Abstract
Over the past two decades, molecular targeted therapy has revolutionized the landscape of cancer treatment due to lower side effects as well as higher anticancer effects. Peroxisome proliferator-activated receptor gamma (PPARγ) is a member of the nuclear hormone receptor which plays a crucial role in cell proliferation and death and the efficacy of PPARγ ligands either as monotherapy or in combination with traditional chemotherapy drugs has been proved by recent studies. In this study, we aimed to investigate the effects of pioglitazone, a well-known PPARγ stimulator, in ALL-derived NALM6 cells by using trypan blue assay, MTT assay, and flow cytometry analysis. Moreover, to investigate the molecular mechanism action of pioglitazone in these cells, we assessed the possible alterations in the expression of some target genes which regulate cell proliferation, apoptosis, and autophagy system. Our result demonstrated that pioglitazone induced a remarkable antileukemic effect on NALM6 cells through a PTEN-mediated manner. Based on the fact that PI3K hyperactivation is one of the main properties of ALL cells, the effects of PI3K inhibition using CAL-101 on pioglitazone-induced cytotoxicity were evaluated by combinatorial experiments. Moreover, the result of cell cycle assay and qRT-PCR demonstrated that pioglitazone-CAL-101 induced antileukemic effect mainly through induction of p21 and p27-mediated G1 arrest. Additionally, our result showed that inhibition of proteasome and autophagy system, two main cellular processes, increased the antileukemic effects of the agents. Taken together, we suggest a novel therapeutic application for PPARγ stimulators as a single agent or in combination with PI3K inhibitors that should be clinically evaluated in ALL patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the most common type of ALL, B-cell acute lymphoblastic leukemia (B-ALL) is characterized by the clonal expansion of poorly differentiated B-cell precursors inside the bone marrow. Even though risk-adapted multi-agent therapy substantially improved the overall survival (OS) of pediatric and young adult ALL to > 90% and 70–80%, respectively, disease prognosis remained poor in patients who are older than 60 years. Moreover, about 30% of all patients experience a relapse, and the median survival following relapse is ranging from < 10% to approximately 25% [1, 2]. Indeed, the majority of the relapsed patients are resistant to conventional treatment options and novel therapeutic approaches which target leukemia-specific molecular determinants are urgently needed [3].
As one of the most critical pathways the in development of various cancers, PI3K signaling pathway is involved in the regulation of cell proliferation, differentiation, and survival. A large body of evidence demonstrated that functional mutations, chromosomal rearrangement, and gene amplification led to a constitutive and aberrant activation of this pathway in B-ALL [4, 5]. Moreover, the expression and activity of PTEN, as the most important negative regulator of PI3K/Akt signaling, is lost in many primary and metastatic human cancers including B-ALL. For instance, Xu et al. reported a statistically decreased expression of PTEN in B-ALL patients [6], and Richter et al. declare that while PTEN mutations and deletions are rare in B-ALL, some cellular mechanisms such as promoter hypermethylation and posttranslational modifications lead to PTEN inactivation [7]. Recent studies showed that pioglitazone, the most studied thiazolidinediones (TZD), demonstrated anticancer impacts in different types of cancers by interfering with different signaling networks in a cancerous cell. Ciaramella et al. reported that pioglitazone can suppress the cell growth and invasion of via inhibition of MAPK and TGFβ/SMAD signaling in NSCLC [8]. In another study conducted by Nemenoff et al., it has been shown that pioglitazone could induce its apoptotic property in lung cancer cells through a Tumor-Necrosis Factor (TNF) and (TNF-Related Apoptosis-Inducing Ligand (TRAIL)-dependent mechanism [9]. Intriguingly, our previous study demonstrated that pioglitazone-induced cytotoxicity was coupled with a significant increase in PTEN expression, suggesting that this agent may induce its antileukemic effects in NB4 through a PTEN-mediated manner [10]. Based on the dysregulation of PTEN expression and activity which is a characteristic of B-ALL, we decided to determine the effects of pioglitazone in pre-B-ALL NALM6 cells. Moreover, hyper-activated PI3K signaling in ALL thrived the question of whether pioglitazone-induced PTEN activation could be exploited to increase the efficacy of CAL-101, a potent and highly selective inhibitor of p110-δ. Notably, we found that pioglitazone potently increased the antileukemic effects of CAL-101 in ALL-derived NALM6 cells through PTEN-mediated PI3K inhibition.
Materials and Methods
Cell Culture and Drug Treatment
NALM6 (human pre-B ALL cells) cells were cultured in RPMI 1640 medium enriched with 10% heat-inactivated fetal bovine serum (Gibco) and 1% penicillin/streptomycin. Cells were stored in an incubator at 37 °C in a humidified atmosphere of 5% CO2. When cells were at appropriate confluency, they were treated with certain concentrations of PPARγ ligand, pioglitazone, which its stock was made by resolving the powder in sterile dimethyl sulfoxide (DMSO) dispensed into aliquots, and kept at − 20 °C. In addition to the negative control (no inhibitor), NALM6 cells were treated with the corresponding concentration of DMSO as an alternative negative control. Also, leukemic cells were subjected to CAL-101 (PI3K inhibitor, Selleckchem), BKM-120 (PI3K inhibitor, Selleckchem), Bortezomib (proteasome inhibitor, Selleckchem) and Chloroquine (autophagy inhibitor, Sigma) either alone or in combination with pioglitazone for more investigations.
Trypan Blue Exclusion Assay
The trypan blue assay was conducted to measure the viability and growth of NALM6 cells after treatment with certain concentrations of pioglitazone, either as a single agent or in combination with BKM-120, CAL 101. Leukemic cells were seeded in a 24-well plate in the medium containing different concentrations of so-called drugs. Following indicated time intervals, 20 µl of each sample was collected, mixed with 20 µl of 0.4% trypan blue (Invitrogen), and incubated at room temperature for 1–2 min. Samples were loaded onto a Neubauer hemocytometer to calculate cell viability by counting viable (unstained) and non-viable (stained) cells. Viability (%) = viable cell count/total cell count × 100.
Metabolic Activity Assessment by MTT Assay
To find out the inhibitory effect of pioglitazone either as a single agent alone or in combination modals, NALM6 cells were treated with pioglitazone and other agents and distributed into the wells of 96-well plates at the density of 5000 cells/well and kept in a humidified 5% CO2 incubator at 37 °C. At indicated time intervals, 100 µl of MTT solution (5 mg/ml in Phosphate buffered saline (PBS)) was added to each well, and cells were incubated for extra 3 h at 37 °C. Consequently, the formazan crystals were dissolved in DMSO and its optical densitometry was measured via the enzyme-linked immunosorbent assay (ELISA) method at the wavelength of 570 nm.
Determination of Combination Index (CI) and Dose Reduction Index (DRI)
To investigate whether the combination of pioglitazone and other drugs has synergic effects or not, the combination index (CI) and dose reduction index (DRI) were calculated using the method developed by Chou and Talalay and the computer software CalcuSyn by the classic isobologram equation. The CI values of > 1, = 1, and < 1 indicate antagonism, additive effect, and synergism of drugs, respectively.
Assessment of Apoptosis Using Flowcytometry
To investigate the effect of pioglitazone on inducing apoptosis in NALM6 cells either alone or combination with other drugs, annexin-V/PI staining was done on the cells. Drug-treated cells were collected after 48 h, washed with PBS, and re-suspended in a total volume of 100 µl of the incubation buffer. 2 µl of Annexin-V-Flous was added to each sample and incubated for 20 min in dark. Fluorescence was then measured using flowcytometry. Annexin V-positive and PI-negative cells are considered as erly apoptotic cells while positive staining for both Annexin-V and PI shows cells in the late apoptosis or necrosis phase.
Assessment of Cell Cycle Distribution Using Flowcytometry
The effect of pioglitazone on cell cycle progression, either as a single agent or in combination with other drugs, was evaluated by propidium iodide (PI) staining. Drug-treated cells were harvested and washed with cold PBS after 48 h incubation. Then fixed in ethanol at 70% concentration overnight. In the following step, cells were centrifuged and washed twice with PBS to remove ethanol. Finally, cells were exposed to DNA staining solution including 1 mg/ml propidium iodide, 0.2 mg/ml RNase, and 0.1% Triton X-100 at 37 °C for 30 min. After quantification of cellular DNA content by flow cytometry, the gathered data were interpreted by FlowJo V10 software.
RNA Extraction and cDNA Synthesis
After 48 h of cell treatment, total RNA was extracted using a high pure RNA isolation kit (Roche, Mannheim, Germany). The concentration of isolated RNA was quantified by a Nanodrop instrument (Nanodrop ND-1000 Technologies), whereas its integrity was checked out by 1% TAE agarose gel. Next, the reverse transcription process of former isolated RNA was carried out using complementary DNA (cDNA) Synthesis Kit (Takara Bio, Shiga, Japan). A 20 µl reaction was conducted comprising 4 µl of 5X PCR buffer, 2 µl of dNTP (10 mmol/l), 1 µl of random hexamers, 1 µl of RNase inhibitor (20 U/µl), 1 µl of M-MuLV RT (200 U/µl), 1 µg total RNA and 1 µl of diethylpyrocarbonate treated water for each reaction. The synthesized cDNAs were stored at -20 °C.
Analysis of Gene Expression by Quantitative Real-Time RT-PCR (qRT-PCR)
To scrutiny the effect of pioglitazone/CAL-101 on the expression of leukemia-related genes, the synthesized cDNAs were undergone Quantitative Real-Time PCR was performed by a light cycler instrument (Roche Diagnostics, Germany) using SYBR Premix Ex Taq technology (Takara Bio, Inc). PCR test was conducted in a closing volume of 20 µl of reaction mixture consisting of 2 µl of prior synthesized cDNA product, 0.5 µl of each forward and reverse primer (10 pmol) 10 µl of SYBR Green master mix, and 7 µl of nuclease-free water. Adapted times and temperature profiles including an initial activation step for 30 s at 95 °C succeeded by 40 repetitive cycles of the following procedure: a denaturation step for 5 s at 95 °C, a combined annealing/extension step for 20 s at 60 °C. Analysis of melting curves were performed to confirm the single PCR product of every single primer ABL housekeeping gene amplified as an internal control, and fold change in the expression of each target mRNA relative to ABL was calculated based on a comparative on 2−ΔΔct relative expression formula. A list of the primers used in this study and their nucleotide sequences is summarized in Table 1.
Detection of Autophagy by Acridine Orange Staining
To label the effect of autophagy suppression on pioglitazone and CAL-101 treatment outcome, NALM6 cells were stained hhhh acridine orange after exposure to chloroquine, a well-known autophagy inhibitor. After 48 h, drug-treated cells were collected and washed 3 times with PBS. For staining, cells were incubated with 1 μg/ml acridine orange (Merck, Darmstadt, Germany) for 15 min at room temperature in darkness. The differences in the acidity of autophagic lysosomes and cytoplasm/nucleolus were observable under a fluorescence microscope (Labomed, Los Angeles).
Statistical Analysis
Data are expressed as the mean ± SD of three independent experiments. All tests were performed in triplicate. The importance of dissimilarities between experimental variables was determined by a two-tailed student’s test and one-way variance analysis using GraphPad Prism Software. To compare between the control and drug-treated samples, the Dennett's multiple comparison test was used. A probability level of P < 0.05 was regarded as statistically consequential.
Results
Pioglitazone-Induced Cytotoxicity Was Coupled with Induction of PTEN Expression
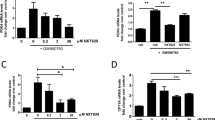
Multiple lines of evidence indicated that the expression of PTEN, dual lipid and protein phosphatase that dephosphorylates the lipid phosphatidylinositol-3,4,5-triphosphate (PIP3), is lower in ALL cells compared to normal peripheral blood mononuclear cells of healthy donors [6]. On another hand, previous studies showed that pioglitazone exerts its anti-leukemic effect through the increasement of PTEN expression. To evaluate the effects of pioglitazone on ALL cells, we aimed to evaluate the effect of this agent on NALM6 cells. To obtain the optimum concentration for further investigations, NALM6 cells were subjected to trypan blue and MTT assay after treatment with pioglitazone. As presented in Fig. 1A, our results demonstrated that pioglitazone can reduce cells viability and metabolic activity of NALM6 cells, and 250 µM was selected for further examinations. Interestingly, the result of qRT-PCR showed that pioglitazone-induced cytotoxicity in NALM6 cells was coupled with a significant increase in PTEN expression. Taken together, our results showed that pioglitazone serves its antileukemic effects in ALL-derived NALM6 cells, at least partially, through a PTEN-mediated manner (Fig. 1B).
The antileukemic effect of pioglitazone on NALM6 cells. A Trypan blue and MTT assays results showed decreased proliferative and survival capacity of NALM6 cells as a consequence of exposure to pioglitazone. B Evaluation the mRNA expression of PTEN demonstrated an increased level of this tumor suppressor gene after treatment of NALM6 cells with pioglitazone. Values are given as mean ± SD of three independent experiments. *P ≤ 0.05 represented significant changes from the control
PI3K Suppression Improved the Inhibitory Effects of Pioglitazone in NALM6 Cells
As another well-studied property of ALL cells, constitutive activity of the PI3K/AKT pathway plays a vital role in promoting the survival and proliferation of leukemic cells and it seems that the hyperactivation of this pathway may play a compensatory role in favor of leukemic cells survival. Interestingly, we found that PI3K signaling blockage using a well-known PI3K inhibitor CAL-101, potentiated the anti-survival effect of the pioglitazone as compared to treatment series using either drug alone (Fig. 2A). To confirm our results, we evaluated the effects of pioglitazone in the presence of BKM-120, a pan-class I PI3K inhibitor. Consistently with the results of CAL-101, it became evident that PI3K inhibition using BKM-120 remarkably sensitized ALL cells to pioglitazone (Fig. 2B). To determine the exact kind of interaction between CAL-101 and pioglitazone, combination index (CI) and dose reduction index (DRI) were calculated, where isobologram analysis demonstrated that all the points are below the line of additive effects (synergistic effect). The fraction-affect (FA) versus combination index (CI) plot also showed a synergistic cytotoxic effect (CI < 1) of CAL-101 combined with pioglitazone (Fig. 2C).
PI3K inhibition elevated the antileukemic capacity of pioglitazone. A The results obtained from Trypan blue and MTT assays after Co-treatment of NALM6 cells with pioglitazone and CAL-101, a PI3Kδ inhibitor, displayed a significant reduction in the survival of these cells. B Replacing CAL-101 by BKM-120, a pan-PI3K inhibitor, resulted in the same outcome, where a remarkable decrease in the survival of NALM6 cells was seen. C The results of combination index (CI) calculation showed a synergistic interaction between pioglitazone and CAL-101. (Dx)1 and (Dx)2 implied the single concentrations of pioglitazone and CAL-101 needed for inhibition a given level of viability index, and (D)1 and (D)2 are the concentrations of pioglitazone and CAL-101 essential to bring out the same effect in combination, respectively. Points below and above of the isoeffect line reflect synergy and antagonism, sequentially
Combination Treatment of pre-B ALL Cells with Pioglitazone and CAL-101 Halted Cell Cycle Progression
Enumeration of viable cells by trypan blue exclusion assay disclosed that while pioglitazone and CAL-101 could remarkably reduce the number of viable cells combinational experiments delineated that combination of pioglitazone and CAL-101 diminished the number of viable cells more strongly (Fig. 3A). To scrutinize the mechanisms by which PI3K inhibition potentiates the efficacy of pioglitazone, the growth-suppressive effects of this combination was also evaluated. Accordantly, the result of cell cycle assay highlighted that while pioglitazone and CAL-101 altered the percentage of cells in different phases of the cell cycle separately, combination of these agents robustly increased stuck cells in the G1 phase of the cell cycle (Fig. 3B). Based on these findings, it was of great interest to evaluate the transcriptional activity of the p21 and p27, two important proteins that control the activity of cyclin-dependent kinase (CDK) and cyclin complexes during the cell cycle [11]. Interestingly, we found a considerable increase in mRNA expression level of the aforementioned genes in pioglitazone-CAL-101-treated cells which was consistent with the results achieved from cell cycle analysis (Fig. 3B).
The antiproliferative effects of pioglitazone was enhanced by PI3K inhibition. A PI3K inhibition by CAL-101 augmented pioglitazone potency to limiting the number of viable cells. B CAL-101 amplified pioglitazone ability in alteration the percentage of cells in different phase of cell cycle which was explicated by a robust G1 arrest. C Data obtained from qRT-PCR denoted that the obstruction occurred in cell cycle was coupled with elevated mRNA level of p21 and p27, two important cyclin-dependent kinase inhibitors. Values are given as mean ± S.D. of three independent experiments. * P ≤ 0.05 represented significant changes from the control
Pioglitazone Enhanced CAL-101-Induced Apoptosis Through Alteration of Apoptosis-Related Genes
Pioglitazone or CAL-101 could increase the percentage of NALM6 cells in the sub-G1 phase either in single or in combinatorial modality. Based on previous studies, late apoptotic cells can be counted in the sub-G1 peak of the PI histogram [12] and this provoked our curiosity to investigate the apoptotic property of our agents using the annexin-V/PI assay. In agreement with the increased cell population in the Sub-G1 phase, both pioglitazone and CAL-101 could lead to a concentration-dependent increase in annexin-V/PI double positive NALM6 cells as compared with the control group. As presented in Fig. 4, while the single agent of pioglitazone and CAL-101 increased the percentage of apoptotic cells to 20.3% and 9.8% respectively, the percentage of these cells reached to 40.3% when pioglitazone was combined with CAL-101. Moreover, the results of qRT-PCR showed that the combination of agents increased the expression of pro-apoptotic genes such as Bid, Bax, FOXO3a, and FOXO4 more vigorously in comparison with each agent alone (Fig. 4).
Inhibition of PI3K enhances pioglitazone apoptotic effect in NALM6 cells. A and B After treatment of NALM6 cells with a certain concentration of pioglitazone in combination with CAL-101, the population of annexin-V-positive cells increased strikingly in comparison to the single-treated groups. The combination of these agents also elevated the number of cells in sub-G1 phase of cell cycle. C The results of qRT-PCR showed that the combination of pioglitazone and CAL-101 increased the expression of pro-apoptotic genes more vigorously compared with each agent alone. Values are given as mean ± S.D. of three independent experiments. * P ≤ 0.05 represented significant changes from the control
Pioglitazone-Plus-CAL-101 Cytotoxicity Was Impeded by NF-κB Signaling Pathway
Unexpectedly, analysis of real-time PCR results demonstrated that mRNA level of Bcl-2 and survivin, two important anti-apoptotic genes, was not significantly changed by pioglitazone and CAL-101 (Fig. 5A). Since the NF-κB signaling pathway plays a crucial role in upregulation of the aforementioned genes, it was suspected that presumably pioglitazone-CAL-101-induced apoptosis, at least partly, is inhibited by activation of this pathway. To examine the hypothesis, we inhibited NF-κB signaling by bortezomib, a well-known NF-κB inhibitor. Of particular interest, we found that the inhibition of NF-κB signaling, as evident by the decreased metabolic activity of the cells (Fig. 5B), not only resulted in concentration-dependent cytotoxicity in NALM6 cells, but also yielded a superior anti-leukemic effect in pioglitazone-CAL-101-treated cells (Fig. 5C); indicating that the activation of NF-κB signaling could decrease the anti-leukemic effects of the agents in ALL cells.
NF-κB signaling served as a compensatory pathway against the antileukemic effects of pioglitazone and CAL-101. A No significant effect on the expression of NF-κB-targeted anti-apoptotic genes was seen after treatment of NALM6 cell by pioglitazone and CAL-101. B Blocking NF-κB signaling via bortezomib, a well-known proteasome inhibitor, reduced the metabolic activity of NALM6 cells in a time-dependent and concentration-dependent manner. C Pioglitazone-plus-CAL-101 induced cytotoxicity was elevated in the presence of bortezomib. Values are given as mean ± S.D. of three independent experiments. *, P ≤ 0.05 represents significant changes from untreated control
Inhibition of Autophagy Enhanced Pioglitazone Plus-CAL-101 Cytotoxicity in pre-B ALL Cells
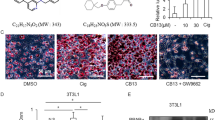
While some studies showed that autophagy exerts a critical role in clearing old organelles and reducing oxidative stress to maintain cell health, and consequently minimizing cancer development, some studies reported that autophagy could activate cell proliferation, survival, and drug resistance in ALL [13]. To determine the role of in ALL-derived NALM6 cells, we treated the cells with chloroquine (CQ), a well-known autophagy inhibitor. As revealed by a conspicuous reduction in the red-to-green fluorescence intensity ratio (Fig. 6A), inhibition of autophagy resulted in a substantial decrease in NALM6 cell, which disclosed that autophagy is in favor of cell proliferation and survival of these cells. Controversially, the results of qRT-PCR showed elevated levels of that ATG7, ATG10, and Beclin-1, as autophagy-triggering genes, markedly increased after treatment of NALM6 cells by pioglitazone-plus-CAL-101 (Fig. 6B). Interestingly, treatment of NALM6 cells with pioglitazone and CAL-101 in the presence of CQ leaded to a robust decrease in metabolic activity of the cells (Fig. 6C), which emphasized that the cytotoxicity of these agents in both single and combined modality in hampered by activation of autophagy.
Superior cytotoxicity of pioglitazone-plus-CAL-101 in the presence of autophagy inhibitor. A Inhibition of autophagy by chloroquine (CQ), as portrayed by a visible reduction in the red-to-green fluorescence intensity ratio, diminished the viability of NALM6 cells. B qRT-PCR analysis clarified that pioglitazone and CAL-101 increased the mRNA expression of autophagy-related genes such as ATG7, ATG10 and Beclin-1 either as a single agent or in a combination modality. C Combinational treatment of pioglitazone-plus-CAL-101 and CQ (20 µM) sensitized NALM6 cells more than using each drug alone. Values are given as mean ± S.D. of three independent experiments. *, P ≤ 0.05 represents significant changes from untreated control
Discussion
As one of the members of the Peroxisome Proliferator-Activated Receptor (PPAR) family of transcription factors, PPARγ plays a vital role in a vast spectrum of cellular process from adipocyte differentiation and lipid metabolism to regulating tumor growth [14]. Activation of PPARγ by a new generation of synthetic compounds such as thiazolidinediones (TZD) has received increasing interest as a potential strategy to treat some types of cancers [15]. Among all PPARγ ligands, pioglitazone is the most studied one in the field of malignancies which multiple preclinical and clinical studies represented promising results of this agent in different cancers such as breast [16], pancreas [17], colorectal [18] prostate and leukemia [19, 20]. For example, Tsubaki et al. evaluated the effects of pioglitazone in a wide range of human cell lines including PC-3, HSC-3, RPMI 8226, K-562, T24, MKN1 and reported that this agent could induce anti-growth effect on these cell lines via different mechanisms [21]. In a pre-clinical in vivo study, Kumari et al. reported anti cancerous effects of pioglitazone in mouse models of endometrium cancer [22]. Indeed, while most of the previous studies evaluating the anti-cancer effects of pioglitazone were at preclinical levels, a few numbers of clinical studies also evaluated the effect of adding pioglitazone to standard chemotherapy. For example, results of a clinical study conducted by Ghadiany et al. showed that adding pioglitazone to cytarabine and daunorubicin standard induction chemotherapy increased the CR rate in AML patients compared to control subjects [23].
PTEN, as one of the main intracellular tumor suppressors, exerts enzymatic activity as a phosphatidylinositol-3,4,5-trisphosphate (PIP3) phosphatase and thus by opposing the activity of PI3K, plays a crucial role in the prevention of cancer development [24]. Owing to several years of investigation about PTEN role in cancer, determining the statue of this tumor suppressor in tumors has gained an important prognostic and therapeutic value. Various studies have reported that loss of function mutations in PTEN is coupled with poor prognosis and lower life expectancy in many cancer patients. For instance, mutated PTEN in HER2 + breast cancers lead to HER2-targeted therapy resistance [25]. Also, abundant studies have introduced PTEN as a prognostic and predictive factor in brain, prostate, and endometrium tumors [26]. In addition, numerous studies have suggested PTEN as a promising therapeutic target in various malignancies including osteosarcoma [27], prostate [28], and gastric neoplasia [29].
Recent studies declare that PTEN is not only dedicated to inhibiting the PI3K pathway, but also through protein–protein interactions, inhibits other growth pathways, thus becoming a major cellular tumor suppressor [30,31,32]. Our previous study demonstrated that the pioglitazone induced antileukemic effect is largely associated with the PTEN statue of leukemic cells. Indeed, while pioglitazone-induced cytotoxicity in wild-type PTEN expressing-NB4 cells was coupled with a significant increase in PTEN-expression, in deficient-PTEN K562 cells, pioglitazone was unable to induce a significant anti-leukemic effect [10]. Given the fact that the expression level of PTEN is markedly decreased in both B-ALL cell lines and patients [6], it seems that the stimulation of PTEN expression by pioglitazone, can be a novel therapeutic tool in this malignancy. Notably, our result showed that pioglitazone cytotoxicity in PTEN-high-expressing NALM6 cells was coupled with a significant increase in PTEN expression. However, hyperactivation of PI3K pathway signaling, as another property of leukemic cells in ALL [33], seems to play a compensatory role in favor of leukemic cells survival. Intriguingly, we found that the blocking of PI3K signaling with CAL-101 or BKM-120 potentiated the anti-survival effect of pioglitazone, shedding light on the fact that hyper-activated PI3K signaling can attenuate the efficacy of pioglitazone in ALL-derived NALM6 cells. As a transcription factor, it has been reported that PPARγ can control the expression of some key regulators of the cell cycle such as p21 and p27 [34]. Göke R et al. reported that pioglitazone could inhibit the growth of carcinoid cells and promotes TRAIL-induced apoptosis by induction of p21 [35]. Consistently, we found that pioglitazone could diminish the proliferation of NALM6 cells through the induction of p21 and p27- mediated G1 cell cycle arrest and this effect was intensified when we inhibited the PI3K signaling via CAL-101. As reported by previous studies, either PTEN activation or PI3K inhibition can convert the anti-apoptotic signaling to pro-apoptotic signaling by shifting the balance of apoptosis-related genes [36, 37]. In harmony, with increase in sub-G1 population in combinatorial modality, while pioglitazone and CAL-101 could increase the expression of pro-apoptotic target genes such as Bax, Bad, Foxo3a, and Foxo4, a combination of these agents dramatically elevated the expression of aforementioned genes. As an unexpected finding, the expression of some PTEN anti-apoptotic target genes such as Bcl2 and Survivin did not change significantly, which raised the doubt of compensatory pathway activation. Based on previous studies, the aforementioned genes are the main anti-apoptotic target genes of the NF-kB pathway [38] and this raised our curiosity to examine the effect of bortezomib, as a well-known proteasome inhibitor on our combination treatment. Noteworthy, we found that the inhibition of NF-κB signaling, not only brought about concentration-dependent cytotoxicity in NALM6 cells, but also exerted a superior anti-leukemic effect in pioglitazone and CAL-101-treated cells; proving that the activation of NF-κB signaling could decrease the anti-leukemic effects of the agents in ALL cells (Fig. 7). As one of the main cellular processes, autophagy has a critical role in cell survival and death and while some studies reported that autophagy could activate cell proliferation, survival and drug resistance in ALL [39], some other studies declared that this system can be an alternative way for cell death in cancers [13]. While Han et al. reported that autophagy inhibition enhances Daunorubicin-induced apoptosis in K562 cells [40], Masui et al. introduced autophagy as a survival mechanism for squamous cell carcinoma cells in endonuclease G-mediated apoptosis [41] Based on our results, inhibition of autophagy substantially decreased the survival of in NALM6 cell, which suggested that autophagy supports cell proliferation and survival in these cells. Controversially, the expression of ATG7, ATG10, and Beclin-1, as the main regulatory genes of the autophagy system [42], significantly increased after treatment of ALL cells with pioglitazone-plus-CAL-101. Interestingly, when CQ was added to this combination, the metabolic activity of the cells robustly decreased, which highlight that the anti-leukemic of the agents is hindered by activation of autophagy. Taken together, our preclinical study showed that stimulating PPARγ by pioglitazone can be a promising therapeutic approach for ALL patients and CAL-101 can reinforce the anti-leukemic effects of this TZD. However, It should be noted that the safety of pioglitazone came under question because of some undesired conditions such as an overall increase in bladder cancer and heart failure risk and supplementary investigations, including clinical trials, are required to ascertain the efficacy of utilizing pioglitazone in ALL patients [43].
Schematic representation suggested for the plausible mechanisms of action of pioglitazone in combination with CAL-101 in NALM6 cells. In the presence of CAL-101, pioglitazone, a synthetic PPARγ activator, more efficiently reduced the survival rate of NALM6 cells through the elevation of PTEN targeted proapoptotic genes and induction of a p21 and p27-mediated G1 cell cycle arrest. However, as presented, expression of antiapoptotic genes as a result of NF-κB signaling, alternatively has bypassed the anti-leukemic signals induced by our combination. Our hypothesis was further strengthened, when the presence of bortezomib, a proteasome inhibitor, significantly resolved this compensatory effect and thereby robust pioglitazone-plus-CAL-101-induced apoptotic cell death in NALM-6 cells
References
DuVall AS et al (2022) Updates in the management of relapsed and refractory acute lymphoblastic leukemia: an urgent plea for new treatments is being answered! JCO Oncology Practice OP. 21.00843
Rasche M et al (2021) Survival following relapse in children with acute myeloid leukemia: a report from AML-BFM and COG. Cancers 13(10):2336
Kasner MT (2010) Novel targets for treatment of adult acute lymphocytic leukemia. Curr Hematol Malig Rep 5(4):207–212
Jiang W, Ji M (2019) Receptor tyrosine kinases in PI3K signaling: the therapeutic targets in cancer. In: Seminars in cancer biology. Elsevier
Gomes AM et al (2014) Adult B-cell acute lymphoblastic leukemia cells display decreased PTEN activity and constitutive hyperactivation of PI3K/Akt pathway despite high PTEN protein levels. Haematologica 99(6):1062
Xu L et al (2018) The expression pattern of Bcl11a, Mdm2 and Pten genes in B-cell acute lymphoblastic leukemia. Asia Pac J Clin Oncol 14(2):e124–e128
Richter A et al (2019) Combined casein kinase II inhibition and epigenetic modulation in acute B-lymphoblastic leukemia. BMC Cancer 19(1):1–11
Ciaramella V et al (2019) Activity and molecular targets of pioglitazone via blockade of proliferation, invasiveness and bioenergetics in human NSCLC. J Exp Clin Cancer Res 38(1):1–13
Nemenoff RA (2007) Peroxisome proliferator-activated receptor-γ in lung cancer: defining specific versus “off-target-effectors. J Thorac Oncol 2(11):989–992
Esmaeili S et al (2021) Stimulation of peroxisome proliferator-activated receptor-gamma (PPARγ) using pioglitazone decreases the survival of acute promyelocytic leukemia cells through up-regulation of PTEN expression. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 21(1):108–119
Chandrasekher G, Sailaja D (2005) Differential regulation of cyclin-dependent kinase inhibitors p21cip (p21) and p27kip (p27) expression by PI–3–kinase (PI–3K)/Akt in lens epithelium. Invest Ophthalmol Vis Sci 46(13):1899–1899
Hansakul P et al (2014) Growth arrest and apoptosis via caspase activation of dioscoreanone in human non-small-cell lung cancer A549 cells. BMC Complement Altern Med 14(1):1–12
Huang F-L, Yu S-J, Li C-L (2021) Role of autophagy and apoptosis in acute lymphoblastic leukemia. Cancer Control 28:10732748211019138
Hernandez-Quiles M, Broekema MF, Kalkhoven E (2021) PPARgamma in metabolism, immunity, and cancer: unified and diverse mechanisms of action. Front Endocrinol 12:624112
Al-Alem L et al (2011) Specific thiazolidinediones inhibit ovarian cancer cell line proliferation and cause cell cycle arrest in a PPARγ independent manner. PLoS ONE 6(1):e16179
Malakouti P et al (2022) Combined effects of pioglitazone and doxorubicin on migration and invasion of MDA-MB-231 breast cancer cells. J Egypt Natl Canc Inst 34(1):1–10
Ninomiya I et al (2014) Pioglitazone inhibits the proliferation and metastasis of human pancreatic cancer cells. Oncol Lett 8(6):2709–2714
Lee C et al (2006) Pioglitazone, a synthetic ligand for PPARγ, induces apoptosis in RB-deficient human colorectal cancer cells. Apoptosis 11(3):401–411
Saiki M et al (2006) Pioglitazone inhibits the growth of human leukemia cell lines and primary leukemia cells while sparing normal hematopoietic stem cells. Int J Oncol 29(2):437–443
Kubota T et al (1998) Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Can Res 58(15):3344–3352
Takahashi N et al (1999) Activation of PPARγ inhibits cell growth and induces apoptosis in human gastric cancer cells. FEBS Lett 455(1–2):135–139
Kumari GK, Kiran A, Krishnamurthy PT (2021) Preliminary evaluation on the beneficial effects of pioglitazone in the treatment of endometrial cancer. Med Oncol 38(6):71
Ghadiany M et al (2019) Adding oral pioglitazone to standard induction chemotherapy of acute myeloid leukemia: a randomized clinical trial. Clin Lymphoma Myeloma Leuk 19(4):206–212
Georgescu M-M (2010) PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer 1(12):1170–1177
Wang Q et al (2016) PI3K-p110α mediates resistance to HER2-targeted therapy in HER2+, PTEN-deficient breast cancers. Oncogene 35(27):3607–3612
Bazzichetto C et al (2019) PTEN as a Prognostic/Predictive Biomarker in Cancer: An Unfulfilled Promise? Cancers 11(4):435
Zheng C et al (2020) PTEN in osteosarcoma: Recent advances and the therapeutic potential. Biochimica et Biophysica Acta (BBA) - Rev Cancer 1874(2):188405
Choudhury AD (2022) PTEN-PI3K pathway alterations in advanced prostate cancer and clinical implications. Prostate 82(S1):S60–S72
Hu M et al (2019) MicroRNAs and the PTEN/PI3K/Akt pathway in gastric cancer. Oncol Rep 41(3):1439–1454
Okumura K et al (2005) Cellular transformation by the MSP58 oncogene is inhibited by its physical interaction with the PTEN tumor suppressor. Proc Natl Acad Sci 102(8):2703–2706
Song MS et al (2011) Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144(2):187–199
Tang Y, Eng C (2006) PTEN autoregulates its expression by stabilization of p53 in a phosphatase-independent manner. Can Res 66(2):736–742
Simioni C et al (2018) Targeting the phosphatidylinositol 3-kinase/Akt/mechanistic target of rapamycin signaling pathway in B-lineage acute lymphoblastic leukemia: an update. J Cell Physiol 233(10):6440–6454
Motomura W et al (2000) Activation of peroxisome proliferator-activated receptor γ by troglitazone inhibits cell growth through the increase of p27Kip1 in human pancreatic carcinoma cells. Can Res 60(19):5558–5564
Göke R et al (2001) Pioglitazone inhibits growth of carcinoid cells and promotes TRAIL-induced apoptosis by induction of p21waf1/cip1. Digestion 64(2):75–80
Koul D et al (2006) PTEN enhances TNF-induced apoptosis through modulation of nuclear factor-κB signaling pathway in human glioma cells. Biochem Biophys Res Commun 350(2):463–471
O’gorman D et al (2000) Sensitisation of HL60 human leukaemic cells to cytotoxic drug-induced apoptosis by inhibition of PI3-kinase survival signals. Leukemia 14(4):602–611
Ghahremanloo A et al (2021) Investigation of the role of neurokinin-1 receptor inhibition using aprepitant in the apoptotic cell death through PI3K/Akt/NF-κB signal transduction pathways in colon cancer cells. BioMed Res Int 2021
Colturato-Kido C et al (2021) Inhibition of autophagy enhances the antitumor effect of thioridazine in acute lymphoblastic leukemia cells. Life 11(4):365
Han W et al (2011) Autophagy inhibition enhances daunorubicin-induced apoptosis in K562 cells. PLoS ONE 6(12):e28491
Masui A et al (2016) Autophagy as a survival mechanism for squamous cell carcinoma cells in endonuclease G-mediated apoptosis. PLoS ONE 11(9):e0162786
Kang R et al (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18(4):571–580
Hillaire-Buys D, Faillie J-L, Montastruc J-L (2011) Pioglitazone and bladder cancer. Lancet 378(9802):1543–1544
Acknowledgements
Authors would like to express their gratitude to Shahid Beheshti University of Medical Sciences (Tehran, Iran) for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All author declares no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mokhtari, Y., Yousefi, AM. & Bashash, D. Inhibition of PI3K Signaling Intensified the Antileukemic Effects of Pioglitazone: New Insight into the Application of PPARγ Stimulators in Acute Lymphoblastic Leukemia. Indian J Hematol Blood Transfus 39, 546–556 (2023). https://doi.org/10.1007/s12288-023-01650-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-023-01650-5