Abstract
Metabolic parameters like uric acid, lipids and homocysteine are influenced by immunopathological mechanisms underlying the autoimmune disease processes. The current study examined the differences in these parameters and the correlation between inflammatory and metabolic variables in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) patients. The cross-sectional prospective study included 24 treatment-naïve patients with moderate to severe diseases-15 subjects had RA and 9 had SLE. Atherogenic index of plasma (AIP) was used to assess the cardiovascular risk of the patients. Spearman’s correlation was performed to verify the relationship between inflammatory and metabolic parameters. A two-tailed P < 0.05 was considered statistically significant for all the analysis. SLE patients had higher uric acid levels, very low density lipoprotein-cholesterol, total cholesterol/high density lipoprotein-cholesterol ratio (TC/HDL-C) and logarithmic ratio of triglycerides to HDL-cholesterol (log[TG/HDL-C]) than RA. Whereas, reduced total lymphocyte count, lipoprotein(a), and low density lipoprotein cholesterol were noted in the former than latter group. Majority of the SLE patients had increased risk of cardiovascular diseases (> 0.24 AIP score) and RA patients in comparison had lower risk. Correlation among serum uric acid, lipid profile constituents and AIP was noted. The immunological process of SLE has greater impact on the metabolic parameters. Higher uric acid levels are suggestive of dysfunctional lipid profile. Understanding the implications of risk factors and its inflammatory role in autoimmune processes may assist in disease management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impact of inflammatory diseases on various metabolic processes including lipid metabolism, glucose metabolism, protein and purine pathways, and endocrinal pathways has been well established [1, 2]. The autoimmune rheumatic disease, predominantly being an inflammatory disorder, has significant influence on different metabolic pathways. The differential influence of cytokines and other immune activation pathways on lipid metabolism, uric acid cycle and metabolic pathways is evident in published literature, and their effects also depend on the triggering and perpetuating factors of inflammation [1, 3]. The autoimmune rheumatic diseases, like rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), have different immunopathogenetic mechanisms. Proliferative process, predominantly driven by TNF, is the basic pathology of RA. Whereas SLE causes tissue necrosis and apoptosis and it is predominantly driven by interferon [4, 5]. RA is predominantly cell-mediated drive, while SLE has significant component of immune complex and antibody-dependent mechanism [6]. The differential CV risks noted in patients with these diseases could be mainly attributed to difference in the immunopathogenic mechanisms [7, 8]. The elevated CV risk is 5–10 times more in SLE and 2–3 times in RA than normal subjects [9, 10].
Dyslipidemia is an indicator of increased incidence of atherosclerosis and coronary heart disease. Studies correlate changes in cholesterol levels with RA disease activity [11]. A recently published literature on early RA suggests such changes to be biphasic i.e. an initial reduction in total cholesterol (TC), triglyceride (TG) and high density lipoprotein (HDL), and their subsequent increase at around 24th week of treatment and further stabilization [1]. There is literature evidence suggesting that the variation in lipid changes is proportional to inflammatory load. Based on these observations, the present study had considered patients with moderate to severe disease.
In addition to cholesterol, changes in uric acid level have been reported as a marker of increased cell turnover accompanied by necrosis [12]. Moreover, the uric acid level has been identified as a surrogate marker of CV risk and as an indicator of possible aggressive autoimmune diseases [13]. The serum uric acid level differs in various autoimmune diseases and it is usually lower in RA. Increase in uric acid levels in SLE is associated with severe clinical manifestations like pulmonary hypertension and renal involvement [14].
Based on these findings, we propose that the effect of inflammation on lipid metabolism, uric acid level and homocysteine is influenced by the immunological process, apart from the extent of inflammation. In order to test this hypothesis, we studied the lipid profile, uric acid and homocysteine levels of treatment-naïve patients with two common autoimmune diseases namely RA and SLE, who had moderate to severe disease. The study attempted to evaluate the correlation of the inflammatory variables like CRP, ESR and neutrophil to lymphocyte ratio (NLR) on metabolic parameters.
Materials and Methods
The cross-sectional, prospective study was conducted at a tertiary care center based in India from June 2014 to January 2015. The study recruited subjects who fulfilled the American College of Rheumatology and the European League against Rheumatism (ACR/EULAR) 2010 criteria for the diagnosis of RA, and Systemic Lupus International Collaborating Clinic (SLICC) criteria for SLE [15, 16]. The selected subjects were treatment-naïve with moderate to severe disease and managed only by NSAIDs or other symptom-modifying treatment. The exclusion criteria considered were: use of steroid or DMARDs at the point of recruitment, having overlapping infection including chronic viral infections like hepatitis B, C and HIV infection, and the use of anti-cholesterol or drugs that can modify either uric acid or homocysteine. The study was approved by the institutional ethics committee and informed consent was obtained from all patients.
The patients were recruited consecutively on their first visit to the institute and the demographic data including age, gender, and duration of disease were recorded. The laboratory parameters considered were: total lymphocyte counts (TLC), neutrophils, lymphocytes, and hemoglobin measured by automated 5-part cell counter; erythrocyte sedimentation rate by Westergren method; c-reactive protein (CRP) by nephelometry; homocysteine by chemiluminescence; lipoprotein(a) by immunoturbidimetry; uric acid by photometric 2,4,6-tribromo-3-hydroxybenzoic acid (TBHBA) method; and lipid profile [TG, TC and lipoproteins: high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), and very low density lipoprotein-cholesterol (VLDL-C)]. TC was estimated by cholesterol oxidase-phenol-aminophenazone (CHOD-PAP) enzymatic method, TG by glycerol phosphate oxidase (GPO)-Trinder’s method, and HDL by direct method. The LDL, VLDL, TC/HDL-C ratio and neutrophil to lymphocyte ratio (NLR) were calculated. Total joint count, swollen joint count and tender joint count of RA patients were assessed and DAS28-CRP was calculated by three variable method [17].
Atherogenic index of plasma (AIP) was calculated as log(TG/HDL-C) in molar concentrations for all the study subjects to assess their CV risk. The AIP values were subsequently classified based on the method put forth by Dobiasova et al. [18,19,20,21]. TG was divided by 89 and HDL-C by 39 to convert TG and HDL-C from mg/dl to mmol/l. Based on the AIP values, the CV risks of the subjects were classified as follows: − 0.3 to 0.1: low, 0.1–0.24: medium and > 0.24: high.
Statistical Analysis
The demographic, clinical and biochemical characteristics of the recruited subjects with RA and SLE were compared. Analysis of continuous variables with normal distribution was done by independent t test and for data without normal distribution by Mann–Whitney U test. Categorical data was analyzed by Chi square test or Fisher’s exact test. Spearman’s rank non-parametric test was used to assess the bivariate correlation for inflammatory and metabolic variables for all the study subjects [22]. P value < 0.05 was considered significant for all the statistical tests. Statistical Package for the Social Sciences (SPSS 22) was used for statistical analysis.
Results
Twenty-four subjects were recruited for the study; 15 patients had RA and 9 had SLE. All the RA subjects had active disease with DAS28-CRP values > 3.5. The demographic characteristics are listed in Table 1. The RA patients (51 ± 13.59) were significantly older than SLE patients (31 ± 11.4). Difference in gender distribution was noted with more females than males in RA (M:F 4:11), and only females in SLE (M:F 0:9) group. RA subjects had longer duration of illness compared to SLE patients; however, the groups did not differ significantly.
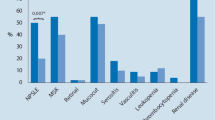
Evaluation of the clinical and biochemical parameters (Table 1) revealed that TLC was significantly higher in RA patients when compared to SLE group. However, the values were within the normal range. Hemoglobin (%) was slightly lower in SLE than RA patients. The lipoprotein(a) was also significantly lower in SLE patients (1.84 mg/dl) than in RA (30 mg/dl). In contrast, uric acid levels were significantly higher in SLE (5.8 mg/dl) group than in RA (4.8 mg/dl). There was no significant difference between the groups with regard to the values of ESR, CRP, NLR, and homocysteine. Interestingly, both the groups had elevated ESR levels. SLE patients had lower CRP levels than RA, and both groups had NLR around 3.
Lipid profile assessment showed that patient groups differed significantly for TC, LDL, VLDL, TC/HDL-C and log (TG/HDL-C). The average values of TC were below normal in both the groups; SLE subjects (114.16 ± 63.23) had significantly lower TC levels than RA (164.25 ± 53.36). Similarly, LDL-C was significantly lesser in SLE patients (49 mg/dl) than in RA (101 mg/dl). However, VLDL-C was higher in SLE patients (38 mg/dl) than in RA (20 mg/dl). The SLE patients also had higher TC/HDL-C values than RA; the average value in SLE was 6.3, which was above normal range.
AIP is a marker of CV risk in patients. The mean value of log (TG/HDL-C) for the SLE group was > 0.24, indicating increased CV risk among these subjects than 0.14 in RA, indicating intermediate risk. The differences among the groups were statistically significant. The categorized AIP index values showed that one patient had intermediate risk and all the remaining patients in SLE group had increased risk (Table 2). The majority of the RA patients (n = 08) had low risk, 2 patients had intermediate and 5 had high risk.
The correlation among the inflammatory and metabolic parameters was evaluated, regardless of the disease groups (Table 3). Spearman’s correlation demonstrated that, among the inflammatory markers, ESR showed non-significant moderate positive correlation with CRP levels. NLR did not show significant correlation with any of the inflammatory parameters. None of the inflammatory parameters had significant correlation with metabolic markers. Among the metabolic variables, uric acid demonstrated significant strong positive correlation with VLDL-C, TC/HDL-C, and log(TG/HDL-C). Among the lipid profile variables, TC had strong positive correlation with LDL-C, VLDL-C with TC/HDL-C and log(TG/HDL-C). TC/HDL-C showed significant positive correlation with log(TG/HDL-C). Homocysteine and lipoprotein(a) did not show correlation with any of the variables.
Discussion
Lipid profiles of both the disease groups showed significant differences from normal population and had higher atherogenic risk. The average value of TC was lesser than the normal population. SLE had higher atherogenicity index than RA. The elevated log (TG/HDL-C) was significant, despite no significant difference in the inflammatory parameters. The changes in TC, TC/HDL-C, log (TG/HDL-C) were not correlated with inflammatory parameters. Moreover, the uric acid levels were higher in SLE and were significantly different from RA. The uric acid correlated directly with VLDL-C, TC/HDL-C, and log(TG/HDL-C). This observation substantiates the differential impact of autoimmune diseases on uric acid and TC, LDL-C, TC/HDL-C, and log(TG/HDL-C).
Literature studies comparing the lipid profiles of SLE and RA are inconsistent with regard to the changes in lipid components. Chung et al. have reported lower levels of TC, LDL-C, TG and higher lipoprotein(a) in SLE. The present study has noted low TC, LDL-C, and lipoprotein(a), and very high VLDL-C in SLE patients. Whereas, in the study by Chung et al. [7] all the parameters were lower than normal values and the primary objective was to verify differential association of insulin resistance with mediators of inflammation in subjects treated for SLE and RA. The lipid profile changes noted in the present study is comparable to that of Santose et al. [23]. The present study has also reported higher atherogenic index for SLE than RA. This was further corroborated by the observation that majority of SLE had high CV risk whereas more number of RA patients had low CV risk.
Several other studies on SLE have reported variable lipid profile presentations. ‘Lupus pattern’ of lipoproteins, i.e. elevated TG and VLDL-C concentrations along with decreased HDL-C levels, has been found during the active phase of SLE, while increased LDL-C and TC concentrations during the period of lower disease activity [24,25,26]. A significant attenuation in lipid protein levels including TC was noted in the present RA subjects. Majority of the studies have reported similar findings and the CV risk ratio tilts towards atherogenesis in such patients. The dyslipidemia frequency noted by Garrido et al. [25] in a RA cohort was 54.9% and it was most frequent between 51 and 60 years of age. The current study, with all the recruited subjects having dyslipidemia, reveals the true status of lipid metabolism, since all of them had moderate to severe disease status, and were not on any lipid-altering medications.
In the current study, RA patients had elevated LDL-C levels when compared to SLE. LDL-C is prone to oxidative modification in the microenvironment of increased oxidative stress such as in autoimmune diseases. Higher levels of oxidized LDL-C (oxLDL) may lead to disruption of endothelial homeostasis via the nitric oxide (NO) pathway. After infiltrating the endothelial arterial wall, oxLDL-C induces the expression of leukocyte adhesion molecules and initiates inflammatory response in the artery wall. The CV risk is aggravated in SLE patients due to the presence of antibodies against oxLDL-C, which accelerate the uptake of LDL-C into the endothelial walls. In addition to LDL-C, the cardiolipin component is also oxidized. Antibodies against oxLDL-C cross-react with IgM and IgG anticardiolipin antibodies and oxLDL-C form complexes with β2-glycoprotein I component of cardiolipin, which in turn enhances the risk of arterial thrombosis [27]. Standard lipid profiles do not reflect oxLDL-C levels. The study by Kim et al. [28] have demonstrated that patients with RA and SLE had significantly lower serum LDL levels and higher serum ox-LDL levels than in age-matched controls. Liao et al. [29] have found lower TC and LDL-C levels in RA patients than the general population. SLE-associated accelerated atherosclerosis due to immune dysregulation plays a pivotal role in modulation of lipid metabolism [30]. Immune dysregulation and systemic inflammation serve as major CV-related risk factors in RA along with treatment regimens including corticosteroids [31]. The CV risk profile noted in the present RA subjects could be due to immune dysregulation and inflammatory load, since the patients were not managed by either DMARDs or steroids.
In contrast to previous literature findings, the current study has reported low lipoprotein(a) levels in SLE patients. Specifically, increased lipoprotein(a) levels noted in RA and SLE have been associated with higher CV risk [32, 33]. Decrease in the level of antioxidants due to alterations in serum paraoxonase and arylesterase activities and formation of autoantibodies to lipoprotein are noted in SLE, which in turn contributes to accelerated atherosclerosis [34,35,36]. The measurements of these enzymes and autoantibodies in the present study subjects were not available to rule out the possibility of its influence in lowering the lipoprotein(a) levels. In addition, literature studies focusing on lipoprotein(a) levels in SLE patients from Indian subcontinent are scarce. However, the RA patients had elevated levels of lipoprotein(a) in concurrence with other studies [37].
None of the inflammatory parameters (ESR, CRP and NLR) correlated with TC, LDL-C, VLDL-C, TC/HDL-C and log (TG/HDL-C). Thus, the pattern of changes can be largely attributed to immunopathological processes rather than the inflammatory status. There are studies reporting direct relationship between CRP and changes in HDL and TC [26]. The present study does not have adequate power to contradict these observations. The changes in lipid proteins do depend on the intensity of inflammation. However, this was not seen in current study subjects and this could be due to selection of patients with uniform level of inflammation; moderate to severe disease. The inflammatory parameters were also not significantly different (Table 1). Hence, it is prudent to presume that the factors influencing the lipid metabolism within the defined narrow spectrum of disease activity to be the immune-pathological process.
The current study has reported uric acid as one of the factors that correlated directly with VLDL-C, TC/HDL-C, and log(TG/HDL-C). The uric acid levels were significantly higher in SLE patients than RA. The higher uric acid levels indicate more skewing of lipid metabolism towards atherogenicity, which may suggest the pattern of immunoinflammation process. Literature evidence suggests that serum uric acid serve as a surrogate marker for screening pulmonary hypertension in patients with SLE, and a value ≥ 7 mg/dl at baseline predicts future risk of developing severe disease (relative risk 8.5, 1.0009 to 72; P = 0.04) [14, 38, 39]. SLE patients with metabolic syndrome have a significantly higher levels of serum uric acid (6.88 ± 2.20 vs. 4.45 ± 1.17, P < 0.001) and certain inflammatory biomarkers such as homocysteine are significantly associated with increased patient organ damage score (3.20 ± 1.97 vs. 1.60 ± 1.67, P = 0.008) [40]. Hyperuricemia in SLE patients is independently associated with the occurrence of stroke, peripheral neuropathy, hypertension, hyperlipidemia, and history of arterial thrombosis [41]. These study findings clearly substantiate the use of uric acid as an indicator of extensive necrotic damage and endothelial injury.
There are studies reporting that the increased serum uric acid level in patients with rheumatic disease is associated with elevated CV risk, whereas the CV risk in gout is independent of serum uric acid levels. Elevated baseline serum uric acid may serve as a strong predictor of first CV events than some traditional CV risk factors or parameters of inflammation [13]. The present study suggests that uric acid level above supraphysiological values indicate a necrotic or a severe inflammatory process reflecting more intense influence on lipid metabolism and increased CV risk.
No significant difference in homocysteine levels between the two groups was noted. Less number of patients in both the groups may reduce the power of the study and it is a major limitation of the study. The recruitment of study population was limited due to inclusion of only treatment-naïve patients. But the subjects selected had inflammation within the narrow range of moderate to severe disease and other possible confounding variables like drugs were eliminated by including treatment-naive patients. This could reduce the limitation of small sample size. Further, the statistical testing of multiple variables could have contributed to falsely significant associations. In order to demonstrate the influence of variables on the outcome, these findings need to be evaluated by a P value corrected for multiple comparisons.
The results were not adjusted for potential confounders, especially those that could affect the lipid levels, such as age and gender [23]. The present study population had more female patients due to the increased female preponderance of SLE and RA. The peak age of onset of SLE and RA were 20–30 and 30–55 years respectively [27, 42]. The SLE patients with an average younger age showed higher AIP values (0.7 ± 0.39) than the RA disease group (0.14 ± 0.26) (Table 1). Studies have shown SLE patients develop CV risk at an younger age [43]. A meta-analysis by Fransen et al. has found that the risk of CVD was relatively higher in younger RA patients (< 50 years) and lower in elderly subjects (> 65 years) [44]. The average age of RA patients (51 years) noted in the present study was comparable to that younger RA patients in meta-analysis study. The risk of atherosclerosis increases with advancing age. In addition to traditional CV risk factors, several disease-related risk factors have been suggested to contribute to the increased atherosclerosis risk in younger SLE and RA patients [31, 45]. These findings did not indicate the influence of advancing age on AIP values, but could be impacted by immunopathological mechanisms underlying the autoimmune disease processes. Further studies with large sample size are required to perform regression analysis to account for various factors influencing the disease process. The study did not consider control samples, as this was primarily designed to examine only the differential influence of immunological process in SLE and RA. Moreover, it was difficult to recruit treatment-naïve controls in a tertiary set-up.
In conclusion, the effect of inflammation on metabolic process depends on the type of immunological process responsible for inflammation. The overall impact on lipid metabolism like reduction in circulating TC is common for both the diseases. The increased levels of VLDL, TC/HDL-C, log (TG/HDL-C) and uric acid appear to be influenced by immune complex-mediated inflammation like SLE, which may serve as a marker of necrosis and apoptosis. The present study may offer a preliminary basis to further elucidate the differential influence of immunological process of autoimmune disease on lipid metabolism, and the use of serum uric acid as a surrogate marker of dysfunctional lipid profile in autoimmune disease. Studies are required to substantiate the use of these metabolic parameters to qualify the impact and pattern of inflammation in developing risk reduction strategies.
References
Charles-Schoeman C, Wang X, Lee YY, Shahbazian A, Navarro-Millán I, Yang S, et al. Association of triple therapy with improvement in cholesterol profiles over two year follow-up in the TEAR Trial. Arthritis Rheumatol. 2016;68(3):577–86.
Wang H, Ye J. Regulation of energy balance by inflammation: common theme in physiology and pathology. Rev Endocr Metab Disord. 2015;16(1):47–54.
Shoenfeld Y, Gerli R, Doria A, Matsuura E, Cerinic MM, Ronda N, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112(21):3337–47.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19.
Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56(7):481–90.
Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. PNAS. 2003;100(5):2610–5.
Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58(7):2105–12.
Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med. 2013;64(1):249–63.
Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42(2):338–46.
Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–7.
Ostojic P, Bartolovic D. Disease activity, obesity, functional disability, and depression in patients with rheumatoid arthritis: impact on lipid status, glycoregulation, and risk for coronary heart disease. Z Rheumatol. 2016;75(7):716–22.
Kono H, Chen C-J, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120(6):1939–49.
Meek IL, Vonkeman HE, van de Laar MA. Hyperuricaemia: a marker of increased cardiovascular risk in rheumatic patients: analysis of the ACT-CVD cohort. BMC Musculoskelet Disord. 2014;15:174.
Huang C, Li M, Liu Y, Wang Q, Guo X, Zhao J, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine (Baltimore). 2016;95(10):e2761.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–86.
Madsen OR. Is DAS28-CRP with three and four variables interchangeable in individual patients selected for biological treatment in daily clinical practice? Clin Rheumatol. 2011;30(12):1577–82.
Bhardwaj S, Bhattacharjee J, Bhatnagar MK, Tyagi S. Atherogenic index of plasma, castelli risk index and atherogenic coefficient-new parameters in assessing cardiovascular risk. Int J Pharm Biol Sci. 2013;3(3):359–64.
Adu EM, Ukwamedu HA, Oghagbon ES. Assessment of cardiovascular risk indices in type 2 diabetes mellitus. Trop Med Surg. 2015;3:184.
Ranjit PM, Guntuku G, Pothineni R. Comparison of lipid profile and new atherogenic indices among the coronary artery disease (CAD)-negative and -positive diabetic dyslipidemia subjects. Int J Med Sci Public Health. 2015;4(11):1574–9.
Dobiásová M. AIP–atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr Lek. 2006;52(1):64–71.
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988.
Santos MJ, Vinagre F, Silva JJ, Gil V, Fonseca JE. Cardiovascular risk profile in systemic lupus erythematosus and rheumatoid arthritis: a comparative study of female patients. Acta Reumatol Port. 2010;35(3):325–32.
Gaál K, Tarr T, Lőrincz H, Borbás V, Seres I, Harangi M, et al. High-density lipopoprotein antioxidant capacity, subpopulation distribution and paraoxonase-1 activity in patients with systemic lupus erythematosus. Lipids Health Dis. 2016;15:60.
Batún GJA, Olán F, Hernández NÉ. Dyslipidemia and atherogenic risk in patients with rheumatoid arthritis. Clin Investig Arterioscler. 2016;28(3):123–31.
Liao KP, Playford MP, Frits M, Coblyn JS, Iannaccone C, Weinblatt ME, et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc. 2015;4(2):e001588.
Toms TE, Panoulas VF, Kitas GD. Dyslipidaemia in rheumatological autoimmune diseases. Open Cardiovasc Med J. 2011;24(5):64–75.
Kim S-H, Lee C-K, Lee EY, Park SY, Cho YS, Yoo B, et al. Serum oxidized low-density lipoproteins in rheumatoid arthritis. Rheumatol Int. 2004;24(4):230–3.
Liao KP, Cai T, Gainer VS, Cagan A, Murphy SN, Liu C, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Rheum. 2013;65(12):2046–50.
Al Gadban MM, Alwan MM, Smith KJ, Hammad SM. Accelerated vascular disease in systemic lupus erythematosus: role of macrophage. Clin Immunol. 2015;157(2):133–44.
Zegkos T, Kitas G, Dimitroulas T. Cardiovascular risk in rheumatoid arthritis: assessment, management and next steps. Ther Adv Musculoskelet Dis. 2016;8(3):86–101.
Haeryfar SMM, Rassaian N, Moslemizadeh M, Hoseini-Gohari L. Elevated lipoprotein(a) in systemic lupus erythematosus. Arch Iran Med. 2002;5(4):230–4.
Missala I, Kassner U, Steinhagen-Thiessen E. A systematic literature review of the association of lipoprotein(a) and autoimmune diseases and atherosclerosis. Int J Rheumatol. 2012;2012:e480784.
Delgado Alves J, Ames PRJ, Donohue S, Stanyer L, Nourooz-Zadeh J, Ravirajan C, et al. Antibodies to high-density lipoprotein and beta2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Arthritis Rheum. 2002;46(10):2686–94.
Kiss E, Seres I, Tarr T, Kocsis Z, Szegedi G, Paragh G. Reduced paraoxonase1 activity is a risk for atherosclerosis in patients with systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1108(1):83–91.
Romero FI, Khamashta MA, Hughes GR. Lipoprotein(a) oxidation and autoantibodies: a new path in atherothrombosis. Lupus. 2000;9(3):206–9.
Govindan KPS, Basha S, Ramesh V, Kumar CN, Swathi S. A comparative study on serum lipoprotein(a) and lipid profile between rheumatoid arthritis patients and normal subjects. J Pharm Bioallied Sci. 2015;7(Suppl 1):S22–5.
Kim K-J, Baek I-W, Park Y-J, Yoon C-H, Kim W-U, Cho C-S. High levels of uric acid in systemic lupus erythematosus is associated with pulmonary hypertension. Int J Rheum Dis. 2015;18(5):524–32.
Castillo-Martínez D, Marroquín-Fabián E, Lozada-Navarro AC, Mora-Ramírez M, Juárez M, Sánchez-Muñoz F, et al. Levels of uric acid may predict the future development of pulmonary hypertension in systemic lupus erythematosus: a seven-year follow-up study. Lupus. 2016;25(1):61–6.
Castejon R, Jimenez-Ortiz C, Rosado S, Tutor-Ureta P, Mellor-Pita S, Yebra-Bango M. Metabolic syndrome is associated with decreased circulating endothelial progenitor cells and increased arterial stiffness in systemic lupus erythematosus. Lupus. 2016;25(2):129–36.
Sheikh M, Movassaghi S, Khaledi M, Moghaddassi M. Hyperuricemia in systemic lupus erythematosus: is it associated with the neuropsychiatric manifestations of the disease? Rev Bras Reumatol Engl Ed. 2016;56(6):471–7.
Amador-Patarroyo MJ, Rodriguez-Rodriguez A, Montoya-Ortiz G. How does age at onset influence the outcome of autoimmune diseases? Autoimmune Dis. 2012;2012:251730.
Scalzi LV, Hollenbeak CS, Wang L. Racial disparities for age at time of cardiovascular events and cardiovascular death in SLE patients. Arthritis Rheum. 2010;62(9):2767–75.
Fransen J, Kazemi-Bajestani SMR, Bredie SJH, Popa CD. Rheumatoid arthritis disadvantages younger patients for cardiovascular diseases: a meta-analysis. PLoS ONE. 2016;11(6):e0157360.
McMahon M, Hahn BH, Skaggs BJ. Systemic lupus erythematosus and cardiovascular disease: prediction and potential for therapeutic intervention. Expert Rev Clin Immunol. 2011;7(2):227–41.
Acknowledgements
We acknowledge the help of www.research-assist.com for editing the manuscript. We thank the ChanRe Diagnostic laboratory, Bengaluru, for blood sample analysis and providing details of reports. The study was approved by the hospital Institutional Ethics Committee (IEC-CRICR/077/14).
Funding
This work was supported by the Immunology and Arthritis Research and Education trust (IARET), Bangalore, an independent trust supporting research in arthritis and education.
Author information
Authors and Affiliations
Contributions
Chandrashekara S conceptualized and monitored the study, Sachin Vithalrao Dhote patient recruitment and data cleaning, and Anupama K R conducted the statistical analysis and interpretation of the data. All the authors had access to anonymous data. All authors contributed for preparation of the manuscript and approved the finalized manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chandrashekara, S., Dhote, S.V. & Anupama, K.R. The Differential Influence of Immunological Process of Autoimmune Disease on Lipid Metabolism: A Study on RA and SLE. Ind J Clin Biochem 34, 52–59 (2019). https://doi.org/10.1007/s12291-017-0715-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-017-0715-9