Abstract
Diabetic retinopathy (DR) is one of the major causes of blindness all over the world. According to the previous studies, impaired glucose tolerance (IGT) has been linked to retinal dysfunction/vascular damage. Decreased retinal function is an initial event of early DR. Although the biochemical and molecular events are not fully understood, glial activation, angiogenesis and oxidative stress are some of the pathways associated with early retinal abnormalities. Since IGT is associated with development of retinal dysfunction/vascular damage; as a preventive strategy, we have studied beneficial effect of Cinnamon as a hypoglycaemic agent on long-term IGT induced retinal abnormalities using neonatal streptozotocin (nSTZ) rat model. Control, IGT rats were maintained on AIN-93M diet alone and another set of IGT rats were maintained on AIN-93M diet with 3% Cinnamon for 8 months. At the end of the study, untreated IGT rats developed retinal functional abnormalities as assessed by electroretinogram (ERG) and the retinal structure did not alter as assessed by H&E staining. Further, increase in expressions of GFAP, VEGF and decreased expression of rhodopsin in untreated IGT rat retinas. 4-HNE, a marker of oxidative stress was also elevated in IGT state. Supplementation of Cinnamon to IGT rats had lowered fasting and postprandial glucose levels and also prevented retinal functional abnormalities. Further, Cinnamon protected photoreceptor cell damage, suppressed glial activation, angiogenesis and oxidative stress as there was an improved rhodopsin expression, inhibited elevated expressions of GFAP, VEGF and 4-HNE respectively. In conclusion, Cinnamon attenuated IGT induced retinal abnormalities probably through its hypoglycemic property.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic Retinopathy (DR) is one of the major microvascular complications of diabetes and the principal cause of extreme vision impairment in working-age adults [1]. Although duration of diabetes and severity of hyperglycemia are majorly known to contribute for onset of DR [2], some of the evidences have shown that even impaired glucose tolerance (IGT) or modest elevations in plasma glucose (impaired fasting glucose or IFG) level have been linked to retinal dysfunction/vascular damage or retinopathy [3,4,5]. In addition, higher postprandial blood glucose is one of the independent risk factors for DR [5, 6]. Reduction or loss of visual function is common feature of DR which also observed in IGT subjects [7].
Loss of retinal function has been associated with early stage of DR and photoreceptor degeneration [8, 9] which is further associated with a decrease of rhodopsin, a rod specific protein in the retina [10, 11]. Müller cells; the primary glial cell type plays an important role in maintaining retinal function and health. Elevated expression of GFAP in muller cells has been associated with retinal injury [12] and retinal degeneration [10, 13]. VEGF is an angiogenic molecule elevated in variety of stress conditions and play an important role in various eye diseases including DR [14], retinal degeneration [10, 11] which are associated with oxidative stress [14, 15].
Management of IGT/IFG or prediabetes and their complications could be possible with appropriate preventive strategies. Several studies reported as a review on plant or dietary based supplements especially spices to explore their beneficial effects for management of prediabetes and diabetes [16]. Cinnamon (Cin) is one of the spices used worldwide including India and it has been used in traditional medicine for centuries. Cinnamon and its aqueous extracts are well known for their hypoglycemic and postprandial glucose lowering effects in diabetic [17, 18] and healthy volunteers [19] and also in experimental models [20]. Earlier we studied the anticataractogenic effect of cinnamon in type-1 diabetic rat model [21]. However, beneficial effects of Cinnamon on IGT induced complications especially retinal abnormalities have not been attempted. Thus, the aim of the present study was to investigate the protective role of Cinnamon on IGT induced retinal abnormalities in nSTZ rat model.
Materials and Methods
Materials
Streptozotocin (STZ), TRI-reagent, HRP conjugated anti-mouse (A9044) and anti-rabbit (A6154) secondary antibodies were purchased from Sigma Chemical Company (St. Louis, USA); VEGF (PA547021), GFAP (PA3-16727), Rhodopsin (MA1-722), Beta actin (MA 1-91399) primary antibodies and HRP conjugated anti-goat (A24452) secondary antibodies were obtained from Thermo Scientific, USA; 4-Hydroxynonenal (4-HNE) primary antibody (Ab46545) was purchased from Abcam, USA; Alexafluor-488 conjugated anti-rabbit, Alexafluor-555 conjugated anti-mouse and Alexafluor-594 conjugated anti-goat antibodies were obtained from Molecular Probes (Eugene, OR, USA); SYBR Green Master Mix was obtained from Applied Biosystems, Warrington, UK, and all other regular chemicals were AR grade and procured from local companies. Cellulose, vitamin and mineral mixture were obtained from MP Biomedicals, California, USA.
Preparation of Cinnamon Powder
Fresh Cinnamon was obtained from the neighbourhood market and powdered. The Cinnamon powder was then combined with the AIN-93M diet.
Induction of IGT and Dietary Regimen
IGT was induced in Sprague–Dawley (SD) rat pups as described earlier [22] and the same is given in the flowchart (Fig. 1). We have followed the individual pregnant rats for their delivery time and date. The delivered mothers (n = 7) along with pups (n = 35) were obtained from the National Center for Laboratory Animal Sciences, National Institute of Nutrition, Hyderabad and maintained in the individual cages on the stock diet. After 48-h of the delivery, a set of male pups (n = 26) were injected with STZ at a dose of 90 mg/kg and the pups were maintained with their respective mothers (4–6 male pups/mother; 5 mothers). Control group pups (n = 9; 4–5 male pups/mother; 2 mothers) received equal volume of citrate buffer and kept with their respective mothers. After 21 days, all the pups (control and STZ injected) were weaned and maintained on AIN-93G diet in an individual cages for 2 months. At the end of 2 months, oral glucose tolerance test (OGTT) was conducted in control and STZ injected animals. Control rats were maintained on AIN-93M diet. STZ injected rats with 2 h OGTT glucose levels equal or more than 140 mg/dL were considered as IGT and further divided into two groups; untreated IGT (n = 12) rats maintained on the AIN-93M diet and another IGT group (n = 12) maintained on a AIN-93M diet containing 3% Cinnamon (IGT + Cin) for a period of eight months. These animals were maintained at a temperature of 22 ± 2 °C, 50% humidity, and 12-h light/dark cycle. Food intake (daily) and bodyweights (monthly) were recorded. Fasting and postprandial glucose levels were monitored every 2 months using a glucometer (One Touch Horizon).
Animal Care
All experimental protocols concerning animals were approved by the Institutional Animal Ethical Committee (IAEC) of the National Institute of Nutrition (P29/IAEC/NIN/2012/7/PS/Rats-SD/Male-81). We adhered to the ARVO Statement for the use of Animals in Ophthalmic and Vision research.
Oral glucose Tolerance Test (OGTT)
OGTT was conducted at second and tenth month after STZ injection on overnight fasted rats by administering glucose orally as a bolus, at a dose of 2.0 g kg−1 of body weight. Blood samples were collected at 0, 30, 60, and 120-min intervals for estimating plasma insulin and glucose concentrations to assess insulin resistance and IGT.
Plasma Glucose and Insulin
Plasma glucose and insulin levels were estimated using the GOD-POD method (Ozone Biomedicals Pvt. Ltd., India) and an ELISA kit (Mercodia, Sweden), respectively as per the manufacturer’s instructions.
Homeostasis Model Assessment (HOMA)
Insulin resistance index was calculated by homeostasis model assessment (HOMA) as described earlier for rats [22].
Electroretinography (ERG)
Animals were dark-adapted for 10–12 h and anesthetized under dim red light illumination. ERG-positive electrode was placed on the cornea of the eye, reference electrode on the ear and a ground electrode on a tail. Pupils were dilated with atropine eye drops 15 min before recording of ERG. Standard ERG was performed by a UTAS Visual Diagnostic System as described earlier [23]. Scotopic and photopic responses were recorded using a series of flash stimuli of − 3.0 to 1.19 log cd-s/m2 using dim white LED with Ganzfeld (BigShot) (LKC Technologies; Gaithersburg, MD, USA). For photopic ERGs, animals were light-adapted for 10 min before photopic recordings. Responses were amplified with UBA-4204 amplifier and analyzed. We used the same photopic and scotopic amplitudes recorded between 0.19 and 1.19 log cd-s/m2 for calculation of Oscillatory potentials (OPs).
Quantitative Real-Time PCR (qRT-PCR)
At the end of the study, all animals were sacrificed and retinas were collected from a set of eye balls and frozen in the liquid nitrogen. Total RNA was extracted from the whole frozen retinas (2–3 retinas from each group) by TRI reagent. The isolated RNA was purified using the RNeasy mini kit (Qiagen). The concentration of total RNA was quantified by measuring the absorbance at 260 nm and 280 nm using Nanodrop spectrophotometer (ND1000) and the integrity was checked by running the RNA samples on denaturing MOPS-Formaldehyde gel. Known quantity (5 µg) of RNA was immediately subjected to reverse transcriptase using cDNA Synthesis Kit (bioline). qRT-PCR was performed using 25 ng of cDNA template and SYBR green master mix by real-time PCR (ABI 7500) using the following gene specific primers: β-Actin forward 5′-gagaagagctatgagctgcc-3′, β-Actin reverse 5′-ctcaggaggagcaatgatct-3′, GFAP forward 5′-tttctccaacctccagatcc-3′, GFAP reverse 5′-agctttaggccctcacactg-3′, Rhodopsin forward 5′-cttcttgatctgctggcttc-3′, Rhodopsin reverse 5′-acagtgtctggccaggctta-3′ VEGF forward 5′- tcaccaaagccagcacatag- 3′, VEGF reverse 5′-gggagtctgtgtttttgcag-3′. The specificity of products generated for each set of primers was examined for each fragment with the use of a melting curve analysis at the end of the run. The relative expression of each targeted gene was normalized by subtracting the corresponding beta-actin threshold cycle (CT) values by using the ∆∆CT values. A total of three samples for each group were used, and each sample was run in triplicate for real-time PCR.
Histology and Immunohistochemistry (IHC)
Another set of eyeballs from each group were kept for overnight in 20 mM phosphate buffer (pH, 7.2) containing 4% paraformaldehyde. These eyeballs were embedded in paraffin blocks and sections of 5 µm thickness were prepared and subsequently mounted on slides. These sections were then used for staining with Hematoxylin & Eosin (H&E) and were observed under light microscope.
The other set of eye ball sections used for immunohistochemistry (IHC) were deparaffinized followed by antigen retrieval by keeping sections in boiled 10 mM Na-citrate buffer pH 6.0 for about 10 min and blocking with 3% goat serum in 0.3% TritonX in PBS. After washing three times with PBS, the sections were incubated in PBS with polyclonal primary antibodies [VEGF (1:50); GFAP (1:500); rhodopsin (1:1000), or 4-HNE (1:100)] for overnight at 4 °C. The slides were washed again with PBS and incubated with secondary antibody conjugated with alexafluor 488 (green)/594 (red). It was then mounted with DAPI antifade mounting medium (Vectashield). These sections were examined for specific staining for above molecules using a fluorescent microscope (Leica Microsystems, LMD6000, Germany).
SDS-PAGE and Immunoblot (IB)
Equal amounts of retinal proteins from pooled retinal lysates of each group were resolved on 12% SDS-PAGE and transferred onto the nitrocellulose (NC) membrane (Pall Corporation, NY, USA). The membrane was immunolabeled with primary antibodies of GFAP (1:1000) or Rho (1:25000) or anti-human recombinant VEGF (1:1000) or 4-HNE (1:500) or beta actin (1:500) followed by incubation with secondary anti goat/anti mouse/anti rabbit IgG conjugated to peroxidase. After thorough washing, immunoreactive bands were developed and visualized using a chemiluminescence kit (Bio-Rad). The bands were quantified by using Image J software.
Statistical Analysis
All statistical analyses were performed using SPSS version 19.0 and quantitative data was presented as mean ± standard error (SE). One-way ANOVA, followed by Tukey HSD test was used to analyze the differences among groups and p < 0.05 was considered as significant.
Results
Food Intake and Body Weights
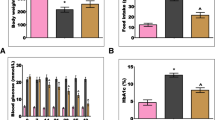
A marginal increase in the average food intake (18.21 ± 1.31 g/rat/day) was observed in untreated IGT group rats when compared to control rats (17.18 ± 0.57 g/rat/day). Despite of the increased food intake, the mean body weights of untreated IGT rats were marginally decreased at the end of the experiment (416 ± 1.9 g) when compared to the control rats (429 ± 3.98 g). However, there were no differences in the mean food intake (18.01 ± 0.81 g/rat/day) and body weight (402 ± 0.52 g) between Cinnamon treated and untreated IGT rats.
Glucose and Insulin Response During OGTT and HOMA-IR
There is a significant high glucose response at all time points except at 0 min in nSTZ rats compared to controls indicating development of IGT at 2 months after STZ injection (Fig. 2a) and maintained this IGT state up to 10 months (Fig. 2c). Cinnamon treated IGT rats showed a significant (p < 0.05) lower plasma glucose levels when compared to untreated IGT rats at all time points except at 0 min (Fig. 2c). The insulin response during OGTT at second month was significantly (p < 0.01) low at 30 and 60 min in nSTZ rats compared to controls (Fig. 2b). Similar response was observed during OGTT at the end of 10 months in untreated IGT group rats compared to controls (Fig. 2d). Interestingly, feeding of Cinnamon to IGT rats showed a significantly high insulin response at 30 and 60 min compared to untreated IGT rats (Fig. 2d).
Glucose and insulin response during OGTT at second month (a, b) and tenth month (c, d) of the experimental period. Values are mean ± SE n = 9–12 animals. The asterisk (*) denotes that data are significantly different from control group and the hash mark (#) denotes that data are significantly different from IGT group
However, there was no difference in HOMA-IR index between control (0.17 ± 0.035) and STZ injected IGT animals (0.18 ± 0.024) at 2 months and even at the end of 10 months as there was no difference in HOMA-IR between control (0.19 ± 0.0135) and STZ injected IGT animals (0.021 ± 0.033) and Cinnamon had no effect on HOMA-IR (0.022 ± 0.014).
Fasting and Postprandial Blood Glucose Levels
Although, the fasting glucose levels of all the three groups falls under normal range (70–100 mg/dL), the mean fasting blood glucose levels were significantly high in untreated IGT group animals compared to controls. Feeding of Cinnamon to IGT rats significantly lowered the fasting glucose levels during the experiment (except at 4th and 8th month) compared to untreated IGT group animals (Fig. 3a). Similarly, the postprandial blood glucose levels were also significantly higher in untreated IGT rats when compared to controls during the experimental period (Fig. 3b). Feeding of Cinnamon to IGT rats significantly lowered postprandial blood glucose levels compared to untreated IGT rats (except at 6th month) (Fig. 3b).
Fasting (a) and postprandial blood glucose levels (b) during the experimental period assessed using glucometer (one touch horizon). All values are mean ± SE, n = 9–12 animals. The asterisk (*) denotes that data are significantly different from control group and the hash mark (#) denotes that data are significantly different from IGT group
Retinal Function
ERG responses in untreated IGT rats showed a significant reduction of scotopic b-wave amplitudes from − 2.4 to 1.19 log cd-s/mm and OPs at 1.19 log cd-s/mm compared to control rats indicating development of retinal functional abnormalities. Cinnamon treatment marginally prevented in reduction of scotopic b-wave, a-wave amplitudes (Fig. 4a, b) and OPs (Fig. 4c, d) compared to untreated IGT group animals.
Electroretinogram data. Scotopic b-wave amplitudes (a), Scotopic a-wave amplitudes (b), Representative wave forms of scotopic oscillatory potentials (c) and representative wave forms of scotopic oscillatory potentials (d) of control, IGT and IGT + Cin groups. Values are mean ± SE. The asterisk (*) denotes that data are significantly different from control group
Retinal Structure
Gross morphological observations indicate that control rats had well maintained retinal integrity as shown in H&E stained retinal sections, whereas, a minimal decrease in outer and inner nuclear layer thickness was observed in the retinas of untreated IGT rats compared to control animals. Cinnamon treatment prevented these gross morphological changes (Fig. 5a).
Morphology of retinas control, IGT and IGT + Cin group stained with H&E (a). Expression of VEGF, Rho, GFAP and 4-HNE in control, IGT and IGT rats fed with cinnamon (IGT + Cin) group rat retinas by IHC (b). White arrow indicated at the site of expression of VEGF, GFAP, rhodopsin and 4-HNE. GCL ganglion cell layer, INL inner nuclear layer, ONL outer nuclear layer
Retinal mRNA and Protein Expression
Immunostaining with VEGF and GFAP antibodies showed a relative increased immunoreactivity in untreated IGT rat retinas, indicating increased angiogenesis and glial cell activation respectively. A decreased rhodopsin-specific immunofluorescence signal was observed in untreated IGT rat retinas when compared to the control rats indicating an association of retinal degeneration in IGT state. Interestingly, feeding of cinnamon to IGT rats had partially prevented these alterations. 4-HNE, a marker of oxidative stress and there is no markable difference in immune staining in retinas of the three groups (Fig. 5b).
The immunofluorescence data were further confirmed by immune blotting to study VEGF, GFAP, rhodopsin and 4-HNE protein expressions in all the three groups. As expected, there was a significant (p < 0.05) increase in VEGF, GFAP and significantly (p < 0.05) decreased rhodopsin expression in untreated IGT rat retinas compared to that of controls further substantiating our IF data. In addition, there was a marginal increase in the relative expression of 4-HNE protein indicating association of oxidative stress in IGT state. Feeding of cinnamon to IGT rats marginally prevented these alterations (Fig. 6a, b).
Expression of VEGF, GFAP, Rhodopsin and 4-HNE at protein level by western blot (a) and densitometry analysis (b) in control, IGT and IGT rats fed with cinnamon (IGT + Cin) group rat retinas. Results are mean ± SE for three independent protein preparations for each group. The asterisk (*) denotes that data are significantly different from control group
Real time PCR analysis showed a significant (p < 0.01) upregulation of VEGF and GFAP expression, and significant (p < 0.01) downregulation of rhodopsin expression in untreated IGT rats compared to that of controls, further supporting our IF and western blot data which indicates the development of retinal abnormalities in IGT state. Interestingly, feeding of Cinnamon to IGT has significantly (p < 0.01) prevented the up-regulation of GFAP and down-regulation of rhodopsin expression (Fig. 7).
Expression of VEGF, GFAP and Rhodopsin at mRNA levels by qRT-PCR in control, IGT and IGT rats fed with cinnamon (IGT + Cin) group rat retinas. Gene expression results for these genes were normalized to beta actin. Results are mean ± SE for three independent mRNA preparations for each group. The asterisk (*) denotes that data are significantly different from control group and the hash mark (#) denotes that data are significantly different from IGT group
Discussion
STZ is widely used drug to induce both type-1 and type-2 diabetes in experimental animals. STZ selectively enters to the pancreatic β cells via a glucose transporter (GLUT2) and causes partial damage of pancreatic β cells through alkylation of DNA which is the main reason of its toxicity and also through oxidative stress by increasing nitric oxide, hydrogen peroxide, and hydroxyl radicals [24]. Earlier our own studies [22] and others [25] found to have IGT, and mild, moderate and severe glycaemia in nSTZ model. In the present study, we demonstrated the protective role of Cinnamon in IGT-induced retinal abnormalities using an nSTZ-induced IGT rat model [22]. As we reported earlier [22], a single intraperitoneal STZ injection to 2 days old SD rat pups significantly increased fasting and postprandial glucose levels and resulted in the development of IGT at 2 months, which was maintained up to 10 months. Fasting and postprandial blood glucose levels were early indicators to identify the development of impaired glucose metabolism, prediabetes and diabetes. Although, the fasting glucose level in all the three groups falls under normal range (70–100 mg/dL), the mean fasting blood glucose levels were significantly high in untreated IGT group animals compared to controls. Supplementation of cinnamon at 3% in the diet to a subset of IGT rats (IGT + Cin group) showed a significant decrease in both fasting and postprandial glucose levels during the experiment probably through its hypoglycemic nature. These results observed in the present study was well correlated with the recent human and animal studies where they observed hypoglycemic effect of cinnamon in healthy volunteers [19], type-2 diabetic subjects [17, 18], in experimental diabetic animals [20]. Supplementation of Cinnamon to the IGT (supplement group) rats showed a significant decrease in the 2 h glucose levels. These results are well correlated with previous studies in healthy volunteers [26] and experimental animals [20].
Since some of the earlier studies have shown the risk and adverse effects of abnormal glucose metabolism on retina or retinal complications [3, 5, 6, 10], we studied the role of cinnamon as an hypoglycemic agent on IGT induced retinal abnormalities. Development of retinal abnormalities in the present study was assessed using ERG and other key molecular markers which are associated with retinopathy and retinal degenerations. Retinal functional abnormalities may develop at early glucose dysregulation [7] and can be detected by ERG [27]. Further, reduced ERG a-wave, b-wave amplitudes and OPs can be used as indicators for studying various types of retinal degeneration related to photoreceptor and/or inner retinal cells in animal studies [27]. In the present investigation, we have observed functional abnormalities as there was a decrease of ERG scotopic b-wave, a-wave amplitudes and OPs in untreated IGT animals when compared to control animals. These alterations may be due to dysfunction of the inner retinal layers (bipolar, muller and amacrine cells). Feeding of Cinnamon to IGT rats had shown a marginal protective effect by inhibiting the scotopic b-wave, a-wave and OPs.
Muller cells are early affected cells of the retina during development of retinopathy and ERG b wave response represents the functions of these cells [28]. GFAP is one of the key molecules and its elevated expression in glial activation was associated with retinal damage [12] and also with retinal degeneration [10]. Since in the present study, we observed a decrease of ERG b-wave, which mainly comes from Muller and bipolar cells; we have studied the expression of GFAP and used as a cellular marker for retinal damage. As expected there was an increased expression of GFAP at mRNA and protein levels in the untreated IGT group retinas when compared to controls indicating retinal damage through glial activation. Feeding of Cinnamon to IGT group rats had partially prevented retinal damage by inhibiting glial activation as there was a significant (p < 0.01) inhibition of GFAP expression when compared to untreated IGT animals.
Photoreceptors of the retina play an important role in the development of early stages of DR and retinal degenerations [8, 29]. Rhodopsin is rod specific protein present in the retina which is important for photoreceptor function. A recent study has also shown a photoreceptor dysfunction (decreased ERG scotopic and photopic a- and b-wave amplitudes) along with a decreased rhodopsin levels in diabetic animals [30]. In the present study, we found a decrease in rhodopsin in the untreated IGT rat retinas along with a decrease in the scotopic b-wave, a-wave amplitudes and OPs indicating photoreceptor cell dysfunction in IGT stage. Interestingly, feeding of Cinnamon at 3% in the diet to a subset of IGT rats has prevented loss of rhodopsin in these animals. These observations are in line with previous study, where they observed a loss of rhodopsin in obesity and IGT associated retinal degeneration rat model [10].
VEGF is an angiogenic molecule which plays an important role in the pathogenesis of diabetic vascular complications including DR [14]. During hyperglycemia, VEGF expression is regulated by retina cells majorly by hypoxia, but it also accumulates in the retina early in diabetes [31], IGT associated Obesity [10, 11], even in non-fasting hyperglycemic/elevated postprandial glucose state [32] which may later contribute to the development of clinical symptoms of retinopathy. In the present study, an elevated expression of VEGF in untreated IGT rats could be due to glial activation as there was an increased expression of GFAP in these animals. In addition to the glial activation, VEGF is also elevated in various stress conditions including oxidative stress [14]. Hence, we have measured 4-HNE in the retinas as an indicator of oxidative stress to study the possible role of oxidative stress in elevated levels of VEGF in IGT state. In the present study, there is an increased 4-HNE in untreated IGT rat retinas compared to that in the control group, indicating oxidative stress and this may be an another contributor for elevated levels of VEGF in IGT rat retina. Feeding of Cinnamon to IGT group rats has marginally inhibited VEGF expression by inhibiting oxidative stress probably through its hypoglycemic property.
Conclusion
Cinnamon protected against long-term IGT-induced retinal abnormalities mainly through its hypoglycemic properties. Our findings may provide a basis for not only preventing long-term IGT-induced retinal abnormalities but also to develop therapeutic strategies in controlling early diabetes and its associated complications.
References
Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298(8):902–16. https://doi.org/10.1001/jama.298.8.902.
Henricsson M, Nilsson A, Groop L, Heijl A, Janzon L. Prevalence of diabetic retinopathy in relation to age at onset of the diabetes, treatment, duration and glycemic control. Acta Ophthalmol Scand. 1996;74(6):523–7. https://doi.org/10.1111/j.1600-0420.1996.tb00727.x.
Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52(12):2867–73. https://doi.org/10.2337/diabetes.52.12.2874.
Wong TY, Barr EL, Tapp RJ, Harper CA, Taylor HR, Zimmet PZ, et al. Retinopathy in persons with impaired glucose metabolism: the Australian diabetes obesity and lifestyle (AusDiab) study. Am J Ophthalmol. 2005;140:1157–9. https://doi.org/10.1016/j.ajo.2005.07.030.
Wang J, Zhang RY, Chen RP, Sun J, Yang R, Ke XY, et al. Prevalence and risk factors for diabetic retinopathy in a high-risk Chinese population. BMC Publ Health. 2013;13:633–9. https://doi.org/10.1186/1471-2458-13-633.
Liu Y, Yang J, Tao L, Lv H, Jiang X, Zhang M, et al. Risk factors of diabetic retinopathy and sight-threatening diabetic retinopathy: a cross-sectional study of 13 473 patients with type 2 diabetes mellitus in mainland China. BMJ Open. 2017;7(9):e016280. https://doi.org/10.1136/bmjopen-2017-016280.
Karadeniz S, Kir N, Yilmaz MT, Ongör E, Dinççag N, Başar D, et al. Alteration of visual function in impaired glucose tolerance. Eur J Ophthalmol. 1996;6(1):59–62.
Kern TS. Do photoreceptor cells cause the development of retinal vascular disease? Vision Res. 2017;139:65–71. https://doi.org/10.1016/j.visres.2017.03.011.
Bravo-Nuevo A, Walsh N, Stone J. Photoreceptor degeneration and loss of retinal function in the C57BL/6-C2J mouse. Invest Ophthalmol Vis Sci. 2004;45(6):2005–12.
Reddy GB, Vasireddy V, Mandal MN, Tiruvalluru M, Wang XF, Jablonski MM, et al. A novel rat model with obesity-associated retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:3456–63. https://doi.org/10.1167/iovs.08-2498.
Godisela KK, Reddy SS, Kumar CU, Saravanan N, Reddy PY, Jablonski MM, et al. Impact of obesity with impaired glucose tolerance on retinal degeneration in a rat model of metabolic syndrome. Mol Vis. 2017;23:263–74.
Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electroretinographical and morphological observations. Exp Eye Res. 2002;74:615–25. https://doi.org/10.1006/exer.2002.1170.
Reisenhofer M, Pannicke T, Reichenbach A, Enzmann V. Characteristics of Müller glial cells in MNU-induced retinal degeneration. Vis Neurosci. 2016;33:e013. https://doi.org/10.1017/S0952523816000109.
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27(4):331–71. https://doi.org/10.1016/j.preteyeres.2008.05.001.
Militante J, Lombardini JB. Age-related retinal degeneration in animal models of aging: possible involvement of taurine deficiency and oxidative stress. Neurochem Res. 2004;29(1):151–60.
Beidokhti MN, Jäger AK. Review of antidiabetic fruits, vegetables, beverages, oils and spices commonly consumed in the diet. J Ethnopharmacol. 2017;201:26–41. https://doi.org/10.1016/j.jep.2017.02.031.
Sahib AS. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: a randomized, placebo-controlled clinical trial. J Intercult Ethnopharmacol. 2016;5(2):108–13. https://doi.org/10.5455/jice.20160217044511.
Zare R, Nadjarzadeh A, Zarshenas MM, Shams M, Heydari M. Efficacy of cinnamon in patients with type II diabetes mellitus: a randomized controlled clinical trial. Clin Nutr. 2018;S0261–5614(18):30114–26. https://doi.org/10.1016/j.clnu.2018.03.003.
Hlebowicz J, Darwiche G, Bjorgell O, Almer LO. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J Clin Nutr. 2007;85:1552–6. https://doi.org/10.1093/ajcn/85.6.1552.
Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108–20. https://doi.org/10.1186/s12937-015-0098-9.
Muthenna P, Akileshwari C, Saraswat M, Bhanuprakash Reddy G. Inhibition of advanced glycation end-product formation on eye lens protein by rutin. Br J Nutr. 2013;107(7):941–9. https://doi.org/10.1017/S0007114511004077.
Patil MA, Suryanarayana P, Putcha UK, Srinivas M, Reddy GB. Evaluation of neonatal streptozotocin induced diabetic rat model for the development of cataract. Oxid Med Cell Longev. 2014. https://doi.org/10.1155/2014/463264.
Machida S, Kondo M, Jamison JA, Khan NW, Kononen LT, Sugawara T, et al. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci. 2000;41(10):3200–9.
Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537–46.
Arulmozhi DK, Veeranjaneyulu A, Bodhankar SL. Neonatal streptozotocin-induced rat model of Type 2 diabetes mellitus: a glance. Indian J Pharmacol. 2004;36(4):217–21.
Solomon TP, Blannin AK. Changes in glucose tolerance and insulin sensitivity following 2 weeks of daily cinnamon ingestion in healthy humans. Eur J Appl Physiol. 2009;105:969–76. https://doi.org/10.1007/s00421-009-0986-9.
Frishman LA, Wang MH. Electroretinogram of human, monkey and mouse. Adler’s Physiol Eye. 2011;24:480–501.
Miller RF, Dowling JE. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970;33:323–41. https://doi.org/10.1152/jn.1970.33.3.323.
Kern TS, Berkowitz BA. Photoreceptors in diabetic retinopathy. J Diabetes Investig. 2015;6(4):371–80. https://doi.org/10.1111/jdi.12312.
Malechka VV, Moiseyev G, Takahashi Y, Shin Y, Ma JX. Impaired rhodopsin generation in the rat model of diabetic retinopathy. Am J Pathol. 2017;187(10):2222–31. https://doi.org/10.1016/j.ajpath.2017.06.007.
Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42(10):2408–13.
Salido EM, de Zavalía N, Schreier L, De Laurentiis A, Rettori V, Chianelli M, et al. Retinal changes in an experimental model of early type 2 diabetes in rats characterized by non-fasting hyperglycemia. Exp Neurol. 2012;236:151–60. https://doi.org/10.1016/j.expneurol.2012.04.010.
Funding
This work was supported by the Department of Biotechnology, Government of India (Grant No: BT/PR3446/BRB/10/969/2011) and National Institute of Nutrition (Indian Council of Medical research, Government of India) through intramural funding (#12-BS11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kommula, S.R., Vadakattu, S.S., Myadara, S. et al. Cinnamon Attenuated Long-Term IGT-Induced Retinal Abnormalities via Regulation of Glucose Homeostasis in Neonatal Streptozotocin Induced Rat Model. Ind J Clin Biochem 35, 442–450 (2020). https://doi.org/10.1007/s12291-019-00842-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-019-00842-z