolic Disorders to screen neonates in five centers in India: Delhi, Mumbai, Chennai, Hyderabad, and Kolkata, from 2007 to 2012 [19]. The overall number of newborns enrolled for screening was 103,849 for CH, and 103,712 for CAH. The incidence of CH overall was 1:1130 newborns, varying from 1:727 in Chennai to 1:1528 in Mumbai. The incidence of CAH overall was 1:5762 newborns, varying from 1:2036 in Chennai to 1:9983 in Mumbai. Similarly, Desai et al. in 1990 in Mumbai, found 1:2804 cases of CH, Pao et al. [20] found 2.83% of G6PD cases in Delhi [21], Dash et al. [22] found 17.5% cases of G6PD in Mizoram, Sanghvi and Diwakar [23], in Kerala, found 1:479 cases of CH, Kaur et al. in 2010, found 0.89% of G6PD, 1:3400 CH, and 1:6813 CAH [xx], Mohanty [25] in Bhuvaneshwar in 2014, found 16.75% of G6PD, Kumar et al.[26] in Bengaluru in 2014, found 1:1042 cases of CH, Shriram et al. in Tamil Nadu in 2014 [27] found 1:900 cases of CH and 1:2000 cases of CAH.
In this study, based on data from two and a half years, we found 1:900 cases of G6PD (only in male babies and none in females), 1:2000 cases of CH, 1:2000 cases of CAH, and one infant with galactosemia. G6PD is an X-linked recessive disorder, and therefore it was found in males only since most females could be carriers. These observations, wherein we screened 8007 infants, were more or less similar to the ICMR study. TSH and 17-OHP were related to birth weight, indicating the necessity to adjust the values to birth weights. We screened 8007 births.
Conclusion
Based on our findings, all hospitals in urban areas in India need to establish NBST for CH, CAH, galactosemia, biotinidase deficiency, and G6PD deficiency, because these are treatable disorders. The existing facilities for confirmation of diagnosis, follow –up, and treatment have been established in our hospital. A nation-wide screening program needs to be initiated by the government of India for these treatable disorders.
We would like to conclude by highlighting the importance of these tests being beneficial in halting the detrimental path of inborn errors of these metabolisms. Had these not been done in the above infants they would have landed in states of mental retardation and organ damage! We also observed that an institutional approach to newborn screening was beneficial, as all the super specialty branches were available under one roof and the diagnosis and treatment of these infants took place rapidly. Furthermore, in doing so, we also strengthen the knowledge and awareness, education of parents in the sense that early diagnosis is of value to the family concerned and, to the extent that it leads to increased use of prenatal diagnosis, may help to reduce the overall burden of disease and continuously improve the quality of the program.
Study strength and limitation: The only study where gender differences in newborn screening test parameters is measured.
The limitation of the study is that one child with a severe high TSH level could not be followed. 18 babies were found to have metabolic disorders. We have screened only 5% of the total live births in Pune city. It is an eye opener to evolve a National policy so that every live birth will be subjected to NBST.
References
van Karnebeek CDM, Stockler S. Treatable inborn errors of metabolism causing intellectual disability: a systematic literature review. Med Metab. 2012;105(3):368–81.
Mathur R. New born screening program in India: ICMR multicentric experience. Mol Cytogenet. 2014;7:140.
Thornell B, Padilla CD. Barriers to implementing sustainable national newborn screening in developing health systems. Int J Paed and Adolesc Med. 2014;1:49–60.
Kumta NB. Inborn errors of metabolism (IEM)—an Indian perspective. Ind J Paediatr. 2005;72:325–32.
Altimimi HA, Aljawadi HF, Ali EA. Inborn errors of metabolism in children with unexplained developmental delay in Misan. Iraq Oman Med J. 2019;34(4):297–301.
Gutrie R. Bloodscreeningforphenylketonuria. JAMA. 1961;178:863.
Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43.
Wilson JMG, Jungner G (1968) Principles and practice of screening for disease. World Health Organization Public Health Papers, No. 34
Dabas A, Bothra M, Kapoor S. CAH newborn screening in India: challenges and opportunities. Int J Neonatal Screen. 2020;6(3):70.
Diepenbrock F, Heckler R, Schickling H, Engelhard T, Bock D, Sander J. Colorimetric determination of galactose and galactose-1-phosphate from dried blood spots. Clin Biochem. 2014;25:37–9.
Frank JE. Diagnosis and management of G6PD Deficiency. Am Fam Phys. 2005;72(7):1277–82.
Heard GS, SecorMcvoy JR, Wolf B. A screening method for biotinidase in newborns. Clin Chem. 1984;30(1):125–7.
Bode HH, Scott AR, Cowley DM, Pardy K, Johnson S. Home monitoring of 17-hydroxy progesterone levels in congenital adrenal hyperplasia with filter paper blood samples. J Ped. 1999;134:185–9.
Kapil U, Jain V, Kabra M, Pandey RM, Sareen N, Khenduja P. Prevalence of neonatal hypothyroidism in Kangra Valley. Himachal Pradesh Eur J Clin Nutr. 2014;68:748–9.
Pandey AS, Joshi S, Rajbhandari R, Kanaskar P, Dhakal S. New born screening for selecteddisorders: a pilot study. Int J Neanat Screen. 2019;5(2):18.
Verma J, Roy P, Thomas DC, Jhingan G, Singh A, Bijarnia-Mahay S, Verma IC. Newborn screening for congenital hypothyroidism, congenital adrenal hyperplasia, and glucose-6-phosphate dehydrogenase deficiency for improving health care in India. J Pediatr Intensive Care. 2020;9(3):40–4.
Bhutani VK. Jaundice due to glucose-6-phosphate dehydrogenase deficiency. NeoReviews. 2012;13:e166–77.
ICMR Multicentric Study.Newborn Screening for Congenital Hypothyroidism and Congenital Adrenal Hyperplasia and High Risk Screening of Infants In: National Task Force for Inborn Errors of Metabolism. Draft Report. New Delhi: ICMR Multicentric Study; 2014.
Desai MP, Upadhye P, Colaco MP, Mehre M, Naik SP, Vaz FE, et al. Neonatal screening for congenital hypothyroidism using the filter paper thyroxine technique. Indian J Med Res. 1994;100(36–42):22.
Pao M, Kulkarni A, Gupta V, Kaul S, Balan S. Neonatal screening for glucose-6-phosphate dehydrogenase deficiency. Indian J Pediatr. 2005;72:835–7.
Dash S, Chhanhimi L, Chhakchhuak L, Zomawaia E. Screening for haemoglobinopathies and G6PD deficiency among the Mizos of Mizoram: a preliminary study. Indian J Pathol Microbiol. 2005;48(17–8):25.
Sanghvi U, Diwakar KK. Universal newborn screening for congenital hypothyroidism. Indian Pediatr. 2008;45(331–2):26.
Kaur G, Srivastav J, Jain S, Chawla D, Chavan BS, Atwal R, et al. Preliminary report on neonatal screening for congenital hypothyroidism, congenital adrenal hyperplasia and glucose 6 phosphate dehydrogenase deficiency: a Chandigarh experience. Indian J Pediatr. 2010;77(969–73):27.
Mohanty MD, Dash DK, Rath B. New born screening for glucose-6- phosphate dehydrogenase deficiency in Orissa, IMS and SUM hospital: a quantitative assay. Experiment. 2014;22:1525–30.
Kumar KR, Ranieri E, Fletcher J. Newborn screening for congenital hypothyroidism in India is overdue. J Neonatal Biol. 2014;3:12.
Shriram V, Shriraam M, Anitharani M, Selvavinayagam, Sathiyasekaran BWC. National health programs in the field of endocrinology and metabolism–Miles to go. Indian J Endocrinol Metab. 2014;18:7–12.
Goyal MG, Garg A, Goyal M, Kapoor S. New born screen for G6PD deficiency: A 2 yrs data from India. Int J public Health. 2015;59(2):145–8.
Kaur G, Thakur K, Singh TR, Chavan BS, Kaur G, Atwal R. Current and future perspectives of new born screen in Indian scenario. J Ped, End Metab. 2016;29(1):5–13.
Kommalur A, Devidas, Kariyappa M, Sabapathy S, Benkappa A, Ganga deep V, Sajjan SV, Laxminarayan SK, Dahshayani B, Chinnapp GD. New born screen for five conditions in a tertiary care government hospital in Bangaluru, S. India-3 yrs experience. J Trop Ped. 2020;66(3):284–49.
Radha Rama Devi A, Naushad SM. New born screen in India. Ind J Ped. 2004;71(2):157–60.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Manuscript prepared by SSN, SN. Technical procedures, done by others.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Consent to participate
Informed consent was obtained from all the parents of the neonates.
Informed consent
A written informed consent was obtained from all the parents of the neonates and were explained about the study and the study procedure.
Human and animals participants
Human Participants and Animals Approval was obtained from the ethics committee of the Deenanath Mangeshkar Hospital & Research Centre, Erandawane, Pune. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Narawade, S., Mahalle, N., Bhavar, S. et al. Birth Prevalence of Endocrine-Metabolic Disorders Detected by Newborn Screening Test in Pune (India) Population. Ind J Clin Biochem (2024). https://doi.org/10.1007/s12291-024-01250-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-024-01250-8