Abstract
The present study was undertaken to evaluate antidiabetic and antioxidant activities of Cassia tora (C. tora) seeds extract against streptozotocin induced diabetes in experimental rats to scientifically validate its use against diabetes. Ethanolic extract of C. tora seeds extract and standard drug (glibenclamide) prepared in aqueous gum acacia (2 %, w/v) suspension and fed orally to streptozotocin induced male adult diabetic rats of Charles Foster strain for 15 days. Biochemical parameters in normal, diabetic control, standard (600 μg/kg bw p.o.) and treated (500 mg/kg bw p.o.) animal groups were quantified and compared. Treatment of streptozotocin induced diabetic rats with ethanolic seeds extract caused significant (p < 0.001) reduction in blood glucose (270–220 mg/dl), total cholesterol (140–104 mg/dl), triglyceride (149–99 mg/dl), phospholipids (100–74 mg/dl), free fatty acid (2.39–2.00 μmol/l), lipid peroxide (9–5.63 nmol MDA/dl) and significantly increased post heparin lipolytic activity (11–14 nmol FFA released/h/l plasma) (p < 0.001). Furthermore, the seeds extract (100–400 μg) when tested for its antioxidant activity in vitro, showed significant (p < 0.001) inhibition in the generation of super oxide anions in enzymic system a (46–37, 33, 23, 21 nmol uric acid formed/min), in enzymic system b (113–91, 77, 60, 51 nmol formazon formed/min), non-enzymic system (324–230, 211, 161, 141 nmol uric acid formed/min) and hydroxyl radicals in enzymic system (544–501, 411, 319, 291 nmol 2,3-dihydroxybenzoate formed/h) and non-enzymic system (28–21, 17, 14, 12). The results of the present study demonstrated antidiabetic, antidyslipidemic and antioxidant activities of C. tora seeds which could help in prevention of diabeticdyslipidemia and related complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a group of syndromes characterized by disturbance in the metabolism of carbohydrate, fats and proteins. Globally, there is increase in number of people suffering from diabetes mellitus in all age groups, from estimated 2.8 % (170 million) in 2000 to 4.4 % (366 million) up to year 2030 [1, 2]. It is also estimated that diabetes causes about 5 % of all deaths globally every year [3, 4]. Diabetes is classified as insulin dependent (type-1), due to reduced insulin secretion by pancreatic β-cells and, insulin independent (type-2), due to low biological activity of the secreted insulin [5]. The main defect in type 2 diabetes is that the cells become insulin resistant and less responsive which leads to impairment in insulin signaling pathway and failure in glucose uptake of target tissues [6]. Currently available treatment for hyperlipidemia and diabetes in modern medicine, fibrats, statins or bile acids sequestraints, glibenclamide and their combinations do not regulate lipid metabolism up to appreciable mark, also have several adverse effects in patients [7]. Herbal formulations are preferred due to lesser side effects and their low cost. Therefore, there is a need to develop safe and effective treatment modalities for hyperlipidemia and diabetes.
Cassia tora Linn (Family: Caesalpiniaceae) commonly known Chakvat, Chakunda and Charota in Hindi, Foetid Cassia in English is an herbaceous foetid annual weed, almost on under shrub, up to 90 cm in height. It grows in tropical and Asian countries especially on way sides and waste places and on hills of low elevations up to 1800 m as well as in plains. Different parts of the plant (leaves, seeds, and roots) are claimed to be effective against a variety of ailments in indigenous medicine [8]. The leaves and seeds are acrid, thermogenic, laxative depurative, antiperiodic, liver tonic, antihelmintic, cardio tonic and are useful in ringworm, pruritis, leprosy, skin disease, jaundice, helminthiasis, flatulence, dyspepsia, intermittent fevers, constipation, ophthalmopathy, cough, bronchitis, cardiac disorders and haemorrhoids [9, 10]. The leaves of Cassia tora (C. tora) are reported to have antirheumatic activity in folklore practice. Decoction of the leaves is used as laxative. The seeds of C. tora have been used in Chinese medicine as vision-improving, cardiotonic, hypolipidemic, aperients, antiasthmic and diuretic agent. Several polyharbal formulations are available in Chinese market for preventing the formation of atherosclerosis plaque [11]. However, antidiabetic, antioxidant and lipid lowering activities of C. tora seeds were not well studied in streptozotocin induced experimental diabetic dyslipidemia. This study was designed to investigate antidiabetic, antidyslipedimic and antioxidant activities of C. tora seeds extract in streptozotocin—induced diabetic rats.
Materials and Methods
Collection of Plant Material
Cassia tora seeds were collected from local area of Lucknow and identified taxonomically by the Department of Pharmacology, Era’s Lucknow Medical College and Hospital, Lucknow and a voucher specimen was also submitted (CT-001/06).
Preparation of Seed Extract
Seeds of C. tora were dried under shade and made into fine powder using laboratory mill. Powder (1000 g) was extracted thrice with 3 × 2000 ml portions of 95 % ethyl alcohol in a laboratory percolator at room temperature. Time allowed for each extraction was 8 h. The extract obtained after third extraction was colorless. All the extracts were mixed; alcohol was distilled out at reduced temperature (20 °C) and reduced pressure (100 psi) in a rotor evaporator. This yielded 20 g (2 % w/w) of crude extract, which was used for in vivo study.
Preparation of Doses
A quantity of 50 mg C. tora seeds extract was suspended/ml tripled distilled water (TDW) containing 2 % (w/v) gum acacia. The suspension was given in a volume of 1 ml/100 g animal bw (500 mg drug/kg bw) by oral intubation. Similarly suspension of glibenclamide (6 mg/dl in TDW containing 2 % (w/v) gum acacia) was prepared and fed in a volume of 1 ml/100 g animal bw (0.6 mg drug/kg bw) as above.
Chemicals
Streptozotocin (STZ), glibenclamide, hypoxanthine, xanthine, xanthine oxidase, nitro blue tetrazolium (NBT) phenazine methosulphate (PMS), nicotinamide adenine dinucleotide reduced (NADH) and heparin were procured from Sigma Chemical CO. ST. Louis MI, USA, Blood glucose (BLG), total cholesterol (TC), triglycerides (TG), phospholipid (PL) were analyzed using standard kits from Erba Diagnostic, (Mannheim GmbH, Germany) by an auto analyzer (Erba Mannheim, EM 360, Germany). Intralipid from victrum AB, in the kabivitrum group, Stockholm, Sweden.
Experimental Animals
Healthy male adult rats of Charles Foster strain (180–225 g) bred in the animal house of the Central Drug Research Institute, Lucknow were used. The animals were kept in controlled conditions; temperature 25–26 °C, relative humidity 60–70 % and 12/12 h light/dark cycle (light from 08:00 AM to 08:00 PM), provide with standard pellet diet (Lipton India Ltd.), and water adlibitum. One group of normal rats without treatment with streptozotocin was used to serve as normal control.
Induction of Diabetes in Charles Foster Rats
Diabetes was induced in overnight fasted rats by single intra peritoneal (ip) injection of streptozotocin (STZ) at a dose of 65 mg/kg bw. It was freshly prepared in 0.1 M cold citrate buffer of pH-4.5. The injected animals were provided with 20 % sterile glucose solution for 24 h to prevent from initial drug induced hypoglycemic mortality. Diabetic animals were identified after 96 h of STZ administration by measuring the vein blood glucose levels by a digital glucometer (one touch select, Johnson and Johnson, USA) based on glucose oxidase peroxidase method. Charles foster rats with blood glucose level above 250 mg/dl were considered as diabetic and used for study [12].
Experimental Design
The rats were divided in four groups having six animals in each as follows: Group 1: normal control (on normal saline); Group 2: diabetic control (on normal saline); Group 3: diabetic control + C. tora seeds extract (500 mg/kg bw); Group 4: diabetic control + glibenclamide (600 μg/kg bw).
Sample Collection
After 15 days of feeding rats were fasted overnight, anaesthetized with thiopental solution, and injected (ip) with 0.1 ml/kg bw of 10 mg/ml solution of heparin. After 15 min blood was withdrawn from the retro-orbital plexus and collected in EDTA coated tubes [12].
Biochemical Parameters
The blood was used for the estimation of glucose level [13], simultaneously plasma was separated and used for the estimations of Total Cholesterol: TC [14], Phospholipids: PL [15], Triglyceride: TG [16] Free Fatty Acids: FFA [17] Plasma posts heparin lipolytic activity [18] by standard spectrophotometeric methods. Plasma level of lipid peroxide was estimated as Thiobarbituric Acid Reactive Substances: TBARS [19].
In Vitro Anti Oxidant Activity
Enzymic and Non Enzymic Generation of Superoxide Anions
The effect of C. tora seeds extract on the generation of superoxide anions (O2 −) in vitro, in an enzymic system of xanthine–xanthine oxidase was investigated. Xanthine oxidase activity in system (A) containing xanthine and different concentrations of C. tora seeds extract (100–400 µg) added with 0.03 μ/ml of xanthine oxidase in phosphate buffer was assayed spectrophotometrically at 295 nm [20]. The change in optical density corresponding to amount of uric acid formed was compared with reaction mixture which did not include with their test substance. The influence of C. tora seeds extract on nitro blue tetrazolium (NBT) reduction by O2 − anions, was measured in a reaction mixture (B) containing xanthine oxidase and NBT in absence or presence of extract (100–400 µg). After incubation, the amount of formazone formed was measured at 560 nm on spectrophotometer. Another system employed for non-enzymic generation of O2 − anions was comprised of phenazine methosulphate, NADH and NBT [21]. After 90 s incubation in absence or presence of test extract 100–400 µg, the amount of formazone formed was read at 560 nm against respective reagent blank.
Enzymic and Non Enzymic Generation of Hydroxyl Radical
Seeds extract of C. tora (100–400 µg) was tested against the formation of hydroxyl radicals (OH−) in vitro in an enzymic system composed of sodium salicylate, FeSO4, hypoxanthine and xanthine oxidase, assayed for 2,3-dihydroxybenzoate formed by OH− mediated hydroxylation of salicylate on spectrophotometer at 510 nm [22]. In another set of experiment, OH− was generated non-enzymically by FeSO4, sodium ascorbate, H2O2 and deoxyribose. After reaction in the absence or presence of seeds extract of C. tora (100–400 µg), incubation mixture was assayed for malondialdehyde formed [23].
Statistical Analysis
One way analysis of variance (ANOVA—New man’s student test) was performed by comparison of values for Streptozotocin treated group with control, Streptozotocin and drug treated groups with Streptozotocin. All hypothesis testing were two tailed. p < 0.05 was considered statistically significant and the results were expressed as mean + SD. The statistical analysis was carried out by the Graph pad INSTAT 3.0 software [24]. Similarly, the generations of oxygen free radicals with different concentrations of C. tora seeds extract were compared with that of their formation without extract. The values were tested for significance at P < 0.05.
Results
Effect of C. tora Seeds Extract in Streptozotocin Induced Hyperglycemia
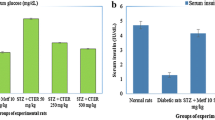
The acute administration Streptozotocin caused marked increase in their plasma levels of blood glucose 212 %, TC 75 %, TG 68 %, PL 39 %, FFA 52 % and lipid peroxide 228 % following decrease in PHLA by 35 %. However, treatment with seeds extract of C. tora seeds extract caused reversal in these levels of blood glucose by 17 %, TC by 25.0 %, PL by 26 %, TG by 33.0 %, FFA by 12.0 %, lipid peroxide by 37 % and reactivation of PHLA by 27 %. The anti-diabetic and lipid lowering activities C. tora seeds extract was comparatively less to that of Glibenclamide (Table 1).
Effect of C. tora Seeds Extract on Generation of Super Oxide Anions In Vitro
The data in Table 2 showed that enzymic oxidation of xanthine to uric acid (A) as well as the generation of O2 − anions in xanthine–xanthine oxidase system, as measured by reduction of NBT to Formazone (B) were inhibited to varying extents by seeds extract of C. tora seeds in a concentration dependent manner and this effect was maximum by 50 and 54 % respectively at 400 µg/ml of test sample. The seed extract also trapped the O2 − anions generated by non enzymic system of NADH—Phenozine–Methosulphate and were responsible for reduction of NBT in the reaction mixture. The effect was dose dependent and was highest by 57 % at 400 µg/ml of test substance.
Effect of C. tora Seeds Extract on Generation of Hydroxyl Radicals In Vitro
The data in Table 2 also showed that C. tora seeds extract inhibited the formation of OH− by enzymic system of hypoxanthine–xanthine oxidase and Fe++. Addition of extract (100–400 µg) inhibited the OH− mediated formation of 2,3-dihydroxybenzoate in concentration dependant manner which was 47 % at 400 µg/ml of test extract. Furthermore, this preparation, when added with reaction mixture containing Fe2+—Sodium ascorbate—H2O2 employed for nonenzymic generation of OH− inhibited fragmentation of deoxyribose into MDA and this effect was maximum by 57 % at peak concentration (400 µg/ml) of seeds extract of C. tora.
Discussion
In the present study, C. tora seeds extract was tested for its anti-diabetic, anti-dyslipidemic and anti-oxidant activities in STZ induced diabetic rats. STZ is a permanent diabetes inducing drug, caused permanent damage to insulin producing β cells found in the pancreas, and that is why this animal model has been used for primary screening of drug. It is well documented that STZ induced pathologic changes also suppresses the synthesis of glucosominaglycons in capillary endothelium surface that lead to defect in LPL binding and consequent poor clearance of VLDL in STZ induced diabetes. This may be due to the diminution of capillary endothelial and hepatic lipases which had been involved to produce hyper β-lipoproteinemia; Hypo α-lipoproteinemia; hyper triglycerdemia and hyper cholestrolemia [25].
However, treatment with seeds extract of C. tora had played a significant role in regulation of lipoprotein metabolism, lowering of blood glucose, lipids and lipid peroxide and recovered PHLA significantly in STZ diabetic rats. The early studies have shown that feeding with seed extract of C. tora caused lowering in blood sugar levels, serum and tissue lipids in alloxan induced diabetic rats [26]. These beneficial effects may be due to bioactive compounds present in C. tora seeds extract like typical alkaloids, Berberine. Palmatine, Tembetarin, Magnoflorine Choline, Tinosporin Isocolubin, Palmatine, Tetrahydropalmatine, Magnoflorine [27].
In conclusion, seeds extract of C. tora might suppress hyperglycemia induced alterations in biochemical path ways that are responsible to cause abnormities with lipid metabolism in STZ induced diabetic rats. Besides its antidiabetic and antioxidant effects, seeds extract of C. tora may have regulated functioning of various enzymes and metabolites to afford a normal lipid metabolism in dyslipidemic animals. This may be due to reactivation of PHLA. The study reveals that seeds extract of C. tora is a better drug as a natural product to regress diabetic-dyslipidemia and oxidative stress in diabetes. Further work to assess the antidyslipidemic activity of different fractions of C. tora plant in STZ induced diabetic rats is under progress to substantiate the present findings. Furthermore seeds extract of C. tora also reduced lipid peroxide levels in above diabetic rats following inhibition of ROS generation in vitro.
Conclusion
Diabetes is the most prevalent endocrine disorder of the world characterized by chronic hyperglycemia and associated complications. Although insulin is one of the important therapeutic agents known to medicine and, technological breakthroughs have improved its access and availability, there is an increased focus on finding insulin substitutes, secretagogues or sensitizer from synthetic or plant source for the treatment of diabetes. It should be pointed out here that plant derived natural compounds have established a proven platform for developing new drug synthesis with fewer side effects [28]. A strong antioxidative and antidiabetic activity of C. tora seeds extract in our study not only validates its use in diabetes management but also demands a wider role, application and research in insulin alternative strategies for diabetes management for it.
References
Fonseca VA. Clinical diabetes: translating research into practice. Philadelphia: Saunders Elsevier; 2006. p. 2–3.
Ashok K, Lakshman K, Nandeesh R, Arun K, Manoj K, Kumar V. In vitro alpha amylase inhibition and in vivo antioxidant potential of Amaranthus spinosus in alloxan induced oxidative stress in diabetic rats. Saudi J Biol Sci. 2011;18:1–5.
Hiremath MB, Jali MV. Diabetes. Ind J Sci Technol. 2010;3:1106–8.
Olubomehin OO, Abo KA, Ajaiyeoba EO. Alpha amylase inhibitory activity of two Anthocleista species and in vivo rat model antidiabetic activities of Anthocleista djalonensis extracts and fractions. J Ethnopharmacol. 2013;146:811–4.
Hosakatte NM, Vijayalaxmi SD, Eun JL, Kee YP. Efficacy of ginseng adventitious root extract on hyperglycemia in streptozotocin induced diabetic rats. J Ethnopharmacol. 2014;153:917–21.
Sujatha S, Anand S, Sangeetha KN, Shilpa K, Lakshmi J, Balakrishnan A, et al. Biological evaluation of (3β)-Stigmast-5-en-3-ol as potent antidiabetic agent in regulating glucose transport using in vitro model. Int J Diabetes Mellit. 2010;2:101–9.
Chattopadhyaya R, Pathak D, Jindal DP. Antihyperlipidemic agents: a review. Indian Drugs. 1996;33:85–97.
Warrier PK, Nambiar VPK, Ramankutty C. A text book of Indian medicinal plants, a compendium of 500 species, vol. 2. Chennai: Orient Longman Private Limited; 2001. p. 26.
Pawar HA, D’mello PM. Cassia tora Linn: an overview. Int J Pharm Sci Res. 2011;2:2286–91.
Chaurasia B, Dhakad RS, Dhakar VK, Jain PK. Preliminary Phytochemical and pharmacological (Antidiabetic) screening of Cassia tora Linn. Int J Pharm Life Sci. 2011;2:759–66.
Kee CH. The pharmacology of Chinese herbs. BocaRaton: CRC Press; 2001. p. 103.
Banskota AH, Nguyen NT, Tezuka Y, Nobukawa TKS. Hypoglycemic effects of the wood of Taxus yunnanensis on streptozotocin induced diabetic rats and its active components. Phytomedicine. 2006;13:109–14.
Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–30.
Deeg R, Ziegenborn J. Kinetic enzymatic method for automated determination of total cholesterol in serum. Clin Chem. 1983;29:1798–803.
Zilversmith DB, Davis DK. Micro determination of plasma phospholipids by trichloroacetic acid precipitation. J Lab Clin Med. 1950;35:155–60.
Buccolo G, David H. Quantitative determination of serum triglyceride by the use of enzymes. Clin Chem. 1973;19:476–80.
Mosinger F. Photometric adaptation of Dole’s microdetermination of free fatty acids. J Lipid Res. 1965;6:157–9.
Wing DR, Robinson DS. Clearing factor lipase in adipose tissue. Biochem J. 1968;29:1798–803.
Okhawa H, Qohishi N. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1978;95:351–8.
Bindoli A, Valante M, Cavallin L. Inhibition of Xanthine oxidase and xanthine dehydrogenase activity. Pharmacol Res Commun. 1985;17:831–9.
McCord JM, Fridovich IJ. Superoxide dismutase; an enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049–55.
Richmond R, Halliwell R, Chauhan J, Darbre A. Suproxide dependent formation of hydroxyl radical by hydroxylation of aromatic compounds. Anal Biochem. 1981;118:320–35.
Halliwell B, Gutteridge JMC, Aroma OI. The deoxyribose method: a simple test tube assay for determination of rate constants for reaction OH-radicals. Anal Biochem. 1987;165:215–9.
Woodson RF. Statistical methods for the analysis of biochemical data. Chichester: Wiley; 1957. p. 315.
Goud BJ, Dwarkanath B, Chikka swamy BK. Streptozotocin—a diabetogenic agent in animal models. Int J Pharm Pharm Res Hum. 2015;3:253–69.
Awasthi VK, Mahdi F, Chander R, Khanna AK, Saxena JK, Singh R, et al. Hypolipidemic activity of Cassia tora seed in hyperlipidemic rats. Ind J Clin Biochem. 2015;30:78–83.
Kumar V, Mahdi F, Chander R, Khanna AK, Singh R, Saxena JK, et al. Cassia tora regulates lipid metabolism in alloxan induced diabetic rats. Int J Pharm Sci Res. 2015;6:3484–9.
Saklani A, Kutty SK. Plant derived compounds in clinical trials. Drug Discov Today. 2008;13:161–71.
Acknowledgments
One of us (Vishnu Kumar) is grateful to the Director, Central Drug Research Institute (CDRI), Lucknow for experimental support, Director Academics, Era’s Lucknow Medical College and Hospital, Lucknow for financial support and Late Dr. Ramesh Chander retired Scientist, Biochemistry, Division, CDRI, Lucknow for his expert guidance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors. The study was approved by the Institutional Animal Ethics Committee of Central Drug Research Institute and was carried out in accordance with the current guidelines set by Organization for Economic Co-operation and Development (OECD), received from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India for the care of laboratory animals.
Rights and permissions
About this article
Cite this article
Kumar, V., Singh, R., Mahdi, F. et al. Experimental Validation of Antidiabetic and Antioxidant Potential of Cassia tora (L.): An Indigenous Medicinal Plant. Ind J Clin Biochem 32, 323–328 (2017). https://doi.org/10.1007/s12291-016-0608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-016-0608-3