Abstract
Currently, alternative cancer remedies, especially herbal-derived medicines, have attracted great interest. Propolis, a honeybee-produced naturopathic formulation, is an available, affordable, and safe example of such remedies with different content according to its geographic location. Findings regarding the protective properties of this resinous substance across numerous pathological conditions are promising. Although the anti-tumor effects of propolis from different origins have been explored to some degree, yet there is no study on the effects of Kermanian propolis in the treatment of hematologic malignancies. Accordingly, the objective of the present experiment was to divulge the anti-tumor potential of this bioactive substance both as monotherapy and in combination with doxorubicin against an acute lymphoblastic leukemia cell line (NALM-6).The viability of cells treated with Kermanian propolis (5–500 μg/mL) and doxorubicin (5–100 μg/mL) was analyzed during 72 h. Based on the MTT results, the best incubation time, IC50 concentrations, and finally the cytotoxicity of the combination therapy were ascertained. Next, the apoptotic rate and expression of apoptosis-related genes (Bcl-2 and Bax) were assessed in mono and combination therapies using flow cytometry and real-time PCR assays, respectively. Kermanian propolis and doxorubicin have impressive tumor-suppressing activity in a dose-dependent manner (IC50 concentrations: 100 and 40 μg/mL respectively). The best incubation time was considered 48 h. For the combination approach, 50 and 10 μg/mL were determined as optimum concentrations of the compounds. The selected concentrations induced notable apoptosis in the studied cells through significant (P < 0.01) upregulation of Bax/Bcl-2 level. The present study clearly suggests that Kermanian propolis, as an adjunct treatment option, has a promising apoptosis-induced cell death potential in the NALM-6 cell line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ALL is the abbreviation for acute lymphocytic leukemia, a hematopoietic cancer that affects a noticeable percentage of people, especially in children [1,2,3]. The proliferation and consequent accumulation of lymphoid progenitor cells in different tissues (especially bone marrow and blood) are the hallmarks of this malignancy, which includes approximately 80% of acute leukemia cases under the age of 15 [1,2,3]. Doxorubicin (DOX)-based chemotherapy is widely applied as a five-step treatment approach for this malignancy: induction, consolidation, interim maintenance, delayed intensification, and maintenance [3]. Two major mechanisms of action are proposed for this medication; as a drug of choice, this compound triggers cell death through topoisomerase II suppression-dependent DNA repair disruption and free radicals-induced oxidative stress [4]. In spite of therapeutic effectiveness, the remarkable dose-dependent DOX-induced toxicity for non-cancerous cells is the major obstacle for its extensive clinical application [5,6,7]. Therefore, it is vital to identify new approaches and/or compounds that can improve the effectiveness and also reduce the cytotoxicity and chemo-resistance to this drug. In this regard, combined-modality therapy, the amalgamation of anticancer agents, is now accepted as a more efficient strategy [8]. Propolis (bee glue), a resinous honeybees-produced substance, has attracted the attentiveness of researchers due to its multiple biological/pharmacological potentials [9,10,11,12]. It has been evidenced that the color, chemical composition, and pharmacological activity of this aromatic waxy-like substance depend on several factors, such as location, plant sources, and extraction solvents [13]. Despite the variable compositions, propolis has gained popularity as an adjunct treatment option or a dietary supplement [13]. Nowadays, it is being extensively employed to ameliorate different illnesses such as cancers, bacterial infections, inflammation, and gastrointestinal tract disorders [9, 13, 14]. Although the anti-tumor efficacy of propolis from different origins has been explored to some degree [14,15,16,17], yet there is no study on the effects of Kermanian propolis in the treatment of hematologic malignancies. Due to this dearth and also the high frequency of ALL, the focus of the present research was to divulge the cytotoxic and apoptosis-inducing potentials of this bioactive substance (as monotherapy and/or in combination with DOX) against an ALL cell line (NALM-6).
Materials and Methods
Substances and Drugs
In this experiment, Kermanian propolis was acquired from a local market and its nature was confirmed by the Department of Pharmacognosy, School of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran. After washing (thrice) and drying, it was crushed, treated with methanol (500 mL; Merck), and incubated at 37 °C for 3 days. The extract was collected on different days, pooled, and then concentrated using a rotary evaporator (Heidolph). To prepare the stock solution (1.6 × 103 µg/mL), 0.016 g of Kermanian propolis was dissolved in 10 µL dimethyl sulfoxide (DMSO, Sigma Aldrich), mixed well, and then the final volume of 10 mL was obtained using the cell culture medium. The stock solution was filtered using a sterile filter (0.22 μm; Orange Scientific Company) and the final concentrations were prepared using the cell culture medium. Regarding DOX (EBEWE Pharma), intended concentrations were attained by the addition of a relevant amount of stock solution (2 mg/mL) to the cell culture medium.
Cell Culture
The ALL cell line, NALM-6 (CRL-3273™), was purchased from the Iranian cell bank of Pasteur Institute and then cultured in RPMI-1640 medium (Gibco Company) enriched with 2 mM L-glutamine, 10% fetal bovine serum (Gibco Company), and 1% penicillin/streptomycin (Sigma Aldrich) in a moistened atmosphere (5% CO2 at 37 °C). The medium was replaced each 48 h and the cells were given subculture after reaching 80–90% confluency. The morphological assessment was done daily using an inverted microscope (ECLIPSE E100; Nikon).
Cell Viability Evaluation
Cells (10 × 103/well) were seeded into wells of 96-well plate containing 0.1 mL of culture medium enriched with pre-defined concentrations of the selected compounds. Desired concentrations for Kermanian propolis were 5, 10, 25, 50, 75, 100, 250, and 500 μg/mL and for DMSO-solved DOX were 5, 10, 20, 40, 80, and 100 μg/mL. Following the stipulated time [24, 48, and 72 h], the plate was centrifuged (500 × g/5 min), the medium was eliminated, and the cell survival rate was analyzed using the MTT assay kit (Sigma Aldrich) according to the instruction. Concisely, after the addition of 100 µL of the 5 mg/mL MTT solution to the wells, incubation (4 h at 37 °C), and centrifugation (700 × g/10 min), the supernatant was discarded and DMSO (100 µL) was added to each well. The plate was shaken and the optical densities (OD) of the wells were read at 570 nm using the ELISA reader ELX808 device (BioTek Company). After the determination of cell viability percentages [18, 19], the IC50 concentrations were specified. It is worth mentioning that the control group contained 0.1% DMSO-treated cells. To evaluate the combined toxic effects and determination of optimal concentrations, NALM-6 cells were treated (48 h) with DOX (5, 10, 20, and 40 μg/mL) and Kermanian propolis (10, 25, 50, 75, and 100 μg/mL) simultaneously. In order to explore possible toxic effects of Kermanian propolis on normal cells, human peripheral blood mononuclear cells (PBMCs) were cultured in the same condition applied for NALM-6 cells and then treated with IC50 concentration of the corresponding extract. All experiments were performed in triplicate (independently), each being repeated 3 times.
Determining the Combination Index (CI) Value
The CompuSyn software version 1.0 (Ting Chao Chou and Nick Martin, Paramus, NJ, 2005) was used to investigate the synergistic effects of Kermanian propolis and DOX based on the results of MTT assays. This software applies the multiple drug effect equation by Chou and Talalay to calculate the CI values: [CI = (D)1/(Dx)1 + (D)2/(Dx)2], where (Dx)1 and (Dx)2 indicate the doses of Kermanian propolis and DOX in a combination required to achieve the same efficacy as that of Kermanian propolis (D1) and DOX (D2) when used singularly. CI values < 1, = 1 and > 1 indicate synergistic, additive, and antagonistic effects, respectively.
Apoptosis Assay
In order to better evaluate the NALM-6-suppressing potential of Kermanian propolis in vitro, ApoFlowEx® FITC kit (ExBio Company) was used. Cells were seeded into cell culture flasks (10 × 104 cells/flask), and 48 h after treatment with Kermanian propolis (100 μg/mL), DOX (40 μg/mL), and Kermanian propolis + DOX (50 and 10 μg/mL), the cells were extracted, washed, suspended in binding buffer, and labeled using propidium iodide (PI) and Annexin V based on the manufacturer’s recipes [18]. After elapse of the incubation time (15 min), the percentage of apoptotic cells (Annexin V+/PI− and Annexin V+/PI+ cells) was quantified using CyFlow® Space flow cytometer (Sysmex Partec). As a general rule, unlabeled cells were used to remove autofluorescence. Trials were repeated 3 times.
RNA Segregation and cDNA synthesis
As per the instructions given by the manufacturers, the harvest of total RNA and then cDNA synthesis from the treated and control groups (following 48 h treatment) were done using TRIzol (Invitrogen) and RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, K1621), respectively. Quality and purity of extracted RNA were evaluated by agarose gel electrophoresis [20] and optical density measurement (A260/A280 ratio) using NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific), respectively.
Expression Profiling of Apoptosis-Related Genes
The relative gene expression analysis was conducted by real-time PCR and using RealQ Plus 2 × Master Mix Green (Ampliqon Company, 5 μL), 1 μL of the cDNA product, 0.5 μL of each primer, and 3 μL of nuclease-free water (total volume of 10 μL). The PCR amplification program using Rotor Gene 6000 Real-time PCR System (Corbett Research) was as follows: denaturation (95 °C/15 min), 40 cycles (95 °C/30 s), and 60 °C for 1 min. A melting curve assessment was applied to affirm the specificity of the products [20]. The housekeeping gene, β-actin, was used as the internal control and the relative Bax and Bcl-2 genes expression was calculated using the comparative Ct method. All trials were repeated 3 times and the list of used primers sequence is available in Table 1.
Statistical analysis
The mean ± standard deviation (mean ± SD) was calculated and the statistical differences between the treatment and the control groups (flow cytometry and real-time PCR) were assessed by t-test using SPSS 20 software. Two-way ANOVA was applied for MTT results analysis and P values of ≤ 0.05 were regarded as statistically significant. Log-Logistic Nonlinear Regression Models were used for the dose–response analysis determining the optimal concentration. To enhance detection, models with 2, 3, 4, and 5 parameters were evaluated and among them, a Log-Logistic Nonlinear Regression with 4 parameters presented the best fit and the least error. Additionally, this model had the least AIC and BIC among all studied models.
Results
Kermanian Propolis Decreases the Metabolic Activity of NALM-6 Cells
The effect of different concentrations of Kermanian propolis, DOX, and Kermanian propolis + DOX on the metabolic activity of cells (an indicator of cell viability) was evaluated by MTT assay. As illustrated in Fig. 1, the viability of the cultured cells after monotherapy was reduced in a dose-dependent mode. As evident from Fig. 2, suggested half-maximal inhibitory concentrations (IC50s) for Kermanian propolis and DOX were 72.99 and 20.39 respectively. Surprisingly, the viability of cells after treatment with these concentrations was more than 50%. Therefore, 100 and 40 μg/mL were selected for subsequent tests instead. In addition, 48 h was selected as the optimum exposure time for the next experiments. During this exposure time, the cytotoxic effect of the combination therapy was concentration-dependent, with approximately 30.9 ± 3.4, 28.2 ± 5.5, 56.6 ± 1.9, 68.1 ± 3.5, 45.6 ± 1.22, 49.4 ± 2.22, 62.1 ± 2.8, 74.5 ± 4.2, 50.9 ± 2.9, 55.6 ± 1.9, 67.9 ± 1.2, 78 ± 0.8, 63.8 ± 1.26, 68.5 ± 2.57, 82.8 ± 1, 90.5 ± 3.04, 84.37 ± 1.9, 89 ± 0.77, 92.38 ± 1.52, and 96.9 ± 1.08% reduction in metabolic activity after treatment with 10 + 5, 10 + 10, 10 + 20, 10 + 40, 25 + 5, 25 + 10, 25 + 20, 25 + 40, 50 + 5, 50 + 10, 50 + 20, 50 + 40, 75 + 5, 75 + 10, 75 + 20, 75 + 40, 100 + 5, 100 + 10, 100 + 20, and 100 + 40 μg/mL of Kermanian propolis along with DOX. Moreover, treating PBMCs with IC50 concentration of Kermanian propolis induced no significant apoptotic effect (P > 0.05, Fig. 1d). This finding suggests the tumor-selective anti-proliferative effect of Kermanian propolis.
Kermanian propolis decreases viability (%) of the NALM-6 cells. The cells were treated (24, 48, and 72 h) with different concentrations of Kermanian propolis (a) and DOX (b) and the MTT assay was applied. After exposure, the metabolic activity (%) of the treated cells was determined relative to the control cells (Cnt) which were set as 100%. As represented, the viability was reduced significantly (compared to the control value) in a dose-dependent mode (IC50 concentrations: 100 and 40 μg/mL). As presented in (c), 48 h exposure of cells with the combination of Kermanian propolis (P) and DOX (D) had a cytotoxic effect on NALM-6 cells, in a concentration-dependent manner. Interestingly, no dramatic apoptotic effect was seen after treatment of PBMCs with IC50 concentration of Kermanian propolis (d). Data are given as the mean ± standard deviation of three independent examinations. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001
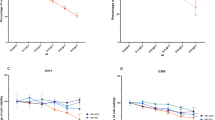
Dose–response curves. The effect of different concentrations of Kermanian propolis (a) and DOX (b) on the viability of Nalm-6 cells. The x-axis represents concentrations and the Y-axis is corresponding to percentages of viability. The IC50 concentrations of Kermanian propolis and DOX were 72.99 and 20.39 μg/mL, respectively
Combination of Kermanian propolis and DOX synergistically decreases cell viability of Nalm-6 cells
Based on MTT results and in order to achieve 50% cell death with the lowest possible concentrations of DOX, 4 pairs of concentrations were selected for detecting synergy indices: P(25) + D(5), P(25) + D(10), P(50) + D(5), and P(50) + D(10). CI analysis and normalized isobolograms indicated synergistic effect (CI < 1) in concentrations 50 µg/mL of Kermanian propolis + 10 μg/mL of DOX in NALM-6 cells (Fig. 3).
Synergistic effect of Kermanian propolis (25 and 50 µg/mL) and DOX (5 and 10 µg/mL) determined by the Compusyn software. The CI/FA curve (a) and concentration-normalized isobologram analysis (b) of Kermanian propolis and DOX combination showed that one of the selected concentration sets was below the additive line and showed CI < 1, confirming a synergistic effect (Kermanian propolis: 50 µg/mL and DOX: 10 µg/mL). (Dx)1 and (Dx)2 refer to the concentration of Kermanian propolis and DOX in a combination needed to achieve the same efficiency as that of compounds used alone (D1 and D2)
The Combination of Kermanian Propolis and DOX Induces Higher Apoptosis in NALM-6 Cell Line
To further assess the cytotoxic effects of Kermanian propolis and DOX, the impact of these substances on cell apoptosis was examined both individually and in combination. To this end, the studied cells were analyzed for Annexin V binding and PI uptake by the corresponding kit and using a flow cytometer. As illustrated in Fig. 4, 48 h incubation of cells with the selected concentrations (100, 40, and 50 + 10 μg/mL), significantly (P < 0.01) increased Annexin V+/PI− and Annexin V+/PI+ cells in the treated groups compared with the control ones, implying the apoptotic effect of Kermanian propolis, DOX, and Kermanian propolis + DOX on NALM-6 cells. Although DOX, with or without Kermanian propolis, had the ability to induce apoptosis in the cells, the level of the impact was greater in the combination treatment. Indeed, the 50% cell death was obtained at a lower concentration of DOX in the combined state. It should be noted that Kermanian propolis had neither cytotoxic nor apoptotic effects against PBMCs.
Apoptotic cell death induction by single and combined therapy approaches in NALM-6 cells. NALM-6 cells were exposed to the selected concentrations of Kermanian propolis (K.p; 100 μg/mL), DOX (40 μg/mL), and K.p + DOX (50 + 10 μg/mL) for 48 h and then the levels of Annexin V binding and PI uptake were assessed using flow cytometer. As shown, combination therapy (64.9 ± 4.35%) increased the Annexin V+/PI− and Annexin V+/PI+ cells compared to monotherapy with DOX (47.03 ± 5.8%) and K.p (43.72 ± 5.29%), respectively. Interestingly, no significant apoptotic effect was seen after treating PBMCs with IC50 concentration of Kermanian propolis. One representative analysis among three independent ones (n = 3) is shown. **P ≤ 0.01 and ***P ≤ 0.001
Kermanian Propolis Regulates the Expression of Apoptosis-Related Genes
To better understand the mechanisms underlying the cytotoxic effect of Kermanian propolis, DOX, and their combination on NALM-6 cells, the expression levels of Bcl-2 and Bax at mRNA level were examined in treated (Kermanian propolis 100 μg/mL, DOX 40 μg/mL, and Kermanian propolis + DOX 50 + 10 μg/mL) and control (DMSO-treated) cells. Based on the results, the expression levels of the studied genes were significantly altered in treated cells compared with the control ones (Fig. 5); the expression levels of Bcl-2 and Bax genes were down- and up-regulated, respectively.
The expression of the studied mRNAs in the control and treated groups. The fold change was measured relative to the control and calculated after adjusting for the ß-actin housekeeping gene using the comparative Ct (2−ΔΔCT) method. After 48 h exposure, the expression of Bcl-2 (a) and Bax (b) genes was significantly reduced and increased, respectively, compared with the corresponding control group. Interestingly, the alteration of Bax/Bcl-2 ratio was more prominent in combination therapy, followed by DOX at selected concentrations (c). The expression of the studied genes in NALM-6 cells underwent modification in DOX and Kermanian propolis treatment alone, but when cells were treated with DOX and Kermanian propolis in combination, the extent of alteration was clearly higher. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. For each gene, we had 3 independent replicates (n = 3)
Taken together, these findings suggest that Kermanian propolis triggers an apoptotic pathway in NALM-6 cells, thereby reduces tumor cells viability and progression. As another cornerstone finding, the amalgamation of Kermanian propolis and DOX further enhances the anti-NALM-6 activity of these substances compared with the monotherapy strategy, probably due to a/an synergistic or additive interaction.
Discussion
Currently, recognition of non-toxic and more effective substances has taken the spotlight in cancer drug development. In this context, honeybee products have attracted a lot of attention [11, 12, 21, 22]. Accordingly, several studies are targeted toward evaluating the health benefits of propolis and have recommended this material for different applications related to gynecological, oral, oncological, and dermatological care [11, 13]. Based on these studies, propolis has been introduced as an immunomodulator and alleviator of oxidative stress, inflammation, sepsis, ulcer, and bacterial infections [9, 13, 14]. This honey-derived substance is mainly composed [11, 23] of vitamins, minerals, resin, wax, enzymes, essential oils, pollen, and various organic elements (esters, flavonoids, terpenes, ß-steroids, aromatic aldehydes, phenolic acids, and alcohols). Although it has been suggested that the bioactive potential of this substance mainly depends on the presence of polyphenols [9, 10, 15], especially flavonoids (quercetin, pinocembrin, acacetin, chrysin, rutin, luteolin, kaempferol, apigenin, myricetin, catechin, naringenin, and galangin), yet the exact mechanisms of actions have not been fully elucidated and further experiments are warranted to explain the exact effective factors. About the tumor-suppressing potential of honey, propolis, and other derivatives, modulation of angiogenesis, insulin signaling, oxidative stress, and inflammation, as well as induction of apoptosis, cell cycle arrest, and mitochondrial outer membrane permeabilization were introduced as the main molecular mechanisms [11, 13]. Regarding propolis, Desamero and colleagues evidenced that crude ethanolic extract of Philippine stingless bee propolis had selective in vitro/vivo anti-cancer effects against differentiated-type human gastric cancer cell lines [17]. The researchers attributed the cytotoxicity to significant modulation of cell cycle-related genes and the consequent cell cycle arrest at the G0/G1 phase. Likewise, another study delineated apoptosis-dependent anti-breast tumor activity of propolis. Another fascinating finding of this experiment was the selective toxicity of this material against tumor cells and not normal cells [24]. Similarly, the ability of Turkish propolis for selective cytotoxicity against human lung cancer cells through the induction of endoplasmic reticulum stress, apoptosis, and caspase activity was approved [16].
Despite current knowledge on the biological activities of propolis and its ingredients, no study has been performed regarding the potential anti-hematopoietic cancer properties of Kermanian propolis and the involved mechanisms. Therefore, the exploration of its apoptosis-dependent cytotoxic effects on NALM-6 cells was aimed in the present experiment. The MTT, flow cytometry, and real-time PCR assays were employed to assess the metabolic activity, apoptotic rate, and expression levels of target genes, respectively, in the treatment and control groups. As illustrated in Figs. 1 and 3, MTT outcomes implied that the Kermanian propolis, DOX, and Kermanian propolis + DOX reduced the viability of the cells in a dose-dependent manner. The IC50 concentrations were 100, 40, and 50 + 10 μg/mL, respectively. To reinforce the obtained results and to better explicate the cytotoxicity mechanisms of the studied compounds, especially Kermanian propolis, the apoptotic rate was determined. The considerable Annexin-PI labeling in treated cells unequivocally supported the MTT findings. Exposure to defined concentrations caused significant apoptosis (P < 0.01) in cells treated with Kermanian propolis, DOX, and Kermanian propolis + DOX (Fig. 4). As apoptosis induction is a desirable characteristic for an anti-cancer drug candidate [18], the observed increase in both early and late apoptotic cells indicated that apoptosis induction was, at least partially, responsible for the cytotoxic effect of the studied compounds. Considering this finding and also to ascertain the mechanism of apoptosis, we decided to analyze the expression of apoptosis-related genes following treatments. Based on the findings (Fig. 5), the underlying mechanism seemed to be the elevated ratio of Bax/Bcl-2 (P < 0.01). Bcl-2 family members, as the cell death regulator proteins, can exert anti-apoptotic or pro-apoptotic functions [25]. Bcl-2 protein is localized to the outer membrane of mitochondria and plays a pivotal role in supporting cell survival and suppressing the functions of pro-apoptotic proteins [25]. In contrast, Bax (a pro-apoptotic protein of the Bcl-2 family) promotes permeabilization of the mitochondrial membrane and also the release of apoptotic signals [25]. A large and growing body of data supports the application of Bax/Bcl-2 ratio as a predictive marker that determines cancer cells’ susceptibility to apoptosis [26, 27]. Interestingly, here we hinted at significant down-regulation of Bcl-2 as well as notable up-regulation of Bax, and thereby the increment of Bax/Bcl-2 ratio in the Kermanian propolis-, DOX-, and noticeably in Kermanian propolis+DOX-treated cells.
The cytotoxic effects of green and red propolis (G12 and G13) on different human leukemic cells (K562, HL60, NB4, Ramos human Burkitt lymphoma, Raji human Burkitt lymphoma, NALM-16, NALM-6, RS4, B15, and REH) has been compared by Franchi and colleagues [28]; higher cytotoxicity of red propolis, induction of 50% apoptosis after 24 and before 48 h as well as different IC50 concentrations (< 40 and > 30 μg/mL for G12; < 20 and > 15 μg/mL for G13) were the most important findings of their study about NALM-6. Unfortunately, these researchers did not investigate the apoptosis-related mechanisms of these compounds. Another interesting study on the propolis-induced apoptosis mechanism was reported by Gunduz et al. [29]. They suggested that inhibition of telomerase function played a notable role in Turkish propolis (ethanolic extract)-induced apoptosis in a T-cell lymphoblastic leukemia (CCFR-CEM) cell line. More fascinating experiments were carried out by Cogulu and Eom. The former evidenced the significant decrease in the expression of human telomerase reverse transcriptase (hTERT) in bone marrow-derived leukemic cells, following treatment with 60 ng/mL Turkish propolis [30]. Eom et al. appraised the cytotoxic and apoptotic effects of ethanolic extract of the Korean propolis on HL-60 cells; they introduced dose-dependent inhibition of cell proliferation, apoptosis induction, caspase-3 activation, and increased levels of cytosolic cytochrome c as the main anti-neoplastic mechanisms of Korean propolis [31].
In summary, our findings unveil that Kermanian propolis, individually or in combination with DOX, is an effective NALM-6-suppressor compound that activates the apoptosis pathway. However, further molecular and cellular investigations are demanded to recognize other possible anti-tumor mechanisms of this natural product.
References
Baljevic MJE, O’Brien S, Kantarjian HM (2016) Acute Lymphoblastic. In: Kantarjian Leukemia HM, Wolff RA (eds) The MD anderson manual of medical oncology, 3rd edn. McGraw-Hill Education, New York
Hunger SP, Mullighan CG (2015) Acute lymphoblastic leukemia in children. N Engl J Med 373(16):1541–1552
Stock W, Luger SM, Advani AS et al (2019) A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood 133:1548–1559
Ghasemimehr N, Farsinejad A, Khalilabadi RM, Yazdani Z, Fatemi A (2018) The telomerase inhibitor MST-312 synergistically enhances the apoptotic effect of doxorubicin in pre-B acute lymphoblastic leukemia cells. Biomed Pharmacother 106:1742–1750
Zhang Y-W, Shi J, Li Y-J, Wei L (2009) Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp 57(6):435–445
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE et al (2011) Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 21(7):440
Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Prasad SVN et al (2014) Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Investig 124(2):617–630
Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B et al (2017) Combination Therapy Combat Cancer Oncotarget 8(23):38022
Campos JF, Santos UPd, Rocha PdSd, Damião MJ, Balestieri JBP, Cardoso CAL et al (2015) Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless bee Tetragonisca fiebrigi (Jataí). Evidence-Based Complement Alternat Med 2015:1–11
Mouhoubi-Tafinine Z, Ouchemoukh S, Tamendjari A (2016) Antioxydant activity of some algerian honey and propolis. Ind Crops Prod 88:85–90
Pasupuleti VR, Sammugam L, Ramesh N, Gan SH (2017) Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxidat Med Cellul Longevit 2017:1–21
Premratanachai P, Chanchao C (2014) Review of the anticancer activities of bee products. Asian Pac J Trop Biomed 4(5):337–344
Silva LMd, Souza PD, Jaouni SKA, Harakeh S, Golbabapour S, de Andrade SF (2018) Propolis and its potential to treat gastrointestinal disorders. Evidence-Based Complement Alternat Med 2018:1–12
Chan GC-F, Cheung K-W, Sze DM-Y (2013) The immunomodulatory and anticancer properties of propolis. Clin Rev Allergy Immunol 44(3):262–273
Brihoum H, Maiza M, Sahali H, Boulmeltout M, Barratt G, Benguedouar L et al (2018) Dual effect of algerian propolis on lung cancer: antitumor and chemopreventive effects involving antioxidant activity. Brazil J Pharmaceut Sci. https://doi.org/10.1590/s2175-97902018000117396
Demir S, Aliyazicioglu Y, Turan I, Misir S, Mentese A, Yaman SO et al (2016) Antiproliferative and proapoptotic activity of Turkish propolis on human lung cancer cell line. Nutr Cancer 68(1):165–172
Desamero MJ, Kakuta S, Tang Y, Chambers JK, Uchida K, Estacio MA et al (2019) Tumor-suppressing potential of stingless bee propolis in in vitro and in vivo models of differentiated-type gastric adenocarcinoma. Sci Rep 9(1):1–13
Vahidi R, Safi S, Farsinejad A, Panahi N (2015) Citrate and celecoxib induce apoptosis and decrease necrosis in synergistic manner in canine mammary tumor cells. Cell Mol Biol (Noisy-le-grand) 61(5):22–8
Pour MSS, Vahidi R, Lashkari M, Derakhshani A, Ameri Z, Farsinejad A (2020) Cord blood serum harvesting by hydroxyethyl starch: a fetal bovine serum alternative in expansion of umbilical cord-derived mesenchymal stem cells. Cytotechnology 72(4):551–567
Pouryazdanpanah N, Vahidi R, Dabiri S, Derakhshani A, Farsinezhad A (2018) Use of some additives for improving mesenchymal stem cell isolation outcomes in non-mobilized peripheral blood. Arch Iran Med 21(8):362–367
Sforcin JM (2016) Biological properties and therapeutic applications of propolis. Phytother Res 30(6):894–905
Sforcin JM, Bankova V (2011) Propolis: is there a potential for the development of new drugs? J Ethnopharmacol 133(2):253–260
Abubakar MB, Abdullah WZ, Sulaiman SA, Ang BS (2014) Polyphenols as key players for the antileukaemic effects of propolis. Evidence-Based Complement Alternat Med 2014:1–11
Xuan H, Li Z, Yan H, Sang Q, Wang K, He Q et al (2014) Antitumor activity of Chinese propolis in human breast cancer MCF-7 and MDA-MB-231 cells. Evidence-Based Complement Alternat Med 2014:1–11
Hardwick JM, Soane L (2013) Multiple functions of BCL-2 family proteins. Cold Spring Harbor Perspect Biol 5(2):008722
Raisova M, Hossini AM, Eberle J, Riebeling C, Orfanos CE, Geilen CC et al (2001) The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Investig Dermatol 117(2):333–340
Prokop A, Wieder T, Sturm I, Eβmann F, Seeger K, Wuchter C et al (2000) Relapse in childhood acute lymphoblastic leukemia is associated with a decrease of the Bax/Bcl-2 ratio and loss of spontaneous caspase-3 processing in vivo. Leukemia 14(9):1606–1613
Franchi GC, Moraes CS, Toreti VC, Daugsch A, Nowill AE, Park YK (2016) Comparison of effects of the ethanolic extracts of Brazilian propolis on human leukemic cells as assessed with the MTT assay. Evidence-Based Complement Alternat Med 2012:1–6
Gunduz C, Biray C, Kosova B, Yilmaz B, Eroglu Z, Şahin F et al (2005) Evaluation of manisa propolis effect on leukemia cell line by telomerase activity. Leuk Res 29(11):1343–1346
Cogulu O, Biray C, Gunduz C, Karaca E, Aksoylar S, Sorkun K et al (2009) Effects of Manisa propolis on telomerase activity in leukemia cells obtained from the bone marrow of leukemia patients. Int J Food Sci Nutr 60(7):601–605
Eom HS, Lee EJ, Yoon BS, Yoo BS (2010) Propolis inhibits the proliferation of human leukaemia HL-60 cells by inducing apoptosis through the mitochondrial pathway. Nat Prod Res 24(4):375–386
Funding
This study gained the support of Kerman University of Medical Sciences by the Grant No. 98000523.
Author information
Authors and Affiliations
Contributions
AF and MM supervised all aspects of the work and proposed the original concept and designed the experiment. MS and RV participated in the data acquisition. MS and ML performed the real-time PCR experiments and interpretation of obtained results. Interpretation of immunoprofiling was performed by MK contributed to the data analysis. RV and BK contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics Approval
The study gained the approval of the ethical committee of Kerman University of Medical Sciences with the Ethics approval code of IR.KMU.REC.1398.397.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masoud, M., Maryam, S.S.p., Mahla, S.B. et al. Elevated Bax/Bcl-2 Ratio: A Cytotoxic Mode of Action of Kermanian Propolis Against an Acute Lymphoblastic Leukemia Cell Line, NALM-6. Indian J Hematol Blood Transfus 38, 649–657 (2022). https://doi.org/10.1007/s12288-022-01522-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-022-01522-4