Abstract
Radiation therapy uses ionizing radiation (IR) to kill cancer cells. However, during radiotherapy normal cells are also damaged and killed by the generation of reactive oxygen species. Polyphenolic compounds are known to mitigate the damaging effects of radiation. Grape (Vitis vinifera) contains a variety of bioactive phytochemicals. We investigated the Ferric reducing antioxidant power assay for commonly available four grape (Vitis vinifera L.) cultivars, including ‘Flame seedless’, ‘Kishmish chorni’, ‘Red globe’ and ‘Thompson seedless’. Grape seed showed the maximum reducing power and antioxidant capacity, followed by its skin, and then pulp of the same cultivars. Kishmish chorni seed showed maximum reducing and antioxidant power. Therefore, we had selected the Kishmish chorni cultivars to determine the protective efficacy against γ-ray irradiated DNA damage and apoptotic gene expression in human peripheral lymphocytes, and their efficacy was compared with widely cultivated Thompson seedless Cultivars. Annexin V-FITC and propidium iodide double staining suggested that apoptosis is a major mode of induction of cell death after irradiation in human lymphocytes. Comet assay revealed that DNA damage in human lymphocytes due to gamma irradiation at a dose of 4-Gy is significantly (P < 0.05) mitigated by pretreatment with grape extracts. Bax and p53 mRNA levels that were up-regulated in gamma irradiated lymphocytes, were significantly down-regulated when irradiated lymphocytes were pretreated with grape extracts. In conclusion, the grape extracts of different cultivars act as an essential source of natural antioxidants at varying degree, which are able to attenuate DNA damage by scavenging free radicals, and regulate apoptosis by modulating apoptotic genes such as p53 and Bax in human lymphocytes induced by IR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ionizing radiation (IR) is used in various purposes, including diagnostic and therapeutic functions. In some cases, radiation may be the best treatment for cancer prevention [1]. However, during radiotherapy of cancer one of the severe side effects is toxicity to normal cells [2]. Low Linear Energy Transfer (LET) radiations act by generating the free radicals [3] which interact with different components of the cells including cell membrane and different intracellular molecules like DNA, RNA and proteins, resulting cellular dysfunction and mortality [3,4,5].
The immune system provides the first line of defense for exposure to environmental risks. The immune system cells are the most radiosensitive cells in the body [6]. Among them lymphocytes are important one [7]. Radio sensitivity of lymphocytes varied among different subtypes. B cells, natural killer (NK) and naive T cells are extremely sensitive to radiation, whereas memory T cells and Treg cells are more resistant to lethal effect of ionizing radiation (IR) [8,9,10,11]. As blood lymphocytes are highly radiosensitive and easily accessible, they are frequently used in biodosimetry [12,13,14].
The search for radioprotectors is an ambitious goal with many practical applications. Currently growth factors derived from granulocyte colony-stimulating factor - filgrastim and keratinocyte are the only approved radioprotectors for general use in humans for the prevention or treatment of radiation-induced haematopoietic injuries [15]. On the other hand, the synthetic thiol compound, the first FDA approved radioprotector amifostine is being used clinically as an adjuvant in radiotherapy of head and neck cancer [16]. While the growth factors are very expensive, the thiols including amifostine has severe side effects [15], limiting their practical applications [17]. Thus, a limited array of available medicinal for the prevention, mitigation or treatment of bodily injuries arising from IR exposure, accentuated the search for either less or non-toxic radioprotective compounds of biological origin. Epidemiological studies have revealed the association between the consumption of antioxidant-rich plant foods and prevention of oxidative-stress-related diseases [18]. Studies suggest that naturally occurring non-toxic phytochemicals, including curcumin, parthenolide, genistein, gossypol, ellagic acid, withaferin, plumbagin, resveratrol etc. may have considerable potential as radioprotectors [19].
Studies on plant extracts and phytochemicals as a radioprotective agents are a new area of research as the synthetic radioprotector possess several side effects. Therefore, it is necessary to assess the protective action of different plant extracts and phytochemicals against radiation induced damage. Grape extracts of different cultivars possess a variety of phytochemicals and are responsible for free radical scavenging activity and antioxidant activity [17, 19]. Compounds possessing free radical scavenging activity and antioxidant activity seem to inhibit or reduce toxicity of free radicals and thus offer protection against radiation [20]. As DNA is the major target of radiation, the ability of grape extracts to prevent radiation-induced DNA damage was investigated using plasmid DNA (pBR322) [21]. In that study, super-coiled pBR 322 plasmid DNA (~ 93%) is completely converted to open circular (~ 97%) and linear (~ 2%) form at a dose of 150 Gy γ-radiation. Pretreatment with 1.6 µg grape extracts reduced the DNA strand breaks as evidenced by an increase in the supercoiled form, with subsequent decrease in open circular form of DNA, indicating recovery of super coiled form [21]. However, to the best of our knowledge, no studies so far have been reported on the protective action of grape extracts of different cultivars against gamma radiation induced DNA damage and apoptosis in human lymphocytes. Therefore, we studied the radioprotective action of different cultivars of grape extracts against gamma radiation induced oxidative stress, DNA damage and gene expression in human lymphocytes. The aim of this study is to evaluate the free radical scavenging activity of grape extracts of commonly available four grape (Vitis vinifera L.) cultivars, including ‘Flame seedless’, ‘Kishmish chorni’, ‘Red globe’ and ‘Thompson seedless’. The radio-protective action of grape extracts of potential cultivar against most commonly available cultivars will be evaluated against γ-radiation (IR)-induced DNA damage and gene expression in human lymphocytes.
Materials and Methods
HiSep LSM 001, Frosted slide from the Hi Media Laboratories (Mumbai, India); Folin–Ciocalteu’s phenol reagent, ethanol from Merck; 2-Propanol, chloroform, isopropyl, heparin, from Sigma-Aldrich; bovine serum albumin (BSA) (fraction V), copper sulphate (CuSO4,5H2O), dimethyl sulphoxide, EDTA, ethidium bromide, low melting agarose, methanol, from SRL; Annexin V-FITC and propidium iodide (PI) double staining kit from Sigma-Aldrich; agarose from Saekem ® Lonza; Trizol from Takara Clontech; tips and PCR tubes for PCR from oxygen Clontech; DEPC from Amresco; DNA Ladder from Thermo Scientific; MMLVRT, Taq polymerase, dNTPS from Invitrogen; primers from Xcleris.
Grapes: Commonly accessible four grape (Vitis vinifera L.) cultivars, including ‘Flame seedless’ (black), ‘Kishmish chorni’ (black with reddish-brown), ‘Red globe’ (red) and ‘Thompson seedless’ (Sonaka, Green) were procured from the local vendors and validated from the Department of Fruits and Orchard Management, Faculty of Horticulture, Bidhan Chandra Krishi Viswavidyalaya, Nadia, West Bengal. Immediately after collection, one set of grapes were stored at 4°C for phytochemical analysis whereas other set of grapes were washed thoroughly and the skin, pulp and seeds were separated by squeezing the fruits, were individually crushed and lyophilized for preservation of the grape samples.
Preparation of Grape Extracts
Grape extracts were prepared by dissolving each lyophilized grape sample in 1X PBS (pH 7.4). Then it was centrifuged and supernatant was used for assays.
Ferric Reducing Antioxidant Power (FRAP) Assay
FRAP is a reducing power assay based on electron transfer in which the presence of reductants (antioxidants) in the sample reduce Fe3+ to Fe2+ complex by electron donation. The Fe2+ formation can be monitored spectrophotometrically by determining Perls Prussian blue colour formation at 700 nm. The increase in absorbance indicates a higher reducing power. For this experiment, 1 ml aqueous grape extract was added in 2.5 ml 200 mM of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml 1% of potassium ferricyanide. This mixture was then incubated for 20 min at 50 °C in water bath. These were then allowed to cool at room temperature, followed by addition of 2.5 ml 10% of TCA and the solution was mixed by vortexing. After centrifugation for 10 min at 3000 g, an aliquot of supernatant (2.5 ml) was added in 2.5ml of milliQ water and 500 µl of FeCl3 (0.1%) solution and the absorbance of the solution was monitored at 700 nm [20].
Blood Sample Preparation
Fresh blood samples were collected aseptically in heparinized tubes from willing healthy male donors (22–30 years) who had no history of disease or recent administration of any drug. Informed consent was obtained from each donor. One hour prior to irradiation, each blood sample was treated with 0.1 mg/ ml concentration of individual chosen grape extracts from seed, skin or pulp of each cultivar. One set of blood sample without any grape extract was irradiated and was labeled as irradiated control, while another set without any treatment (neither grape extract nor irradiation) was labeled as normal control. 0.1 mg/ ml concentrations of grape extracts were used for comet assay and mRNA expression of p53 and BAX.
In vitro γ-irradiation
Irradiation of the blood samples were carried out in a 60Co γ-radiation unit at a dose of 4 Gy (dose rate of 3.05 kGy/ h) at the UGC-DAE Consortium for Scientific Research, Kolkata Centre, Salt Lake City, Kolkata. The study was permitted by the Institutional Ethics Committee (No. F-24/ Pr/ CMJNMH/ IEC/ 14/ 93(4)) as per the ICMR guideline (ECR/674/Inst/WB/2014, dated 31/10/2014). After irradiation, samples were incubated at room temperature for one hour or two hour depending on the experiment, and then transferred to the laboratory on ice. Lymphocytes were isolated from blood samples by density gradient centrifugation using the HiSepLSM 001.
Determination of Viability and Apoptosis in Control and Treated Lymphocytes
The cytotoxic effect of plasma isolated from control and irradiated blood was assayed using an trypan blue exclusion technique [22]. Apoptosis in control and treated cells was determined by using Annexin V-FITC and propidium iodide (PI) double staining kit using a fluorescence microscope. At least 300 cells in the labeled cell suspensions were counted randomly. Cells showing only green fluorescence were considered apoptotic whereas cells showing only red or both green and red fluorescence were considered to be necrotic [23].
Comet Assay or Alkaline Single Cell Gel Electrophoresis
Evaluation of DNA damage was performed by comet assay under alkaline condition with few modifications [24]. In short, lymphocytes were suspended in low melting agarose (0.6%) and then layered over a frosted slide which was previously coated with a layer of normal melting agarose (0.75%) to ensure firm gripping. For solidification, slides were then placed at 4 °C. In order to lysis of the cell membrane and the nuclear membrane slides were submerged in lysis buffer (pH 10) and left overnight. Next day, slides were first wash with distilled water and then presoaked in electrophoresis buffer (alkaline solution of 0.3 M NaOH, 1 mM Na2EDTA; pH ≥ 13) for 30 min for unwinding of DNA. Then electrophoresis was carried out at 20 V, 300 mA for 20 min. After that the slides were washed for three times with neutralizing buffer (Tris 400 mM, pH7.5) to remove alkali and stained by ethidium bromide (concentration 40 µg/ml) and visualized under fluorescence microscope (Olympus, 1 × 81). Acquisition, processing and comet tail length measurement was done with Micromanager and ImageJ software.
Semi-quantitative Reverse Transcriptase PCR
Isolation of RNA from human lymphocytes was done using Trizol reagent (Takara Clontech). Complementary DNA (cDNA) was then synthesized from 600 ng total RNA using MMLV Reverse transcriptase (Invitrogen). Amplification of cDNA was carried out by polymerase chain reaction for 29 cycles with an initial hot start followed by denaturation, then annealing, and finally extension ( 94 °C for 30 s, 55 °C for 30 s, 72 °C for 90 °C, 28–29 cycles) with the use of the primers specific to p53 gene (forward primer sequence 5′CACCCTTCAGATCCGTGGGC3’ and reverse primer sequence 5′AAACCCAAAATGGCAGGGGA3′) to yield a product of 250 bp and specific to Bax gene (forward primer sequence 5′TCATGGGCTGGACATTGGAC3’ and reverse primer sequence 5′GGCCTCAGCCCATCTTCTTC3′) gene to yield a product of 118 base pair (bp). For Positive control GAPDH specific primer (forward primer sequence 5′TGATGACATCAAGAAGGTGGTGAAG3′ and reverse primer sequence 5′TCCTTGGAGGCCATGTGGGCCAT3′) to yield a product of 240 bp was used. Cycle number was controlled in order to prevent saturation of the amplification level and get amplicons at the exponential phase of amplification. Analysis of PCR product was done by electrophoresis on agarose gel (2%) along with a 100 bp DNA ladder. The pictures of the gel were then examined under the gel Documentation System (Chemidoc, Syngene) and band intensities were measured by gene tools Syngene software and were normalized to respective GAPDH band intensities.
Statistical Analysis
FRAP assay, Comet assay, and semi-quantative PCR were performed three times, and all the results are expressed as the mean ± standard error (SE). Statistical significance was established by t-test using SPSS version 13.0. P value < 0.05 was set to establish the significance level.
Results
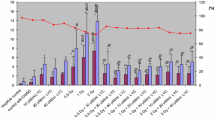
The antioxidant properties of grape extracts were measured through metal reducing capacity. The ferric reducing power assay was determined spectrophotomectically based on the reduction of Fe metal ion. Among different cultivar and different parts, grape seed showed the maximum reducing power and antioxidant capacity, followed by its skin, and then by its pulp in the same cultivars (Fig. 1). Among the skin of selected Flame seedless (black) cultivar skin showed the highest reducing power and antioxidant capacity, followed by Kishmish chorni cultivars, and then Red globe cultivars; while Thompson seedless cultivars showed least capacity in this study (Fig. 1). Kishmish chorni seed possesses maximum antioxidant activity.
Antioxidant capacity of grape extract measured through ferric reducing antioxidant power (FRAP) assay. Values are mean ± SE of 3 experiments
Abbreiations: TS: Thompson seedless skin, TP: Thompson seedless pulp, FS: Flame seedless skin, FP: Flame seedless pulp, RS: Red globe skin, RP: Red globe pulp, KS: Kishmishchorni skin, KP: Kishmishchorni pulp
Ionizing radiation caused increase in Annexin V-positive cells, suggesting induction of apoptosis in these cells. The number of necrotic cells was low in all treatment groups, suggesting apoptosis as a major mode of induction of cell death after irradiation (Fig. 2).
Effects of grape extract on viability and apoptosis in control and treated lymphocytes by Annexin V-FITC and propidium iodide (PI) double staining
Radiation caused increased in Annexin V-positive cells, suggesting induction of apoptosis in these cells. The number of necrotic cells was low in all treatment groups, suggesting apoptosis as a major mode of induction of cell death after irradiation
Single cell gel electrophoresis (comet assay) was performed to determine the DNA damage protecting ability of the Kishmish chornin grape extracts in irradiated cells and compared with non-treated (IR) and non-irradiated (control) cells. The results were compared with widely available Thompson seedless cultivar. Comet assay is highly effective technique to measure strand breaks in DNA in a single cell. Protective efficacy of grape extracts in different cultivars against DNA damage induced by γ radiation in human lymphocytes isolated from blood was analyzed by measuring the comet tail length (Fig. 3). A significant increase (P < 0.05) in comet tail length was observed in γ-irradiated lymphocytes compared to the non-irradiated control lymphocyes. Grape extract pretreated lymphocytes showed significant decrease (P < 0.05) in comet tail length in varying degree depending on the part of the extract and the cultivars. Seed extracts showed maximum level of protection followed by skin and followed by pulp. Kishmish chorni cultivars showed higher DNA damage protection than Thompson seedless cultivars.
DNA protection ability of the grape extracts by Comet assay and by measuring the tail length. Control: untreated sample, IR: only 4 Gy radiation treatment, TS + IR: Pretreatment with Thompson seedless skin prior to 4 Gy radiation, TP + IR: Pretreatment with Thompson seedless pulp prior to 4 Gy radiation, KS + IR: Pretreatment with Kishmish chorni skin prior to 4 Gy radiation, KP + IR: Pretreatment with Kishmish chorni pulp prior to 4 Gy radiation. The graph represents the respective tail length of DNA. Values are represented as mean ± SE of 100 observarions. P values: #<0.05 compared to the un-irradiated control group, *<0.05 compared to control and 4 Gy treated groups
Further two important cell markers were determined by semi-quantitative PCR method. The significant uprise (P < 0.05) of p53 and Bax were noticed in non-treated irradiated cells. Post 1 h of 4 Gy γ-irradiation p53 m RNA level was significantly upregulated, whereas, Bax mRNA level was upregulated post 2 h of irradiation compared to untreated control group (Figs. 4 and 5). Pretreatment of lymphocytes with extract of different grape cultivars significantly (P < 0.05) protected the cells by down-regulating radiation-induced p53 mRNA level and Bax mRNA level. Among the tested cultivars and extracts, seed extracts of Kishmish chorni cultivar showed maximum protection.
Effect of grape extracts on p53 expression. a Reverse transcription followed by PCR showing p53 gene expression in human lymphocytes. b Graphical representation of p53 expression. Control: untreated sample, IR: only 4 Gy radiation treatment, TS + IR: Pretreatment with Thompson seedless skin prior to 4 Gy radiation, TP + IR: Pretreatment with Thompson seedless pulp prior to 4 Gy radiation, KS + IR: Pretreatment with Kishmish chorni skin prior to 4 Gy radiation, KP + IR: Pretreatment with Kishmish chorni pulp prior to 4 Gy radiation. Values are represented as mean ± SE of 3 experiments. P values: #<0.05 compared to the un-irradiated control group, *<0.05 compared to control and 4 Gy treated groups
Effect of grape extracts on Bax expression. a Reverse transcription followed by PCR showing BAX gene expression in human lymphocytes. b Graphical representation of Bax expression. Control: untreated sample, IR: only 4 Gy radiation treatment, TS + IR: Pretreatment with Thompson seedless skin prior to 4 Gy radiation, TP + IR: Pretreatment with Thompson seedless pulp prior to 4 Gy radiation, KS + IR: Pretreatment with Kishmish chorni skin prior to 4 Gy radiation, KP + IR: Pretreatment with Kishmish chorni pulp prior to 4 Gy radiation. Values are represented as mean ± SE of 3 experiments. P values: #<0.05 compared to the un-irradiated control group, *<0.05 compared to control and 4 Gy treated groups
Discussion
Naturally occurring reductants are involved in oxidative metabolisms and the reducing capacity is directly linked with their potential antioxidant activity [25, 26]. The reducing capacity of seed extracts followed by skin extracts of grape in this study indicates their degree of potential antioxidant activity. FRAP assay showed that Kishmish chorni seed possess maximum reducing and antioxidant power. On the contrary, Thompson seedless is the most widely cultivated grape cutivar in India. Therefore, we had selected the Kishmish chorni cultivars for the study related to DNA damage and gene expression, and their efficacy was compared with Thompson seedless Cultivars.
A number of studies have been done on the protective effect of polyphenols and antioxidants on gamma radiation induced human lymphocytes at cellular and molecular level using doses at 1–4 Gy [27,28,29]. We have selected 4 Gy γ-radiation dose based on our previous study [17] that induces higher damage, and modification by the presence or absence of other agent is easily identifiable.
Apoptosis and necrosis reflect the program of cell death contributed by a dying cell and the ultimate stage of death, respectively. Whereas apoptosis is characterized as a physiological, highly organized cell death process, necrosis is usually deemed to be accidental and uncontrolled. Physiological and weak pathological death stimuli conventionally induce apoptosis, while harsh non-physiological insults often immediately instigate (primary) necrosis [30]. One of the early events occurring at the cell membrane during apoptosis is the translocation of phosphatidylserine from the inner side of the plasma membrane to the outer layer. These phosphatidylserine groups can be bound by fluorescein isothiocyanate (FITC)-labelled annexin V [31]. Combining it with propidium iodide (PI) staining is used to distinguish early versus late apoptotic or necrotic events [32]. The observed apoptosis as a major mode of induction of cell death after irradiation in our study is in agreement with other study, where 2 Gy γ-IR exposure to cultured human endothelial cells (ECs) increased the number of annexin V positive cells that activated apoptosis in ECs [33].
The neutral comet assay not only measure double-stranded DNA breaks, but it has also been used to measure apoptosis based on its characteristic DNA fragmentation patterns. There is still ambiguity about the reliability of this method [34]. We have provided evidence by evaluating both Annexin V/ PI and the comet assay for detecting apoptosis in this study. While the Annexin V/ PI assay measures higher amounts of apoptosis because it can detect cells in an earlier stage of the apoptotic pathway, the comet assay measures the late stages of apoptosis [34]. Significant (P < 0.05) decrease in tail length by pre-treatment with grape extracts in IR-induced lymphocytes in this study implies that grape extracts can protect peripheral blood lymphocyte DNA from irradiation. It has also been reported that antioxidants and polyphenols ameliorate gamma radiation induced DNA damage in human lymphocytes [29, 35]. Since DNA damage induced by radiation is caused by generation of reactive oxygen species (ROS) [36], grape extracts containing polyphenols, flavonoids and antioxidants with free radical scavenging activity [17, 19] may protect from IR-induced DNA damage in human lymphocytes.
It was reported that the IR induces a complex signaling apoptotic cascade that eliminate injured cells through the intrinsic pathway [37]. Interestingly, one study shows that the grape product rich in dietary fibre with natural antioxidants have the capacity to modulate the mucosal apoptosis by modulating the redox environment in the cell [38]. We have also demonstrated that pretreatment with grape extracts attenuated the oxidative stress induced by 4 Gy γ-radiations in human lymphocytes in vitro; and modulated apoptosis by attenuating γ-radiation-induced elevated caspase 3/7 activity [17]. Whereas, another study reported that polyphenols present in grape seed could defend against apoptosis of the cardiac cell by inducing the endogenous enzymatic antioxidants [39].
Several studies concentrate on the transcriptional response of lymphocytes to IR to describe the direct effect of IR on lymphocytes by gene expression analysis. These studies revealed that a major portion of the strongly activated genes are p53 and p53 targets, like the Bcl-2 associated gene Bax etc. are involved in repair of DNA and regulation of apoptosis [40,41,42]. Real-time RT-PCR is the most sensitive powerful tool for quantitative measurement of gene expression [43]. Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) is a relatively simple, inexpensive, extremely sensitive and specific common tool to determine the expression level of target genes and is widely used in biomedical science research. The basic principles for RT-PCR and real-time PCR are the same [44]. Semi-quantitative and quantitative RT-PCR has significant advantages over traditional RNA assays, such as Northern blot, ribonuclease protection, RNA blot, and solution hybridization assays [45]. Two important genes, such as p53 and Bax expression were studied by semi-quantitative PCR method.
Major function of p53 is to maintain the integrity of genome [46]. p53 helps in removing damaged and mutated cells from dividing cell population by promoting cell cycle arrest [47]. It is a significant factor in aging [48]. It can instigate apoptosis in case of irreparable DNA damage. More than 100 p53 target genes involved in regulation of the cell cycle arrest, transcription, cell death and DNA repair have been discovered [49]. p53 level was up regulated and caspase 3 and 6 activity was increased at 5 Gy dose gamma irradiated mouse thymocytes in one study [50]. Reduction in p53 expression upon treatment by grape extracts of irradiated lymphocytes in this study suggests that the grape extracts are able to reduce the IR-induced DNA damage and thereby reducing the expression of p53.
Multiple Bcl-2 family proteins including B-cell lymphoma 2 (Bcl-2), B-cell lymphoma-extra large [Bcl-X(L)], and Bcl-2-associated X protein (Bax), orchestrate key life and death decisions in lymphocytes [51]. The balance between antiapoptotic and proapoptotic proteins of the Bcl-2 family is critical in determining the fate of T cells in response to death stimuli. Proapoptotic genes, such as Bax, are generally regulated by the p53 family of transcription factors, whereas NF-kappaB subunits can activate the transcription of antiapoptotic Bcl-2 members [52]. It was found that pretreatment with antioxidants and free radical scavengers attenuated the irradiation-induced ROS generation, increased cell viability and down regulate Bax protein in irradiated lymphocytes [50, 53, 54]. Our study also demonstrated that pretreatment with grape extract modulated of Bax expression to protect peripheral blood lymphocytes from gamma irradiation-induced apoptosis.
Thus, we have observed variation in degree of protection offered by the skin, pulp and seed extracts of the tested cultivars in different parameters in this study. These differences in degree of protection offered by the skin, pulp and seed extracts of the tested cultivars in different parameters may be due to different mechanism of action at molecular and cellular level.
Interestingly, over the past several years, different preclinical in vitro and ex vivo models have been developed that facilitated to comprehend some of the critical aspects of health and disease. Yet, the translation to the human in vivo condition remains challenging. For example, the comet assay offers the opening to determine both DNA damage and repair. There are diverse reports regarding the regulation of DNA repair by environmental and dietary factors. However, decisive issues about the factors regulating DNA repair and inter-individual variation remain unanswered [55]. Moreover, in vitro studies may be a useful preliminary screening method to identify promising plant cultivars [56], while, the low bioavailability of most phytochemicals limits their translation to humans [56]. The approaches follows in preclinical in vitro and ex vivo models fail to fully imitate the multifactorial and intricate in vivo environment [57].
In spite of above limitations, our study suggested that, the grape extracts of different cultivars act as an essential source of natural antioxidants, and is able to attenuate DNA damage and apoptosis by regulating apoptotic genes such as p53 and Bax in human lymphocytes induced by IR. In addition, the protective activity of grape depends on the part of the extract i.e. seed, skin or pulp, and the type of cultivars. However, further studies, particularly human clinical trials, is necessary to confirm the beneficial function of relevant nutraceuticals and to explore the safe limits of human supplementation and the risk of side effects.
References
Jagetia GC. Radioprotective potential of plants and herbs against the effects of ionizing radiation. J Clin Biochem Nutr. 2007;40(2):74–81.
Hari Kumar KB, Sabu MC, Lima PS, Kuttan R. Modulation of haematopoetic system and antioxidant enzymes by Emblica officinalis gaertn and its protective role against gamma-radiation induced damages in mice. J Radiat Res. 2004;45(4):549–55.
Pandey BN, Mishra KP. Modification of thymocytes membrane radiooxidative damage and apoptosis by eugenol. J Environ Pathol Toxicol Oncol. 2004;23(2):117–22.
Georgieva S, Popov B, Bonev G. Radioprotective effect of Haberlearhodopensis (Friv.) leaf extract on-radiation-induced DNA damage, lipid peroxidation and antioxidant levels in rabbit blood. Indian J Exp Biol. 2013;51(1):29–36.
Rajan I, Rabindran R, Jayasree PR, Kumar PR. Antioxidant potential and oxidative DNA damage preventive activity of unexplored endemic species of Curcuma. Indian J Exp Biol. 2014;52(2):133–8.
Manda K, Glasow A, Paape D, Hildebrandt G. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front Oncol. 2012;2:102.
Trowell OA. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. 1952;64(4):687–704.
Yao Z, Jones J, Kohrt H, Strober S. Selective resistance of CD44hi T cells to p53-dependent cell death results in persistence of immunologic memory after total body irradiation. J Immunol. 2011;187(8):4100–8.
Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81(4):1128–35.
Qu Y, Jin S, Zhang A, Zhang B, Shi X, Wang J, et al. Gamma-ray resistance of regulatory CD4+ CD25+ Foxp3+ T cells in mice. Radiat Res. 2010;173(2):148–57.
Bogdándi EN, Balogh A, Felgyinszki N, Szatmári T, Persa E, Hildebrandt G, Sáfrány G, Lumniczky K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat Res. 2010;174(4):480–9.
Chua ML, Rothkamm K. Biomarkers of radiation exposure: Can they predict normal tissue radiosensitivity? Clin Oncol (R CollRadiol). 2013;25(10):610–6.
Vandevoorde C, Depuydt J, Veldeman L, De Neve W, Sebastià N, Wieme G, et al. In vitro cellular radiosensitivity in relationship to late normal tissue reactions in breast cancer patients: a multi-endpoint case-control study. Int J Radiat Biol. 2016;92(12):823–36.
Ozsahin M, Crompton NE, Gourgou S, Kramar A, Li L, Shi Y, et al. CD4 and CD8 T-lymphocyte apoptosis can predict radiation-induced late toxicity: a prospective study in 399 patients. Clin Cancer Res. 2005;11(20):7426–33.
Singh VK, Seed TM. Pharmacological management of ionizing radiation injuries: current and prospective agents and targeted organ systems. Expert Opin Pharmacother. 2020;21(3):317–37.
Yahyapour R, Shabeeb D, Cheki M, Musa AE, Farhood B, Rezaeyan A, et al. Radiation protection and mitigation by natural antioxidants and flavonoids: implications to radiotherapy and radiation disasters. Curr Mol Pharmacol. 2018;11(4):285–304.
Singha I, Das SK. Grapevine extract protect against radiation-induced oxidative stress and apoptosis in human lymphocyte. Indian J Exp Biol. 2015;53(11):753–61.
Singha I, Das SK. Antioxidant potential of different grape cultivars against Fenton-like reagent-induced liver damage ex-vivo. Indian J Biochem Biophys. 2014;51(5):372–7.
Singha I, Das SK. Scavenging and antioxidant properties of different grape cultivars against ionizing radiation-induced liver damage ex vivo. Indian J Exp Biol. 2016;54(4):280–5.
Saxena S, Verma J, Gautam S. Potential prophylactic properties of apple and characterization of potent bioactive from cv. Granny Smith displaying strong antimutagenicity in models including human lymphoblast TK6(+/-) cell line. J Food Sci. 2016;81(2):H508-518.
Singha I, Saxena S, Gautam S, Das SK. Grape extract protect against ionizing radiation-induced DNA damage. Indian J Biochem Biophys. 2020;57(2):219–27.
Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001; Appendix 3: Appendix 3B.
Vasumathy R, Pandey BN, Mishra KP. Oxidative stress-mediated apoptotic death in human peripheral blood lymphocytes treated with plasma from gamma-irradiated blood. J Environ Pathol Toxicol Oncol. 2012;31(1):1–6.
Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–91.
Meir S, Kanner J, Akir B, Philosoph-Hadas S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem. 1995;43:1813–9.
Philip JP, Madhumitha G, Mary SA. Free radical scavenging and reducing power of Lawsonia inermis L. seeds. Asian Pac J Trop Med. 2011;4(6):457–61.
Srinivasan M, Rajendra Prasad N, Menon VP. Protective effect of curcumin on gamma-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat Res. 2006;611(1–2):96–103.
Tiwari P, Kumar A, Balakrishnan S, Kushwaha HS, Mishra KP. Radiation-induced micronucleus formation and DNA damage in human lymphocytes and their prevention by antioxidant thiols. Mutat Res. 2009;676(1–2):62–8.
Chen L, Liu Y, Dong L, Chu X. Edaravone protects human peripheral blood lymphocytes from γ-irradiation-induced apoptosis and DNA damage. Cell Stress Chaperones. 2015;20(2):289–95.
Janko C, Munoz L, Chaurio R, Maueröder C, Berens C, Lauber K, et al. Navigation to the graveyard-induction of various pathways of necrosis and their classification by flow cytometry. Methods Mol Biol. 2013;1004:3–15.
Louagie H, Cornelissen M, Philippe J, Vral A, Thierens H, De Ridder L. Flow cytometric scoring of apoptosis compared to electron microscopy in gamma irradiated lymphocytes. Cell Biol Int. 1998;22(4):277–83.
Plesca D, Mazumder S, Almasan A. DNA damage response and apoptosis. Methods Enzymol. 2008;446:107–22.
Vu HT, Kotla S, Ko KA, Fujii Y, Tao Y, Medina J, et al. Ionizing radiation induces endothelial inflammation and apoptosis via p90RSK-Mediated ERK5 S496 phosphorylation. Front Cardiovasc Med. 2018;5: 23.
Wilkins RC, Kutzner BC, Truong M, Sanchez-Dardon J, McLean JR. Analysis of radiation-induced apoptosis in human lymphocytes: flow cytometry using annexin V and propidium iodide versus the neutral comet assay. Cytometry. 2002;48(1):14–9.
Ghosh D, Pal S, Saha C, Chakrabarti AK, Datta SC, Dey SK. Black tea extract: a supplementary antioxidant in radiation-induced damage to DNA and normal lymphocytes. J Environ Pathol Toxicol Oncol. 2012;31(2):155–66.
Koukourakis MI. Radiation damage and radioprotectants: new concepts in the era of molecular medicine. Br J Radiol. 2012;85(1012):313–30.
Furlong H, Mothersill C, Lyng FM, Howe O. Apoptosis is signalled early by low doses of ionising radiation in a radiation-induced bystander effect. Mutat Res. 2013;741–742:35–43.
López-Oliva ME, Agis-Torres A, Goñi I, Muñoz-Martínez E. Grape antioxidant dietary fibre reduced apoptosis and induced a pro-reducing shift in the glutathione redox state of the rat proximal colonic mucosa. Br J Nutr. 2010;103(8):1110–7.
Du Y, Guo H, Lou H. Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes. J Agric Food Chem. 2007;55(5):1695–701.
Mori M, Benotmane MA, Tirone I, Hooghe-Peters EL, Desaintes C. Transcriptional response to ionizing radiation in lymphocyte subsets. Cell Mol Life Sci. 2005;62(13):1489–501.
Gruel G, Voisin P, Vaurijoux A, Roch-Lefevre S, Grégoire E, Maltere P, et al. Broad modulation of gene expression in CD4+ lymphocyte subpopulations in response to low doses of ionizing radiation. Radiat Res. 2008;170(3):335–44.
Gridley DS, Rizvi A, Luo-Owen X, Makinde AY, Pecaut MJ. Low dose, low dose rate photon radiation modifies leukocyte distribution and gene expression in CD4+ T cells. J Radiat Res. 2009;50(2):139–50.
Lukasiak S, Breuhahn K, Schiller C, Schmidtke G, Groettrup M. Quantitative analysis of gene expression relative to 18S rRNA in carcinoma samples using the LightCycler instrument and a SYBR GreenI-based assay: determining FAT10 mRNA levels in hepatocellular carcinoma. Methods Mol Biol. 2008;429:59–72.
Mo Y, Wan R, Zhang Q. Application of reverse transcription-PCR and real-time PCR in nanotoxicity research. Methods Mol Biol. 2012;926:99–112.
Yoshikawa M, Nakayama H, Ueno S, Nishimine N, Furuya H. Novel quantitative reverse-transcribed polymerase chain reaction of mu opioid receptor mRNA level. Brain Res Brain Res Protoc. 2001;7(2):147–53.
Wu D, Prives C. Relevance of the p53-MDM2 axis to aging. Cell Death Differ. 2018;25(1):169–79.
Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8(21):2540–51.
Papazoglu C, Mills AA. p53: at the crossroad between cancer and ageing. J Pathol. 2007;211(2):124–33.
Bisio A, De Sanctis V, Del Vescovo V, Denti MA, Jegga AG, Inga A, et al. Identification of new p53 target microRNAs by bioinformatics and functional analysis. BMC Cancer. 2013;13: 552.
Chen Y, Stanford A, Simmons RL, Ford HR, Hoffman RA. Nitric oxide protects thymocytes from γ-irradiation-induced apoptosis in correlation with inhibition of p53 upregulation and mitochondrial damage. Cell Immunol. 2001;214(1):72–80.
Dallman C, Packham G. Purification of primary malignant B-cells and immunoblot analysis of bcl-2 family proteins. Methods Mol Med. 2005;115:1–13.
Cianfrocca R, Muscolini M, Marzano V, Annibaldi A, Marinari B, Levrero M, et al. RelA/NF-κB recruitment on the bax gene promoter antagonizes p73-dependent apoptosis in costimulated T cellsB recruitment on the bax gene promoter antagonizes p73-dependent apoptosis in costimulated T cells. Cell Death Differ. 2008;15(2):354–63. https://doi.org/10.1038/sj.cdd.4402264. (Epub 2007 Nov 23).
Ma ZC, Hong Q, Wang YG, Tan HL, Xiao CR, Liang QD, et al. Ferulic acid protects lymphocytes from radiation-predisposed oxidative stress through extracellular regulated kinase. Int J Radiat Biol. 2011;87(2):130–40.
Park E, Lee NH, Joo HG, Jee Y. Modulation of apoptosis of eckol against ionizing radiation in mice. Biochem Biophys Res Commun. 2008;372(4):792–7.
Azqueta A, Langie SAS, Boutet-Robinet E, Duthie S, Ladeira C, Møller P, et al. DNA repair as a human biomonitoring tool: comet assay approaches. Mutat Res Rev Mutat Res. 2019;781:71–87.
Dias DM, Costa NMB, Nutti MR, Tako E, Martino HSD. Advantages and limitations of in vitro and in vivo methods of iron and zinc bioavailability evaluation in the assessment of biofortification program effectiveness. Crit Rev Food Sci Nutr. 2018;58(13):2136–46.
Rahman S, Ghiboub M, Donkers JM, van de Steeg E, van Tol EAF, Hakvoort TBM, et al. The progress of intestinal epithelial models from cell lines to gut-on-chip. Int J Mol Sci. 2021;22(24):13472.
Acknowledgements
Financial assistance from the Department of Atomic Energy- Board of Research in Nuclear Studies (DAE-BRNS 2012/35/37) is gratefully acknowledged. The authors also thank Dr. Abhijit Saha, UGC-DAE –CSR, Salt Lake City, Kolkata for extending necessary irradiation facility and Ritabrata Ghosh, IISER Kolkata for his technical assistance in microscopy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Ethical Approval
The study was approved by the Institutional Ethics Committee (No. F-24/ Pr/ CMJNMH/ IEC/ 14/ 93(4)) as per the ICMR guideline (ECR/674/Inst/WB/2014, dated 31/10/2014). The same is mentioned in Materials and Method section.
Informed Consent
Fresh blood samples were collected aseptically in heparinized tubes from willing healthy male donors (22–30 years) who had no history of disease or recent administration of any drug. Informed consent was obtained from each donor. The same is mentioned in Blood sample preparation section.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singha, I., Poria, D.K., Ray, P.S. et al. Role of Grape (Vitis vinifera) Extracts of Different Cultivars Against γ-Radiation Induced DNA Damage and Gene Expression in Human Lymphocytes. Ind J Clin Biochem (2023). https://doi.org/10.1007/s12291-023-01154-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-023-01154-z