Abstract
Angiotensin-1-converting enzyme (ACE) gene has established substantial attention in the recent years as a candidate gene for hypertension, cardiovascular diseases and type 2 diabetes. The aim of the present study was to investigate the association of ACE (I/D) polymorphism with coronary artery disease (CAD) in a north Indian population. A total of 662 subjects (330 CAD patients and 332 healthy controls) were examined for association of ACE gene (I/D) polymorphism and environmental risk factors. The mean age of the CAD patients and control subjects was 60.53 ± 8.6 years and 56.55 ± 7.7 years, respectively (p = 0.000). Anthropometric and demographic data showed BMI values significantly higher among CAD patients and control subjects (26.98 ± 4.9 vs 24.04 ± 4.7, p = 0.000). We observed pronounced central obesity in both CAD patients and controls, even at the lowest BMI values (<23 kg/m2). Dyslipidemia was highly prevalent in CAD patients compared to control subjects. Genotypic data showed significantly higher frequency of DD genotype in CAD patients than that of control subjects (40 vs 28.3 %). No significant difference was observed in the distribution of ID genotypes between CAD patients and control subjects. Logistic regression analysis of data demonstrate that DD genotype was associated with 1.8 fold increased risk of development of CAD in Asian Indians (OR 1.8; 95 % CI 1.22–2.66; p = 0.003). The frequency of D allele was significantly higher in CAD patients (p = 0.001). No significant difference was observed in the clinical and biochemical characteristics of CAD patients and controls when the data was stratified according to the genotypes of ACE gene. In conclusion, DD genotype of ACE gene may be associated with increased risk of CAD in Asian Indian population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among the Asian population [1]. According to World Health Organization, the number of deaths from CVDs in 2004 were 17.1 million and it is predicted that by 2030, about 23.6 million deaths will be reported due to CVDs, mainly from coronary artery disease (CAD) and stroke [2]. Multiple risk factors such as environmental, genetic and clinical risk factors seems to play principle role in predisposition of CADs [3]. The development of CAD is well known to be controlled by smoking, alcohol intake, physical inactivity, diabetes, dyslipidemia, hypertension, obesity, and psychosocial conditions [4–7]. These modifiable risk factors, account for more than 90 % of myocardial infarction (MI) cases and more than 80 % of stroke cases worldwide [8, 9]. Over the past few decades, regional changes in dietary intake and habits have led to a compelling decrease in serum cholesterol levels in North American and western Europe, counterbalanced by an increase everywhere in developing regions in Southeast Asia and the Pacific [10]. Current studies have also proposed that various risk factors conventionally thought as solely environmental, might have a genetic predisposition [11–15]. The interactions of genetic and modifiable risk factors with each other effect cardiovascular risk [16].
The renin-angiotensin system (RAS) is a hormonal signaling mechanism implicated in the regulation of blood pressure and cardiovascular homeostasis. Through modulation of gene expression, growth, fibrosis, and inflammatory response, it also plays an important role in the pathological changes preceding kidney damage [17]. The angiotensin-1–converting enzyme (ACE) gene, consists of 26 exons and spans 21 kb, located on chromosome 17q23. A very common polymorphism in ACE gene was identified by Rigat et al. [18]. The 287–base pair Alu insertion/deletion (I/D) polymorphism in intron 16 is the most studied polymorphism and has been related to ACE levels [19]. Serum ACE concentrations were significantly higher in homozygotes with the “shorter” deletion allele (D/D) than in heterozygotes (I/D) or in homozygotes with the “longer” insertion allele (I/I) [18]. Previous studies demonstrated the association of this polymorphism with susceptibility to hypertension [20], central obesity [21], elevated glucose [22] and hypertriglyceridemia [23]. Therefore, ACE I/D polymorphism might be involved in the pathogenesis of Metabolic syndrome (MetS). However, results have been inconsistent [24–32]. The present study was planned to investigate the association of angiotensin converting enzyme (insertion/deletion) gene polymorphism with CVD in north Indian population.
Research Design and Methodology
Human Subjects
A total of 662 subjects (330 CAD patients and 332 healthy controls) recruited from North Indian population. This sample is a part of our ongoing study, started in 2013 with the objectives to investigate the risk factors associated with coronary artery disease in Asian Indian population. All the subjects were originated from similar geographical locations. The diagnosis of the occurrence of CAD was performed by cardiologists, based on the clinical symptoms, characteristic ECG changes, cardiac enzyme levels, and the findings in coronary angiography and/or echocardiography. Informed written consent was obtained from all the study participants. The study was approved by the ethical committee of the Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Clinical and Biochemical Measurements
Standard anthropometric measurements were performed including height, weight, waist and hip circumferences and blood pressure. Body Mass Index (BMI) was calculated as [weight (kg)/height (meter)2] and Waist Hip Ratio (WHR) as ratio of waist to hip circumferences. According to WHO Expert Consultation 2004, the following BMIs have been proposed to indicate risk of developing weight-related diseases in Asian populations: <23 kg/m2 for low risk, 23–27.5 kg/m2 for increased risk, and >27.5 kg/m2 for high risk. Abdominal obesity was measured according to the new cutoffs proposed for South Asian Indians (WHR >0.89 for men and WHR >0.81 for women).
Blood samples were drawn in EDTA coated and plain vials. All the biochemical measurements such as Glucose, Total Cholesterol (TC), Triglycerides (TG), High Density Lipoprotein (HDL) and Creatinine levels were done in serum using standard kits. Body fat percentage was calculated in all the subjects using a previous method [33].
Amplification of Insertion/Deletion Polymorphism of ACE Gene
The genomic DNA was extracted from peripheral blood leukocytes using phenol/chloroform method. PCR amplification of ACE (I/D) polymorphism was done using the following primer sequences. Forward primer: 5′-CTGGAGACCACTCCCATCCTTTCT-3′ and reverse primer: 5′-GATGTGGCCATCACATTCGTCAGAT-3′. The PCR was carried out in a final volume of 25 µl containing 1xPCR buffer, 100–200 ng of genomic DNA, 1.5 mmol/l MgCl2, 0.2 mmol/l of each dNTP (Eppendorf, Germany), 250 pmol of each primer (IDT, USA), 1.25 units Taq DNA polymerase (NEB, Boverly, MA) and 5 % of DMSO to avoid mistyping. The cycling conditions were as follows: an initial denaturation at 94 °C for 5 min, followed by 30 cycles of annealing at 55 °C for 1 min, extension at 72 °C for 1.5 min, denaturation at 94 °C for 1 min, and final extension at 72 °C for 10 min. PCR amplified products were then separated on 2 % agarose-gel containing ethidium bromide (Metaphor agarose, FMC Bioproducts, Rockland, ME). The wild type homozygote, II yielded only the 490 bp fragment and mutant type homozygote, DD yielded only the 190 bp fragment. PCR product from a heterozygote yielded both the 190 and 490 bp fragments. The assays were performed in blind to the phenotype. Mistyping of ID heterozygotes as D homozygotes may occur due to the preferential amplification of the D allele and inefficiency in the amplification of the I allele [34]. To increase the specificity of DD genotyping, PCR amplifications were also performed with an insertion specific primer pair (5′-TGGGACCACAGCGCCCGCCACTAC-3′ and 5′-TCGCCAGCCCTCCCATGCCCATAA-3′) in all samples with DD genotype. Briefly, insertion-specific amplification was carried out in a final volume of 25 µl containing 1xPCR buffer, 100–200 ng of genomic DNA, 1.5 mmol/l MgCl2, 0.2 mmol/l of each dNTP, 250 pmol of each primer, 1.0 units Taq DNA polymerase and 5 % of DMSO. The cycling conditions were as follows: an initial denaturation at 94 °C for 5 min, followed by 30 cycles of annealing at 55 °C for 1 min, extension at 72 °C for 1.5 min, denaturation at 94 °C for 1 min, and final extension at 72 °C for 10 min. PCR amplified products were then separated on 2 % agarose-gel containing ethidium bromide. Only “I” allele produced a 335 bp fragment. The reaction yields no products in samples with DD genotype.
Statistical Analysis
Results were expressed as mean ± SD. Chi square analysis was applied to test the significance of differences in genotypic and allelic frequencies. Comparisons between CAD patients and control subjects were done using Chi square tests, and group comparisons were done using unpaired t tests. One-way ANOVA test was used for comparison of normally distributed variables among genotypes. All the p values <0.05 (two-tailed) were considered as significant difference. Logistic regression analysis was carried out to correlate various clinical parameters with genotypes. Statistical analysis was performed using the Statistical Package of Social Sciences (SPSS) for Windows, version 16.0 (SPSS, Inc., Chicago, IL).
Results
Baseline Characteristics of the Study Subjects
A total of 662 subjects (330 CAD patients and 332 healthy controls) were examined in the present case control study. The baseline characteristics of all the participants are summarized in Table 1. The mean age of the CAD patients and control subjects was 60.53 ± 8.6 years and 56.55 ± 7.7 years, respectively (p = 0.000). All the CAD patients were regularly taking lipid lowering drugs under the guidance of cardiologist. The mean BMI values was significantly higher among CAD patients and control subjects (26.98 ± 4.9 vs 24.04 ± 4.7, p = 0.000). CAD patients and control subjects did not significant difference in abdominal adiposity as reflected by their waist circumference and WHR (Table 1). Following stratification of the data based on the BMI cutoffs by WHO Expert Consultation (2004) for Asians, we observed pronounced central obesity (measured by WHR) in both CAD patients and controls, even at the lowest BMI values (<23 kg/m2). No significant difference in blood pressure (systolic and diastolic) was observed in CAD patients and control subjects. The biochemical parameters are shown in Table 2. There were significantly higher levels of total cholesterol, triglycerides, LDL and VLDL were observed in control subjects compared to CAD patients. However, no significant difference was observed in HDL and creatinine level among the CAD patients and controls (Table 2).
Genotype Distribution and Allele Frequencies
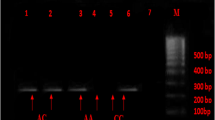
The distribution of genotypes and allele frequencies of insertion/deletion polymorphism of ACE gene are shown in Table 3. Both patients and control populations were in Hardy–Weinberg equilibrium. Figure 1 shows the II, ID and DD genotypes of ACE gene polymorphism. The distribution of II genotype was higher in control subjects than the CAD patients (32.5 vs 25.5 %). Significantly high frequency of DD genotype (40 %) was observed in CAD patients compared to control subjects (28.3 %). The frequency of D allele was significantly higher in CAD subjects (p = 0.001). No significant difference was observed in the distribution of ID genotypes between CAD patients and control subjects. Logistic regression analysis of data demonstrate that DD genotype was associated with 1.8 fold increased risk of development of CAD in north Indians (OR 1.8; 95 % CI 1.22–2.66; p = 0.003). The clinical characteristics of the CAD patients and control subjects according to the genotypes of ACE (I/D) polymorphism are shown in Table 4. Although significant differences were observed in various metabolic characters in CAD patients and control subjects as shown in Tables 1 and 2, but no significant differences were observed amongst all the genotypes (II, ID and DD) of ACE gene in both CAD patients and control subjects and metabolic traits in our population when the data was stratified according to the genotypes of ACE gene.
Discussion
The incidence and prevalence of CAD is rising in developing countries. It is the single major cause of mortality in the developed countries and a leading cause of death and disability in developing countries [35]. It is a multifactorial disorder in which both genetic and environmental factors play a pivotal role in its pathogenesis [36]. Conventional risk factors such as hypertension and dyslipidemia are associated with the risk of CAD in Asian Indians [37]. The present case–control study was undertaken to establish the risk factors associated with CAD in an urban population of North India. Asian-Indians have been identified as one of the ethnic groups with a high prevalence of cardiovascular diseases. The findings described in this paper confirm and extend our knowledge of the dynamics of the present epidemic of CVD in North Indians [38]. The improved socioeconomic conditions in India have resulted in a decrease in physical activity and an increase in obesity, which has led to the increase in the prevalence of CVD and T2DM in urban Indians [16]. The present study subjects depict an unusual clinical picture of uneven distribution of adiposity and dyslipidemia in CAD patients as well as control subjects. The mean age of onset of CAD in north Indians was a decade earlier than the Caucasians but is consistent with the age of onset in other Asian populations.
Abdominal obesity can be considered one of the key factors for causing insulin resistance and progression to the development of CAD, T2DM and its complications [38, 39]. Our data showed a strong tendency toward upper body adiposity (waist circumference and WHR), even in control groups. Compared to western population, North Indian diabetic subjects were leaner and had relatively lower BMI. Body fat percentage at a given BMI is comparatively higher in Asian Indians. It has been suggested that thrifty genes that provided a survival advantage in previous eras now result in central obesity and T2DM and CAD in populations living in the rapidly modernizing environment of India. Dyslipidemia is not very much prominent in our study population because most of the CAD patients were taking lipid lowering drugs in routine. Therefore, the upper body adiposity along with physical inactivity and abnormalities in the HDL-C level may induce insulin resistance in diabetic subjects, which in turn results in increased prevalence of CAD, T2DM and other complications in North Indians.
In the present case–control study, ACE (I/D) gene polymorphism is significantly associated with the risk of CAD in Asian Indian population. Our findings were in line with the findings of previous studies carried out in different populations. [40–43]. The ethnic background seems to influence the allele frequencies in ACE (I/D) gene polymorphism worldwide. However, some epidemiological studies did not show association of ACE (I/D) gene polymorphism with the risk of CAD [44, 45]. Some studies have shown the association of ACE (I/D) gene polymorphism with CVD in type 2 diabetic subjects [41–43, 46, 47], while the association of the I/D polymorphism with CVD has not been confirmed in other studies [48, 49]. This polymorphism also affects the level of circulating ACE, but there is great individual variation, even between those with the same genotype [44]. The CAD subjects carrying DD genotype have higher levels of ACE, relative to II individuals, while ID subjects have intermediate ACE levels. Apart from CAD, ACE (I/D) gene polymorphism was found to be associated with Type 2 diabetes and its complications [50–58]. ACE (I/D) is also a risk factor for hypertension [52]. The ethnic background appears to influence the ACE (I/D) gene polymorphism globally. It demonstrates the importance of using a homogeneous population in the selection of the study samples, making possible the identification of more exact distributions of the ACE genotypes among racial populations. In conclusion, our data suggested that the DD genotype of ACE gene might be a significant risk factor for the development of CAD in Asian Indian population.
References
Prabhakaran D, Jeemon P, Roy A. Cardiovascular Diseases in India: current Epidemiology and Future Directions. Circulation. 2016;133(16):1605–20. doi:10.1161/CIRCULATIONAHA.114.008729.
WHO. Cardiovascular diseases (CVDs). WHO fact sheet. 2011.
Cooney MT, Dudina A, D’Agostino R, Graham IM. Cardiovascular risk-estimation systems in primary prevention: do they differ? Do they make a difference? Can we see the future? Circulation. 2010;122(3):300–10. doi:10.1161/CIRCULATIONAHA.109.852756.
Broeckel U, Hengstenberg C, Mayer B, Holmer S, Martin LJ, Comuzzie AG, et al. A comprehensive linkage analysis for myocardial infarction and its related risk factors. Nat Genet. 2002;30(2):210–4. doi:10.1038/ng827.
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47.
Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA, J Am Med Assoc. 2003;290(7):898–904. doi:10.1001/jama.290.7.898.
Bhatti GK, Bhadada SK, Vijayvergiya R, Mastana SS, Bhatti JS. Metabolic syndrome and risk of major coronary events among the urban diabetic patients: north Indian Diabetes and Cardiovascular Disease Study-NIDCVD-2. J Diabetes Complications. 2016;30(1):72–8. doi:10.1016/j.jdiacomp.2015.07.008.
O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–23. doi:10.1016/S0140-6736(10)60834-3.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi:10.1016/S0140-6736(04)17018-9.
Farzadfar F, Finucane MM, Danaei G, Pelizzari PM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;377(9765):578–86. doi:10.1016/S0140-6736(10)62038-7.
Ingelsson E, Larson MG, Vasan RS, O’Donnell CJ, Yin X, Hirschhorn JN, et al. Heritability, linkage, and genetic associations of exercise treadmill test responses. Circulation. 2007;115(23):2917–24. doi:10.1161/CIRCULATIONAHA.106.683821.
Keskitalo K, Knaapila A, Kallela M, Palotie A, Wessman M, Sammalisto S, et al. Sweet taste preferences are partly genetically determined: identification of a trait locus on chromosome 16. Am J Clin Nutr. 2007;86(1):55–63.
Whitfield JB, Zhu G, Madden PA, Neale MC, Heath AC, Martin NG. The genetics of alcohol intake and of alcohol dependence. Alcohol Clin Exp Res. 2004;28(8):1153–60.
Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE. Molecular genetics of nicotine dependence and abstinence: whole genome association using 520,000 SNPs. BMC Genet. 2007;8:10. doi:10.1186/1471-2156-8-10.
Walia R, Bhansali A, Ravikiran M, Ravikumar P, Bhadada SK, Shanmugasundar G, et al. High prevalence of cardiovascular risk factors in Asian Indians: a community survey-Chandigarh Urban Diabetes Study (CUDS). Indian J Med Res. 2014;139:252–9.
Shanker J, Kakkar VV. Contribution of classical and emerging risk factors to coronary artery disease in Asian Indians. Int J Cardiol. 2016;214:97–106. doi:10.1016/j.ijcard.2016.03.012.
Nicholls MG, Richards AM, Agarwal M. The importance of the renin-angiotensin system in cardiovascular disease. J Hum Hypertens. 1998;12(5):295–9.
Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Investig. 1990;86(4):1343–6. doi:10.1172/JCI114844.
Villard E, Tiret L, Visvikis S, Rakotovao R, Cambien F, Soubrier F. Identification of new polymorphisms of the angiotensin I-converting enzyme (ACE) gene, and study of their relationship to plasma ACE levels by two-QTL segregation-linkage analysis. Am J Hum Genet. 1996;58(6):1268–78.
Ji LD, Zhang LN, Shen P, Wang P, Zhang YM, Xing WH, et al. Association of angiotensinogen gene M235T and angiotensin-converting enzyme gene I/D polymorphisms with essential hypertension in Han Chinese population: a meta-analysis. J Hypertens. 2010;28(3):419–28. doi:10.1097/HJH.0b013e32833456b9.
Strazzullo P, Iacone R, Iacoviello L, Russo O, Barba G, Russo P, et al. Genetic variation in the renin-angiotensin system and abdominal adiposity in men: the Olivetti Prospective Heart Study. Ann Intern Med. 2003;138(1):17–23.
Irvin MR, Lynch AI, Kabagambe EK, Tiwari HK, Barzilay JI, Eckfeldt JH, et al. Pharmacogenetic association of hypertension candidate genes with fasting glucose in the GenHAT Study. J Hypertens. 2010;28(10):2076–83. doi:10.1097/HJH.0b013e32833c7a4d.
Vallejo M, Martinez-Palomino G, Ines-Real S, Perez-Hernandez N, Juarez-Rojas JG, Vargas-Alarcon G. Relationship between the angiotensin I-converting enzyme insertion/deletion (I/D) polymorphism and cardiovascular risk factors in healthy young Mexican women. Genet Test Mol Biomark. 2009;13(2):237–42. doi:10.1089/gtmb.2008.0105.
Lee YJ, Tsai JC. ACE gene insertion/deletion polymorphism associated with 1998 World Health Organization definition of metabolic syndrome in Chinese type 2 diabetic patients. Diabetes Care. 2002;25(6):1002–8.
Milionis HJ, Kostapanos MS, Vakalis K, Theodorou I, Bouba I, Kalaitzidis R, et al. Impact of renin-angiotensin-aldosterone system genes on the treatment response of patients with hypertension and metabolic syndrome. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2007;8(4):181–9. doi:10.3317/jraas.2007.027.
Alvarez-Aguilar C, Enriquez-Ramirez ML, Figueroa-Nunez B, Gomez-Garcia A, Rodriguez-Ayala E, Moran-Moguel C, et al. Association between angiotensin-1 converting enzyme gene polymorphism and the metabolic syndrome in a Mexican population. Exp Mol Med. 2007;39(3):327–34. doi:10.1038/emm.2007.36.
Nikzamir A, Nakhjavani M, Golmohamadi T, Dibai L. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with metabolic syndrome in Iranians with type 2 diabetes mellitus. Arch Iran Med. 2008;11(1):3–9.
Dankova Z, Sivakova D, Luptakova L, Blazicek P. Association of ACE (I/D) polymorphism with metabolic syndrome and hypertension in two ethnic groups in Slovakia. Anthropologischer Anzeiger; Bericht uber die biologisch-anthropologische Literatur. 2009;67(3):305–16.
Sivakova D, Lajdova A, Basistova Z, Cvicelova M, Blazicek P. ACE insertion/deletion polymorphism and its relationships to the components of metabolic syndrome in elderly Slovaks. Anthropologischer Anzeiger; Bericht uber die biologisch-anthropologische Literatur. 2009;67(1):1–11.
Sesal C, Ciloglu F, Peker I, Sayar N. Role of angiotensin converting enzyme, paraoxonase 1 55, 192 gene polymorphisms in syndrome X and coronary heart disease. Pak J Biol Sci PJBS. 2009;12(1):46–51.
Procopciuc LM, Sitar-Taut A, Pop D, Sitar-Taut DA, Olteanu I, Zdrenghea D. Renin angiotensin system polymorphisms in patients with metabolic syndrome (MetS). Eur J Intern Med. 2010;21(5):414–8. doi:10.1016/j.ejim.2010.06.001.
Fiatal S, Szigethy E, Szeles G, Toth R, Adany R. Insertion/deletion polymorphism of angiotensin-1 converting enzyme is associated with metabolic syndrome in Hungarian adults. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2011;12(4):531–8. doi:10.1177/1470320310394231.
Lean ME, Han TS, Bush H, Anderson AS, Bradby H, Williams R. Ethnic differences in anthropometric and lifestyle measures related to coronary heart disease risk between South Asian, Italian and general-population British women living in the west of Scotland. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 2001;25(12):1800–5. doi:10.1038/sj.ijo.0801823.
Shanmugam V, Sell KW, Saha BK. Mistyping ACE heterozygotes. PCR Methods Appl. 1993;3(2):120–1.
Gupta R. Burden of coronary heart disease in India. Indian Heart J. 2005;57(6):632–8.
Roberts R, Stewart AF. Genetics of coronary artery disease in the 21st century. Clin Cardiol. 2012;35(9):536–40. doi:10.1002/clc.22002.
Raj R, Bhatti JS, Badada SK, Ramteke PW. Genetic basis of dyslipidemia in disease precipitation of coronary artery disease (CAD) associated type 2 diabetes mellitus (T2DM). Diabetes/Metab Res Rev. 2014. doi:10.1002/dmrr.2630.
Bhatti JS, Bhatti GK, Joshi A, Rai S, Mastana SS, Ralhan SK, et al. Identification of the risk factors for the high prevalence of type 2 diabetes and its complications in a Punjabi population: North Indian Diabetes Study: A case-control study. Int J Diabetes Dev Ctries. 2007;27(4):108–15.
Bhatti JS, Bhatti GK, Mastana SS, Ralhan S, Joshi A, Tewari R. ENPP1/PC-1 K121Q polymorphism and genetic susceptibility to type 2 diabetes in North Indians. Mol Cell Biochem. 2010;345(1–2):249–57. doi:10.1007/s11010-010-0579-2.
Sobti RC, Maithil N, Thakur H, Sharma Y, Talwar KK. Association of ACE and FACTOR VII gene variability with the risk of coronary heart disease in north Indian population. Mol Cell Biochem. 2010;341(1–2):87–98. doi:10.1007/s11010-010-0440-7.
Akar N, Aras O, Omurlu K, Cin S. Deletion polymorphism at the angiotensin-converting enzyme gene in Turkish patients with coronary artery disease. Scand J Clin Lab Invest. 1998;58(6):491–5.
Gardemann A, Fink M, Stricker J, Nguyen QD, Humme J, Katz N, et al. ACE I/D gene polymorphism: presence of the ACE D allele increases the risk of coronary artery disease in younger individuals. Atherosclerosis. 1998;139(1):153–9.
Samani NJ, Thompson JR, O’Toole L, Channer K, Woods KL. A meta-analysis of the association of the deletion allele of the angiotensin-converting enzyme gene with myocardial infarction. Circulation. 1996;94(4):708–12.
Ljungberg L, Alehagen U, Lanne T, Bjorck H, De Basso R, Dahlstrom U, et al. The association between circulating angiotensin-converting enzyme and cardiovascular risk in the elderly: a cross-sectional study. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2011;12(3):281–9. doi:10.1177/1470320310391326.
Shafiee SM, Firoozrai M, Salimi S, Zand H, Hesabi B, Mohebbi A. Angiotensin converting enzyme DD genotype not associated with increased risk of coronary artery disease in the Iranian population. Pathophysiology. 2010;17(3):163–7. doi:10.1016/j.pathophys.2009.10.001.
Lei HP, Chen HM, Zhong SL, Yao QZ, Tan HH, Yang M, et al. Association between polymorphisms of the renin-angiotensin system and coronary artery disease in Chinese patients with type 2 diabetes. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2012;13(2):305–13. doi:10.1177/1470320311435533.
Narne P, Ponnaluri KC, Singh S, Siraj M, Ishaq M. Relationship between angiotensin-converting enzyme gene insertion/deletion polymorphism, angiographically defined coronary artery disease and myocardial infarction in patients with type 2 diabetes mellitus. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2012;13(4):478–86. doi:10.1177/1470320312448947.
Agerholm-Larsen B, Nordestgaard BG, Steffensen R, Sorensen TI, Jensen G, Tybjaerg-Hansen A. ACE gene polymorphism: ischemic heart disease and longevity in 10,150 individuals. A case-referent and retrospective cohort study based on the Copenhagen City Heart Study. Circulation. 1997;95(10):2358–67.
Lindpaintner K, Pfeffer MA, Kreutz R, Stampfer MJ, Grodstein F, LaMotte F, et al. A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med. 1995;332(11):706–11. doi:10.1056/NEJM199503163321103.
Parchwani DN, Palandurkar KM, Hema Chandan Kumar D, Patel DJ. Genetic predisposition to diabetic nephropathy: evidence for a role of ACE (I/D) gene polymorphism in type 2 diabetic population from Kutch region. Indian J Clin Biochem IJCB. 2015;30(1):43–54. doi:10.1007/s12291-013-0402-4.
Baroudi T, Bouhaha R, Moran-Moguel C, Sanchez-Corona J, Ben Maiz H, Kammoun Abid H, et al. Association of the insertion/deletion polymorphism of the angiotensin-converting enzyme gene with type 2 diabetes in two ethnic groups of Jerba Island in Tunisia. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2009;10(1):35–40. doi:10.1177/1470320309102314.
Ramachandran V, Ismail P, Stanslas J, Shamsudin N, Moin S. Mohd Jas R. Association of insertion/deletion polymorphism of angiotensin-converting enzyme gene with essential hypertension and type 2 diabetes mellitus in Malaysian subjects. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2008;9(4):208–14. doi:10.1177/1470320308097499.
Movva S, Alluri RV, Komandur S, Vattam K, Eppa K, Mukkavali KK, et al. Relationship of angiotensin-converting enzyme gene polymorphism with nephropathy associated with Type 2 diabetes mellitus in Asian Indians. J Diabetes Complicat. 2007;21(4):237–41. doi:10.1016/j.jdiacomp.2006.07.001.
Thomas GN, Tomlinson B, Chan JC, Sanderson JE, Cockram CS, Critchley JA. Renin-angiotensin system gene polymorphisms, blood pressure, dyslipidemia, and diabetes in Hong Kong Chinese: a significant association of tne ACE insertion/deletion polymorphism with type 2 diabetes. Diabetes Care. 2001;24(2):356–61.
Arzu Ergen H, Hatemi H, Agachan B, Camlica H, Isbir T. Angiotensin-I converting enzyme gene polymorphism in Turkish type 2 diabetic patients. Exp Mol Med. 2004;36(4):345–50. doi:10.1038/emm.2004.45.
Rahimi Z, Hasanvand A, Felehgari V. Interaction of MTHFR 1298C with ACE D allele augments the risk of diabetic nephropathy in Western Iran. DNA Cell Biol. 2012;31(4):553–9. doi:10.1089/dna.2011.1364.
Lu Y, Ge Y, Hu Q, Shi Y, Xue C, Chen S, et al. Association between angiotensin-converting enzyme gene polymorphism and diabetic retinopathy in the Chinese population. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2012;13(2):289–95. doi:10.1177/1470320311432187.
Stephens JW, Dhamrait SS, Acharya J, Humphries SE, Hurel SJ. A common variant in the ACE gene is associated with peripheral neuropathy in women with type 2 diabetes mellitus. J Diabetes Complicat. 2006;20(5):317–21. doi:10.1016/j.jdiacomp.2005.07.010.
Acknowledgments
The present study was supported by University Grant Commission, New Delhi, India. We would like to thank all the volunteers for their support during the recruitment. We are also very thankful to all the participants of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Bhatti, G.K., Bhatti, J.S., Vijayvergiya, R. et al. Implications of ACE (I/D) Gene Variants to the Genetic Susceptibility of Coronary Artery Disease in Asian Indians. Ind J Clin Biochem 32, 163–170 (2017). https://doi.org/10.1007/s12291-016-0588-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-016-0588-3