Abstract
C677T (rs1801133) and A1298C (rs1801131) MTHFR gene polymorphisms and/or nutritional deficiency of folate/vitamin B12 leading to hyperhomocysteinemia is an established risk factor for CAD. The objective of this study was to evaluate the clinical usefulness of association between MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphisms with serum homocysteine, folate and vitamin B12 in addition to conventional cardiovascular risk factors in patients with young CAD. Genomic DNA was isolated from the whole blood. Genotyping of MTHFR C677T (rs1801133) and MTHFR A1298C (rs1801131) polymorphisms in young CAD patients and healthy controls was performed by ARMS-PCR method. Serum homocysteine, vitamin B12 and folate were estimated by CMIA and lipid profile parameters were measured by automated chemistry analyzers. Serum homocysteine levels were significantly higher but serum folate and vitamin B12 levels were not significantly different among young CAD group as compared to control group. Statistically significant hyperhomocysteinemia was observed in carriers of T allele for MTHFR 677C/T (rs1801133) genotype in young CAD group but this association was not significant for MTHFR 1298A/C (rs1801131) polymorphism. The association between hyperhomocysteinemia and CAD in young group was not independent of conventional cardiovascular risk factors. Risk of hyperhomocysteinemia and young CAD could be monitored by MTHFR polymorphism detection followed by serum homocysteine, folate and vitamin B12 measurements. The findings could help to prevent or delay the occurrence of young CAD through appropriate measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis leading to coronary artery disease (CAD) in young Indian population less than 45 years of age is looming large as a cause of morbidity and mortality in recent years. In young CAD patients, established risk factors like hypertension, diabetes mellitus, smoking, central obesity, family history of heart disease, dyslipidemia are as important as in old CAD patients [1]. But many variations are observed in the prevalence of these risk factors in young CAD leaving the blank arena for the research. An elevated level of serum homocysteine (> 15 µmol/L) is an independent established risk factor for CAD due to its role in the initiation of endothelial dysfunction [2]. Mild to moderate elevation in serum homocysteine levels could be due to nutritional factors like deficiency of vitamin B12 (cobalamin) and folate. This is because of their involvement as cofactor and methyl group donor respectively in the methylation of homocysteine to methionine. Methylene tetrahydrofolate reductase (MTHFR) enzyme is responsible for reducing serum homocysteine levels by converting it into methionine. Methyltetrahydrofolate is methyl group donor in homocysteine re-methylation metabolism. Genetic factors like mutation in the gene coding for the MTHFR, the regulatory enzyme in the reduction of 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, is responsible for hyperhomocysteinemia [2, 3].
MTHFR gene is located on the short arm of chromosome 1 at 1p36.3. A common single nucleotide polymorphism (SNP) C677T (rs1801133), where cytosine is replaced by thymine at 677 position (exon 4) of MTHFR gene which results in substitution of an alanine to a valine at codon 222 in N-terminal catalytic domain of protein. The encoded protein has reduced activity at temperature 37 °C and higher hence called thermolabile variant [4]. MTHFR TT genotype has been associated with increased serum homocysteine level which is an established risk factor for CAD. Another polymorphism in MTHFR gene is seen in exon 7 at 1298 position where adenine (A) is replaced by cytosine(C) with subsequent substitution of a glutamate codon at position 429 to an alanine in amino acid sequence within C-terminal regulatory domain of protein. A1298C (rs1801131) mutation in MTHFR has also been described as a cause of reduced enzyme activity though it is considered less than conferred by C677T [4, 5]. Several studies have reported that C677T (rs1801133) and A1298C (rs1801131) MTHFR gene polymorphisms are crucial in determining the serum homocysteine levels, which if elevated, can lead to CAD [3,4,5]. However, there is paucity of data on the serum homocysteine, folate and vitamin B12 levels and their association with MTHFR C677T (rs1801133) and A1298C (rs1801131) gene polymorphism in young CAD patients. Hence, the present study was carried out to evaluate the association between serum homocysteine levels with MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphism in young CAD patients. Association between C677T (rs1801133) polymorphism and serum homocysteine levels are also affected by dietary folate intake [4, 5]. Hence, additionally, we also evaluated the correlation between serum homocysteine and serum folate as well as vitamin B12 and studied association between serum folate and vitamin B12 levels and MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphism in young CAD patients.
Materials and Methods
In the present case—control study, we included total ninety participants further divided into two groups with forty five patients with young CAD as cases and forty five healthy volunteers as controls. The young adult CAD patients included in this study were diagnosed first time for CAD at the Cardiovascular Department of our University affiliated Medical College and Tertiary Care Hospital, Pune, India, between June 1, 2018 and September 30, 2019. The study protocol was approved by institutional ethical committee and informed written consent was obtained from all patients and healthy volunteers prior to their enrollment. Inclusion criteria for young CAD group was confirmed diagnosis of CAD by diagnostic percutaneous coronary angiography with maximum luminal narrowing estimated by visual analysis of 15 coronary segments by the cardiologist. Clinically relevant CAD was defined as the occurrence of ≥ 1 stenosis of ≥ 40% in ≥ 1 of 15 coronary segments. Patients enrolled in young CAD group were 34 males and 11 females in between age group of 18–45 years. Control group was formed by healthy volunteers (males = 34, females = 11) of age group ranging 18–45 years who attended the institute for routine yearly health check-up. Patients with diabetes mellitus, hepatic and kidney diseases, family history of heart disease or on vitamin supplementation which could affect serum homocysteine levels were excluded from the study. 2.5 ml of blood sample was collected from ante-cubital vein in EDTA vacutainer and 2 ml in plain vacutainer under all aseptic precautions. Serum was separated after centrifugation at 2000 g for 10 min from plain vacutainer.
Estimation of Serum TC, TG, HDL-C, Vitamin B12, Folate and Homocysteine
Serum total cholesterol (TC) levels were measured by cholesterol oxidase‐peroxidaseaminoantipyrine (CHOD‐PAP) enzymatic colorimetric method [6]. Serum high‐density lipoprotein cholesterol (HDL‐C) levels were measured by direct enzymatic method [7]. The glycerol phosphate oxidase‐peroxidaseaminoantipyrine (GPO‐PAP) enzymatic colorimetric test was used to estimate serum triglyceride levels [8]. Friedewald formula was used to calculate serum low‐density lipoprotein cholesterol (LDL‐C) levels. Serum homocysteine, vitamin B12 and folate were measured by Chemiluminiscent Microparticle Immuno Assay (CMIA) according to instructions of kit manufacturers [9]. The required kits were purchased from Abbott ARCHITECT. All the parameters were analysed on automated biochemistry analyzers in NABL (National Accreditation Board for Testing and Calibration Laboratories) accredited Biochemistry department of central clinical laboratory of our hospital.
Isolation of Genomic DNA and MTHFR Polymorphism Analysis by ARMS PCR
Genomic DNA was isolated from the whole blood collected in EDTA vacutainer using commercially available DNA isolation kit (GeNei ™ Whole Blood DNA Extraction Kit, Genei Laboratories Private Laboratories) according to protocols of the manufacturer [10]. Genotyping of MTHFR C677T (rs1801133) and MTHFR A1298C (rs1801131) polymorphisms was performed by amplification-refractory mutation system- polymerase chain reaction (ARMS-PCR) method using a commercially available kit (GeNei ™ PCR Master Mix, Genei Laboratories Private Laboratories). The primer sequences were:
For MTHFR- rs1801133 (C/T) Forward inner primer-A (T allele): TTGAAGGAGAAGGTGTCTGCGGGCGT,
Reverse inner primer –B (C allele): CAAAGAAAAGCTGCGTGATGATGAAATAGG,
Forward outer primer- C (5'—3'):CCCAGCCACTCACTGTTTTAGTTCAGGC,
Reverse outer primer- D (5'—3'): GTGAGAGTGGGGTGGAGGGAGCTTATG.
For MTHFR- rs1801131 (A/C) Forward inner primer- A (A allele): GTGGGGGGAGGAGCTGACCAGTGAGGA Reverse inner primer-B (C allele): GGTAAAGAACGAAGACTTCAAAGACACCTG,
Forward outer primer- C (5'—3'): GGCCTGCAGACCTTCCTTGCAAATACAT,
Reverse outer primer- D (5'—3'): ACTTACCCTTCTCCCTTTGCCATGTCCA.
The PCR protocol followed was: Initial denaturation at 94 °C for 5 min, then 40 cycles (Denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s), and final extension at 72 °C for 7 min. In post-PCR process, 5 µl of PCR product was loaded on 3% agarose gel along with DNA ladder to confirm size of PCR product and results were interpreted. In C677T (rs1801133) genotyping, TT mutant genotype amplicon size was 189 bp while for CC Wild it was 273 bp. In case of A1298C (rs1801131) genotyping, AA wild genotype amplicon size was 232 bp while for CC mutant it was 334 bp.
Statistical Analysis
Data collected by using a structured form was entered in MS excel sheet. Using SPSS 24.0 version IBM USA data was analyzed. Normality test was carried out by using kolmogrov Smirnov test where the p value was 0.2 which was statistically not significant and hence data was normally distributed. Large variance can exist depending upon the observed values and wide normal range of few parameters in this study like for serum folate and serum vitamin B12. Qualitative data was expressed in terms of percentages and proportions. Quantitative data was expressed in terms of Mean ± standard deviation. Chi square test was used to determine association between two qualitative variables. Comparison of mean and SD between two groups was done by using unpaired ‘t’ test to assess significance of the mean difference between groups. Descriptive statistics of each variable was presented in terms of mean, standard deviation and standard error of mean. Correlation between two quantitative variables was assessed by using Pearson’s correlation coefficient test (r). P ≤ 0.05 was considered as statistically significant. P value of < 0.001 was considered as statistically highly significant.
Results
In the present study, we observed statistically significantly higher levels of systolic blood pressure, diastolic blood pressure and waist to hip ratio in the cases as compared to controls. However, there was no statistically significant difference in BMI between cases and controls (Table 1). Mean age of control group was 32.49 ± 7.11 years and of cases was 36.46 ± 6.98 years. P value of comparison was 0.008 making the difference statistically significant.
Further, significantly higher serum levels of total cholesterol (Sr. TC), triglycerides (Sr. TG) and VLDL cholesterol were observed in the cases as compared to levels in controls. However, serum HDL cholesterol (Sr. HDL-C) and serum LDL cholesterol (Sr. LDL-C) between patients and control group were not statistically significantly different. However, young CAD group had significantly higher TC/HDL-C ratio and LDL-C/HDL-C ratio as compared to ratio in control group (Table 2).
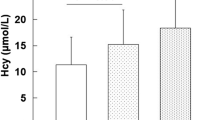
Significantly higher serum homocysteine levels were found in the young CAD group as compared to levels in the control group. However, serum folate and serum vitamin B12 were not statistically significantly different in cases as compare to levels in controls (Table 3).
For MTHFR 677 C/T (rs1801133) polymorphism the frequencies of genotype distribution CC, CT and TT were 75.6% versus 62.2%, 20% versus 37.8% and 4.4% versus 0.0% in cases versus controls respectively. However, no statistically significant difference was observed between young CAD group and control group for C677T (rs1801133) polymorphism (P = 0.23, chi square = 2.87). For MTHFR 1298 A/C (rs1801133) variant, the frequencies of AA, AC and CC were 8.9% versus 13.3%, 62.2% versus 77.8% and 28.9% versus 8.9% in young CAD patient group versus control group respectively. The most frequent genotype in both cases (62.2%) and controls (77.8%) was AC. Whereas CC mutant phenotype was found in much higher frequency in cases (28.9%) as compared to controls (8.9%). When we did overall comparison for A1298C (rs1801131) polymorphism in both groups we got P value of 0.051 (chi square = 5.94) which is slightly greater than the level of significance 0.05 making this association statistically not significant but this may be due to the small sample size used in our study.
When we compared results of serum homocysteine, folate and vitamin B12 with MTHFR genotyping, we found that for MTHFR 677 C/T (rs1801133) genotyping, carrier of T allele has higher mean concentration of serum homocysteine in both cases and controls. However, this association was statistically significant in only cases of young CAD patients (P = 0.019). While for MTHFR1298 A/C genotyping, significant association between serum homocysteine and three genotypes was noted in control group (P = 0.003), while in cases, this association was not statistically significant (P = 0.82), though serum homocysteine was higher in concentration in cases as compared to controls (Table 4). For MTHFR 677 C/T (rs1801133) genotyping, carrier of T allele has lower serum folate (Table 5) and serum vitamin B12 levels (Table 6) in both cases and controls which go with the finding of higher serum homocysteine observed with T allele, however, this association was not statistically significant. For MTHFR1298 A/C (rs1801131), carrier of C allele has lower serum vitamin B12 values in both cases and controls. However, no genotypic association was statistically significant in both cases and controls.
Using Pearson’s correlation coefficient test, we observed that serum homocysteine levels were negatively correlated with both serum folate (r = −0.333, P = 0.025) and serum vitamin B12 (r = −0.202, P = 0.188) in cases but this negative correlation was statistically significant with only serum folate. In the control, statistically significant negative correlation was found between serum homocysteine and serum folate as well as serum vitamin B12 (r = −0.411, p = 0.006 and r = −0.492, P < 0.001 respectively). Negative correlation of serum homocysteine with serum folate and vitamin B12 marks the importance of role of both folate and vitamin B12 in homocysteine metabolism.
Discussion
Hyperhomocysteinemia is a strong and independent risk factor for coronary artery disease. Serum homocysteine levels are affected by various genetic and/or nutrient related factors playing important role in trans-sulfuration or remethylation reactions of homocysteine [2, 3].
In the present study, statistically significantly higher levels of serum homocysteine were observed in young CAD patients (cases) as compared to controls which were supported by many studies [2, 3, 11, 12]. Previous studies have reported positive association between C677T (rs1801133) polymorphism with cardiovascular diseases [13, 14] while others found no association between C677T (rs1801133) polymorphism and risk of CAD [15, 16] and the available data on MTHFR C677T (rs1801133) polymorphisms and risk of cardiovascular disease is conflicting. In this study, we did not find any association between C677T (rs1801133) polymorphism and serum homocysteine levels which is consistent with some earlier studies [3, 15,16,17]. However, in our study, we found higher serum homocysteine levels among the carrier of T allele in cases and controls (Table 4). Ilhan N et al. [18] concluded in their study that homocysteine level was higher in TT genotypes in CAD patients compared with CC and CT genotypes and MTHFR gene polymorphism is an independent risk factor for essential hypertension but not for CAD. In the present study also homozygous mutant TT polymorphism in group I (n = 2), were having serum homocysteine levels of 50 µmol/L.
Vitamin folate and B12 plays important role in homocysteine metabolism. In this study, lower levels of serum folate and vitamin B12 were found in carrier of T allele in both cases and control groups. When we compared average serum folate and B12 levels, no significant difference was found between cases and controls (Tables 5 and 6) but when we correlated serum homocysteine levels with serum folate and B12, negative association was found between them in cases as well as controls. This negative association was statistically significant for serum folate in cases as well as for serum folate and B12 in controls. John C. Chambers has shown elevated serum homocysteine levels associated with low serum folate and B12 levels in Asian population which could be due to genetic or environmental factors [17,18,19]. Moreover, they reported that MTHFR C677T (rs1801133) mutation does not contribute to elevated plasma homocysteine concentrations or increased CHD risk in Indian Asians compared with European whites [17]. Kadziela J et al. [20] reported that serum folic acid and vitamin B12 levels were not related to the MTHFR genotype but homozygotes TT had significantly higher homocysteine concentration than heterozygotes (CT) and homozygotes (CC). Plasma homocysteine concentration was significantly higher in patients with CAD compare to controls and correlated significantly with folic acid and vitamin B12. The findings of our study were consistent with their reports. Some non-Indian population studies have shown that hyperhomocysteinemia and the T/T genotype was a risk factor for early onset of CAD [21, 22]. However, some studies reported no association between C677T (rs1801133) mutation and risk of CAD [23, 24].
The genotype polymorphism comparison for MTHFR A1298C (rs1801131) among young CAD patients and controls were very near to significant level but could not attain it which could be due to small sample size of our study. When MTHFR A1298C (rs1801131) polymorphism was compared with serum homocysteine, folate and vitamin B12 levels, no significant association was observed between them (Tables 4, 5, 6). However, serum homocysteine concentration was higher in young CAD patients as compared to control group (Table 4). Contradictory to C677T (rs1801133) polymorphism findings, serum folate and vitamin B12 levels were lower in control group in C allele carrier for A1298C (rs1801131) polymorphism (Table 6). Kolling K et al. [25] demonstrated that MTHFR gene C677T (rs1801133) or A1298C (rs1801131) polymorphisms were not associated with the presence of angiographic CAD. Although, an apparent association was noted between elevated level of homocysteine and CAD, it was not independent of conventional cardiovascular risk factors. Spiroski I et al. [26] also could not confirm a significant association of MTHFR C677T (rs1801133) and MTHFR A1289C (rs1801131) polymorphisms with occlusive artery disease or deep venous thrombosis in Macedonians, except for the safeguarding effect of MTHFR/CA:CC diplotype against occlusive artery disease. Domagala TB et al. [27] reported that the polymorphisms C677T(rs1801133) and A1298C(rs1801131) of MTHFR and fasting plasma homocysteine levels do not seem to be significant risk factors for venous thromboembolic disease. Hanson NQ et al. [28] have shown that the prevalence of the C677T (rs1801133) and A1298C (rs1801131) polymorphisms did not differ among individuals with CAD, DVT, or those without documented vascular disease. In contrast to the C677T (rs1801133) polymorphism, the A1298C (rs1801131) polymorphism was not associated with increased fasting total serum homocysteine. Our findings were in similar line with their reports. The prevalence of MTHFR C677T (rs1801133) and A1298 C (rs1801131) polymorphism was subjected to variation in different geographical regions, ethnicity, races, environmental influences, dietary folate intake and other life‐style factors giving conflicting results. Friso S et al. [5] reported no additional effect of A1298C (rs1801131) on total plasma homocysteine in 148 combined heterozygotes compared with 98 heterozygotes for the C677T alone. These findings emphasize the hypothesis that MTHFR genotypes may interfere with coronary artery disease risk only when an unbalanced nutritional status leads to raised total plasma homocysteine levels. We found significantly elevated serum homocysteine levels in young CAD group as compare with control group which was significantly associated with C677T (rs1801133) polymorphism in young CAD patients but not in A1298C (rs1801131) polymorphism. This supports the hypothesis that C677T (rs1801133) mutation affects the thermoliability and decreases activity of MTHFR, the regulatory enzyme in remethylation of homocysteine and thus leading to hyperhomocysteinemia which is independent risk factor for CAD. We did not find significant difference between serum folate and vitamin B12 levels among young CAD patients and control group but negative association was found between serum homocysteine and folate as well as vitamin B12 levels in young CAD group and control group, earmarking the role of vitamin folate and B12 in homocysteine metabolism.
In addition to this, in the present study, we found significantly higher levels of serum total cholesterol, triglycerides, TC/HDL-C and LDL-C/HDL-C ratio. Serum LDL-C was higher and HDL-C was lower in young CAD group as compare to controls but it was not statistically significant (Table 2). These findings were much similar to the reports of Gupta S et al. [6]. Moreover, significantly higher systolic BP, diastolic BP and w/h ratio was observed in young CAD group as compared to control group (Table 1). These conventional risk factors might also be contributing to the pathogenesis of CAD among young age patients.
We were among very few researchers who could successfully show that MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphisms in young CAD patients could lead to hyperhomocysteinemia which fills the role of cardiac biomarker in prediction of young CAD risk. The novelty of this study was that we have studied association between serum folate, vitamin B12 status along with traditional risk factors with MTHFR polymorphisms as well as hyperhomocysteinemia in young CAD for the first time. This would help to shift focus of preventive cardiology test panel on single nucleotide gene polymorphisms especially in young CAD which has got polygenic pathophysiology in different ethnic population. The limitation of our study was that we have conducted preliminary study with a small sample size to establish link between MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphism with serum homocysteine, folate and B12 levels in young CAD group. Additional studies with larger sample size are needed to define the influence of MTHFR C677T (rs1801133) and A1298C (rs1801131) genotyping in pathogenesis of young CAD patients.
In conclusion, the findings of our study suggests that elevated serum homocysteine levels were associated with increased risk of CAD in young age group (< 45 years) independent of MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphisms. The significant hyperhomocysteinemia (> 15 µmol/L) was associated with C677T (rs1801133) polymorphism in young CAD group. This association between hyperhomocysteinemia and CAD in young group was not independent of conventional cardiovascular risk factors. Young age population being important part in population pyramid of any country, risk of hyperhomocysteinemia and CAD could be monitored by MTHFR polymorphism detection followed by serum homocysteine, folate and vitamin B12 measurement and appropriate measures could be taken to prevent or delay the occurrence of CAD. In further scope, longitudinal follow up of group I participants could be done for predicting development of future cardiac event.
References
Iyengar SS, Gupta R, Ravi S, Thangam S, Thomas A, Manjunath CN, et al. Premature coronary artery disease in India: coronary artery disease in the young (CADY) registry. Indian Heart J. 2017;69(2):211–6.
Ma Y, Peng D, Liu C, Huang C, Luo J. Serum high concentrations of homocysteine and low levels of folic acid and vitamin B12 are significantly correlated with the categories of coronary artery diseases. BMC CardiovascDisord. 2017;17(1):37.
Gupta SK, Kotwal J, Kotwal A, Dhall A, Garg S. Role of homocysteine & MTHFR C677T gene polymorphism as risk factors for coronary artery disease in young Indians. Indian J Med Res. 2012;135(4):506–12.
Butler S, Young A, Akam EC, Sinha N, Agrawal S, Mastana S. Association of methylenetetrahydrofolatereductase (MTHFR) C677T and A1298C polymorphisms with coronary artery disease (CAD) in a North Indian population. Cogent Med. 2018;5(1):1–11.
Friso S, Girelli D, Trabetti E, Stranieri C, Olivieri O, Tinazzi E, et al. A1298C methylenetetrahydrofolatereductase mutation and coronary artery disease: relationships with C677T polymorphism and homocysteine/folate metabolism. Clin Exp Med. 2002;2(1):7–12.
Roeschlau P, Bernt E, Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974;12(5):226.
Warnick GR, Nauck M, Rifai N. Evolution of methods for measurement of HDL-cholesterol: from ultracentrifugation to homogeneous assays. Clin Chem. 2001;47(9):1579–96.
Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19(5):476–82.
Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteinevs vitamin B(12) and folate. Clin Chem. 2000;46(8 Pt 2):1277–83.
Chacon-Cortes D, Griffiths L. Methods for extracting genomic DNA from whole blood samples: current perspectives. J Biorepos Sci Appl Med. 2014;2:1–9. https://doi.org/10.2147/BSAM.S46573.
Angeline T, Aruna RM, Ramadevi K, Mohan G, Jeyaraj N. Homocysteine status and acute myocardial infarction among Tamilians. Indian J Clin Biochem. 2005;20(1):18–20.
Shenoy V, Mehendale V, Prabhu K, Shetty R, Rao P. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem. 2014;29(3):339–44.
Kluijtmans LA, Van den Heuvel LP, Boers GH, Frosst P, Stevens EM, Van Oost BA, et al. Molecular genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylenetetrahydrofolatereductase gene is a genetic risk factor for cardiovascular disease. Am J Hum Genet. 1996;58(1):35–41.
Falchi A, Giovannoni L, Piras IS, Caló Maria C, Moral Pedro, Vona Giuseppe, et al. Prevalence of genetic risk factors for coronary artery disease in Corsica island (France). ExpMolPathol. 2005;79(3):210–3.
Ma J, Stampfer MJ, Hennekens CH, Frosst P, Selhub J, Horsford J, et al. Methylenetetrahydrofolatereductase polymorphism, plasma folate, homocysteine, and risk of myocardial infarction in US physicians. Circulation. 1996;94(10):2410–6.
Schmitz C, Lindpaintner K, Verhoef P, Gaziano JM, Buring J. Genetic polymorphism of methylenetetrahydrofolatereductase and myocardial infarction. A Case-Control Study Circ. 1996;94(8):1812–4.
Chambers JC, Ireland H, Thompson E, Reilly P, Obeid OA, Refsum H, et al. Methylenetetrahydrofolatereductase 677 C–>T mutation and coronary heart disease risk in UK Indian Asians. Arterioscler Thromb Vasc Biol. 2000;20(11):2448–52.
Ilhan N, Kucuksu M, Kaman D, Ilhan N, Ozbay Y. The 677 C/T MTHFR polymorphism is associated with essential hypertension, coronary artery disease, and higher homocysteine levels. Arch Med Res. 2008;39(1):125–30.
Chambers JC, Obeid OA, Refsum H, Ueland P, Hackett D, Hooper J, et al. Plasma homocysteine concentrations and risk of coronary heart disease in UK Indian Asian and European men. The Lancet. 2000;355(9203):523–7.
Kadziela J, Janas J, Dzielinska Z, Szperl M, Gazdzik D, Chotkowska E, et al. The C677T mutation in methylenetetrahydrofolatereductase gene, plasma homocysteine concentrationand the risk of coronary artery disease. Kardiol Pol. 2003;59(7):17–26.
Mager A, Battler A, Birnbaum Y, Magal N, Shohat M. Plasma homocysteine, methylenetetrahydrofolatereductase genotypes, and age at onset of symptoms of myocardial ischemia. Am J Cardiol. 2002;89(8):919–23.
Mager A, Lalezari S, Shohat T, Birnbaum Y, Adler Y, Magal N, et al. Methylenetetrahydrofolatereductase genotypes and early-onset coronary artery disease. Circulation. 1999;100(24):2406–10.
Zee RYL, Mora S, Cheng S, Erlich HA, Lindpaintner K, Nader R, et al. Homocysteine, 5,10-methylene tetrehydrofolatereductase 677 C>T polymorphism, nutrient intake and incident cardiovascular disease in 24968 initially healthy women. Clin Chem. 2007;53:845–51.
Lin PT, Huang MC, Lee BJ, Chang CH, Tsai TP, Huang YC, et al. High plasma homocysteine is associated with the risk of coronary artery disease independent of methylene tetrahydrofolate reductase 677 C→T genes. Asia Pac J ClinNutr. 2008;17:330–8.
Kolling K, Ndrepepa G, Koch W, Braun S, Mehilli J, Schomig A. Methylenetetrahydrofolatereductase gene C677T and A1298C polymorphisms, plasma homocysteine, folate, and vitamin B12 levels and the extent of coronary artery disease. Am J Cardiol. 2004;93(10):1201–6.
Spiroski I, Kedev S, Antov S, Arsov T, Krstevska M, Dzhekova-Stojkova S, et al. Association of methylenetetrahydrofolatereductase (MTHFR-677 and MTHFR-1298) genetic polymorphisms with occlusive artery disease and deep venous thrombosis in Macedonians. Croat Med J. 2008;49(1):39–49.
Domagala TB, Adamek L, Nizankowska E, Sanak M, Szczeklik A. Mutations C677T and A1298C of the 5,10-methylenetetrahydrofolate reductase gene and fasting plasma homocysteine levels are not associated with the increased risk of venous thromboembolic disease. Blood Coagul Fibrinolysis. 2002;13(5):423–31.
Hanson NQ, Aras O, Yang F, Tsai MY. C677T and A1298C polymorphisms of the methylenetetrahydrofolatereductase gene: incidence and effect of combined genotypes on plasma fasting and post-methionine load homocysteine in vascular disease. Clin Chem. 2001;47(4):661–6.
Acknowledgements
We would like to thank Dr. Anant A. Takalkar, MBBS, MD, Professor in Community Medicine and consultant Statistician, Dept. of Community Medicine, MIMSR Medical College, Latur (Maharashtra) for his valuable help in statistical analysis of this project.
Funding
This work was supported by Bharati Vidyapeeth (Deemed to be) University, Pune (Grant number: BVDU/Research Cell/2016–17/929).
Author information
Authors and Affiliations
Contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Corresponding author
Ethics declarations
Conflict of interests
Authors state no conflict of interest.
Informed consent
Informed consent was obtained from all individuals included in this study.
Ethical approval
Research involving human subjects complied with all relevant national regulations, institutional policies and has been approved by the institutional ethical committee of Bharati Vidyapeeth (Deemed to be) University Medical college, Pune [BVDUMC/IEC/9A dated 30/04/2018].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shivkar, R.R., Gawade, G.C., Padwal, M.K. et al. Association of MTHFR C677T (rs1801133) and A1298C (rs1801131) Polymorphisms with Serum Homocysteine, Folate and Vitamin B12 in Patients with Young Coronary Artery Disease. Ind J Clin Biochem 37, 224–231 (2022). https://doi.org/10.1007/s12291-021-00982-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-021-00982-1