Abstract
Secondary hypogammaglobulinemia (SHG) is characterized by a decrease in total serum immunoglobulin (Ig) levels and can lead to immunodeficiency associated with recurrent and severe infections and is a common complication of chronic lymphocytic leukaemia (CLL). SHG also increases with the treatment of CLL. Ibrutinib is one of these treatments and acts by inhibiting bruton tyrosine kinase. Twenty-seven patients with relapsed/refractory (R/R) CLL who received ibrutinib monotherapy were included. IgG levels, stage, bulky disease, previous treatments, genetics and laboratory features, overall survival (OS) and progression free survival (PFS) were compared with and without SHG. Nine patients (33.3%) had SHG and 18 patients (66.6%) didn’t have SHG. The mean IgG levels after ibrutinib treatment first, third, 6th and 12th months were 684, 531.3, 452 and 360 mg/dL respectively in SHG arm (p < 0.001) and 1156, 1058.2, 1012.8 and 886.9 mg/dL respectively in without SHG arm (p < 0.001). All patients with SHG had ibrutinib related other adverse effects(AEs) but 2 (11.1%) patients without SHG had AEs (p < 0.001). In SHG arm 7 (77.7%) had complete and partial remission but in other arm only 6 (33.3%) had (p: 0.029). There was no significant difference in OS and PFS (p values 0.95 and 0.64, respectively). IgG levels at the beginning of ibrutinib treatment is the best predicted value for SHG development in our study (p = 0.001). As a result, we reported a significant decrease in IgG values after ibrutinib monotherapy in R/R CLL patients. This decrease occurs every month after ibrutinib use, but after a maximum of 1 year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults. CLL cells coexpress the B-cell antigens CD19, CD20, and CD23 together with CD5 [1]. Infections are the most important cause of morbidity and mortality in CLL patients [2]. Approximately 50% of deaths in CLL patients are caused by infections [3].
Hypogammaglobulinemia (HG) is characterized by a decrease in total serum immunoglobulin (Ig) levels and can lead to immunodeficiency associated with recurrent and severe infections and is a common complication of B-cell malignancies, such as CLL [4]. In many studies, the frequency of HG development in CLL patients was shown to be between 20–70% [5, 6]. In a different study, the frequency of HG at the onset of CLL was 25%, and 25% of CLL patients with normal baseline IgG values subsequently developed HG [6].
HG can develop not only during the natural process of CLL but it can also develop due to CLL treatments. Ibrutinib is one of these treatments and acts by inhibiting Bruton’s tyrosine kinase (BTK). BTK is a non-receptor tyrosine kinase and plays a very important role in B cell receptor (BCR) signalling. CLL cells depend on tonic BCR signaling for tumor expansion and proliferation. Ibrutinib binds to the cysteine-481 amino acid of the BTK enzyme and blocks BCR signalling, induces apoptosis, and inhibits adhesion of malignant B-cells to microenvironment cells [7].
In the current guidelines, ibrutinib treatment in CLL patients is increasingly recommended. At present, ibrutinib is recommended for both fit and frail patients in the first and other lines of treatment [8]. The purpose of our study is to evaluate the development and frequency of secondary HG (SHG) after ibrutinib, since the most common cause of mortality and morbidity in CLL is infection.
Materials and Methods
Twenty seven CLL patients were included in this single center retrospective study. CLL patients who were in Erciyes University Hematology Department between May 2013 and January 2020 were eligible to be included in this study in the following cases: (a) they had a diagnosis of relapsed/refractory (R/R) CLL, (b) they were treated with ibrutinib monotherapy, (c) they used ibrutinib for at least 1 year, (d) their Eastern Cooperative Oncology Group (ECOG) performance status was ≤ 2. All information of the patients were recorded from the patient files and hospital automation system.
All patients received 420 mg oral ibrutinib once daily. IgG levels were measured at the beginning of ibrutinib treatment and after first, second, third, sixth, ninth and twelfth month of ibrutinib treatment. IgG and IgA measurement was performed with Siemens, BN II System device using the nephelometric method, which is the measurement of the scattered light intensity at a fixed angle of 13–24 degrees. Intravenous immunoglobulin(IVIg) treatment was initiated in patients with frequent infections and IgG levels below 500 mg/dL. All of our patients with IgG levels < 500 mg/dL (n = 9, 33.3%) had frequent infections. Two (22.2%) of the infections were grade 4, 4 (44.4%) were grade 3 and 3 (33.3%) were grade 2. Six (66.6%) patients required hospitalization and all received iv antibiotic therapy. Only one (11.1%) patient required iv antifungal therapy. IVIg treatment was given to all of them, according to ESMO 2015 criteria [9]. We named this group of patients as SHG and compared it with those without SHG.
Age, gender, laboratory features, the follow up time, bulky disease, types and number of before ibrutinib treatments were compared. As genetic features, del (11q), del (13q), del (17p) and trisomy 12 were evaluated and detected by fluorescence in situ hybridization. ECOG performance status [10] and Cumulative Illness Rating Scale (CIRS) [11] was used to define performance status and comorbidities, respectively. Rai system was used for staging at baseline. For definition of response, relapse and refractory disease we used International Workshop on Chronic Lymphocytic Leukemia (IWCLL) 2018 criteria [1]. In these criteria, patients were categorized as complete remission (CR), partial remission (PR), progressive disease (PD) and stable disease (SD). In addition, increased lymphocytes alone are not a sign of treatment failure or cannot be considered PD [1]. Toxicity grading was conducted according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (U.S. Department of Health and Human Services, National Institutes of Health National Cancer Institute).
Our primary objective was to investigate the development and frequency of SHG in R/R CLL patients after ibrutinib monotherapy. Our secondary objectives were to assess overall survival (OS), progression-free survival (PFS) and disease response status after ibrutinib monotherapy. All the patients signed written informed consent allowing the use of their medical data in clinical research. This study was approved by Erciyes University Ethics Committee with the number of 2020/106.
Kolmogorov Smirnov test was used to assess the distribution of continous variables. Mann Whitney U test was used to compare nonparametric data; Student t test was used to compare parametric data. Parametric variables were expressed as Mean ± SD, non parametric variables were expressed as Median (Range). Chi-square or Fisher’s Exact test was used to compare categorical data. Binary logistic regression analysis was used to detect independent predictors. ROC curves were produced to assess the optimal predictive capability of baseline independent variables among dependent outcomes. The data were analysed by using Statistical Package for the Social Sciences (SPSS) for Windows computer program (release 25.0; SPSS Inc., Chicago, IL, USA). A two tailed p value of less than 0.05 was considered statistically significant in all tests.
Results
Twenty seven patients with R/R CLL were included in our study. Nine of them (33.3%) had SHG and received IVIg replacement, 18 of them (66.6%) didn’t have SHG. The median age of the patients with SHG, 5 of whom (55.5%) were female, was 61.1 ± 9.8 years, while the mean age of the non-SHG patients, 12 of whom(66.6%) were female, was 63.06 ± 9.2 years (p values 0.62 and 0.41 respectively). Baseline characteristics and laboratory values are provided in Table 1. The mean IgG level at the beginning of ibrutinib treatment was 830 mg/dL in the SHG arm, 1304 mg/dL in the without SHG arm (p = 0.004).
All patients underwent serological testing for HBV, HCV and HIV. Trimethoprim/sulfamethoxazole 800/160 mg 3 times a week for Pneumocystis jiroveci Pneumonia prophylaxis and valacyclovir 500 mg daily for herpetic infections prophylaxis were given to all patients. Antifungal prophylaxis treatment was not given as it interacts with ibrutinib via CYP3A.
Bulky disease, Rai stage, ECOG and CIRS are presented in Table 2. Both groups had similar characteristics in these regards (p > 0.05 for all). In order to evaluate genetic characterization, we performed del(17p), del(11q), del(13q) and trisomy 12 and there was no statistically significant difference (p values 0.14, 0.54 and 0.25 respectively). Although current guidelines recommend the IGHV mutation status assessment in every CLL patient [1], unfortunately we could not perform the IGHV mutation status in any of our patients since it was not studied in our clinic.
We evaluated IgG levels after ibrutinib monotherapy. After first, third, 6th and 12th months, mean IgG levels were 684, 531.3, 452 and 360.1 mg/dL respectively in SHG arm (p < 0.001) and 1156, 1058.2, 1012.8 and 886.9 mg/dL respectively in without SHG arm (p < 0.001) (Fig. 1) (Supplemental Table S2). It was observed that IgG levels decreased significantly in both groups after ibrutinib treatment.
IgA values were evaluated before initiation of ibrutinib treatment and one, six, and twelve months after initiation. The median IgA levels were 55 (23–182), 53 (22–112), 59 (20–85) and, 60 (20–199) mg/dL respectively in SHG arm and 173.5 (33–751), 152.5 (24–697), 147 (24–765) and, 155 (24–754) mg/dL respectively in without SHG arm (Supplemental Table S3). In the all patient population, the median IgA levels were 93 (22–697) at 1 month after ibrutinib and 104 (20–765) mg/dL at 6th month of ibrutinib (r = 0.94, p < 0.001) and, 108 (20–574) mg / dL at 12th month (r = 0.876, p = 0.001). Unlike IgG, there was a significant improvement in IgA at 6 months and 12 months after ibrutinib started.
Grade 3–4 adverse event (AE)s were observed in 11 (40.7%) of 27 patients. In SHG arm, all of patients had other ibrutinib related AEs but in other arm only 2 of them (11.1%) had (p < 0.001). The most common AE is pneumonia (Supplemental Table S1). Ibrutinib dose modification was performed in 8 (88.8%) patients in the SHG arm and 2 (11.1%) patients in the without SHG arm (p < 0.001), a total of 10 (37%) patients. Among 10 patients, 8 (80%) patients received 280 mg/d (2 patients due to grade 3 neutropenia, 2 patients due to grade 3 rash, 2 patient due to grade 3 diarrhea, 1 patient due to grade 3 trombocytopenia, and 1 patient due to grade 3 atrial fibrillation) and 1 (14.3%) patient received 140 mg/d due to grade 4 trombocytopenia and 1(14.3%) patient discontinued after intracranial hemorrhage. Dose modification was observed significantly more in the SHG arm. It was thought that the dose modification was due to the fact that more other AEs were observed in patients in the SHG arm and therefore the need for more modification.
One (11.1%) patient had CR in SHG arm but no patient in the other arm had. Six (33.3%) patients had PR in without SHG arm, 6 (66.6%) patients had PR in SHG arm. Eleven (61.1%) patients had SD in without SHG arm. One (5.5%) had PD in without SHG arm and 2 (22.2%) had PD in SHG arm. For comparison, CR was combined with PR and SD was combined with PD. In SHG arm 7 (77.7%) had CR + PR, in without SHG arm 6 (33.3%) patients had CR + PR. Two (22.2%) patients had SD + PD in SHG arm and 12 (66.6%) had SD + PD in without SHG arm (p = 0.029) (Table 2). Other ibrutinib related AEs were more frequent in patients with SHG after ibrutinib, but response to treatment was better.
SHG patients had significantly lower PLT and IgG levels at the beginning of ibrutinib treatment and higher follow up time (p < 0.05 for all, Table 1). However, in univariate and multivariate regression analysis, no difference was observed between two groups in PLT (p values 0.051 and 0.761 respectively) (Table 3). In multivariate analysis, a significant difference was observed for follow-up time and for the IgG value at the beginning of ibrutinib treatment (p values 0.004 and 0.001 respectively).
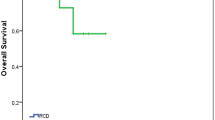
The median overall survival (OS) was 110.5 ± 8.8 months (95% CI 93 to 127.9) in the SHG arm and 123.4 ± 7.3 months (95% CI 109 to 137.7) in the without SHG arm (p = 0.951, log-rank test) (Fig. 2a). The median progression free survival (PFS) was 62.3 ± 11.2 months (95% CI 40.3 to 84.2) in the SHG arm and 71 ± 6.6 months (95% CI 58 to 83.9) in the without SHG arm (p = 0.637, log-rank test) (Fig. 2b). Three (11.1%) patients had progression and 2 of them had SHG and 1 of them had without SHG and all of their treatment switched to venetoclax.
IgG levels at the beginning of ibrutinib treatment is the best predicted value for SHG development in multivariate analysis (p = 0.001), also in ROC- curve analysis AUC:0.87 ± 0.6 (95% CI 07–1.0), (p = 0.002) (Supplemental Figure S1). The cut off value of the IgG level at the beginning of the ibrutinib treatment is 650 mg/dL and its negative predictive value is 94.4%.
Discussion
CLL is characterized by immune defects such as SHG, T and natural killer(NK) cell dysfunction and phagocytic variances [12]. SHG is one of the most common causes of immune dysregulation. The frequency of SHG development in CLL has been investigated in several studies. In the study of Shvidel et al., 11% of patients had low IgG levels [13]. In two different studies, they reported HG in 10% to 44% of previously treated CLL patients [14, 15]. In other studies, while 25% of the patients diagnosed had SHG, it was observed that it increased up to 85% during the course of the disease [2, 3, 6, 16]. In our study, the rate of SHG was 33.3%.
SHG may develop either due to CLL itself or treatments used in CLL. Treatments such as anti-CD20 antibodies, BTK inhibitors and phosphoinositide 3 kinase δ inhibitors have effective results and are increasingly used in CLL, but can cause SHG [12, 17]. Treatment of CLL has changed drastically until today, and its registration to these drugs is why SHG in CLL should be investigated in more detail.
Ibrutinib is an orally bioavailable, irreversible and selective potent inhibitor of the BTK enzyme [7, 18]. In both our study and all other studies, ibrutinib treatment has been shown to have robust efficacy in R / R CLL patients [19,20,21]. Ibrutinib can cause SHG due to its mechanism of action. Some studies have shown that SHG development does not occur during the use of ibrutinib in CLL patients [18, 19, 22]. But Sun et al. showed that IgG levels remained stable in the first 6 months after ibrutinib, but there was a significant decrease after 1 year, this decrease deepened in 24 months but remained at similar levels in 24 and 36 months [12]. In our study, the IgG levels decreased every month after ibrutinib treatment, and this decrease was profound after 12 months (p < 0.001) (Fig. 1). Unlike IgG, there was a significant improvement in IgA levels 6 months and 12 months after ibrutinib started, consistent with other studies [18, 22]. Sun et al. observed an increase in IgA levels in 45% of patients after 12 months of ibrutinib started and in 64% after 24 months [12].
SHG is very important in CLL patients because it predisposes to infections. Infections are the most important cause of mortality and morbidity in patients with CLL. Infections are responsible for 30 to 50% deaths [2, 3]. The recommended treatments to prevent infection in SHG include prophylactic antibiotics, vaccination and Ig replacement [23]. Among these treatments, only Ig replacement has been shown to reduce major infections in SHG [24]. In our study, frequent infections decreased in 7 (77.7%) patients after IVIg. IVIg reduces infections with complement-mediated pathogen effects, toxin opsonization and inactivation and bactericidal neutralization [25]. IVIg also has in vivo advantageous immunomodulatory effects on CLL cells. But the most important problem is the cost of Ig replacement. IVIg's cost effectiveness is still controversial. Therefore, when and how IVIg treatment will be given to CLL patients have been investigated in randomized clinical trials [26, 27]. In all of these trails, IVIg has been shown to significantly reduce the rate of bacterial infections and prolong the time to the first infection. And the recommended dose is 0.4 g/ kg/ 3 to 5 weeks. IVIg was given to our patients with SHG as 0.4 g/kg/4 weeks.
In current studies and guidelines, IVIg treatment is recommended when there is severe hypogammaglobulinemia (IgG level below than 400 or 500 mg/dL) and recurrent infections. We gave IVIg treatment when our patients had IgG levels below 500 mg/dL and recurrent infections. In multivariate analysis, the IgG level at the beginning of the ibrutinib treatment was most effective value in predicting SHG development (p = 0.001) (Table 3). In a study related to Rituximab, it was shown that those with low IgG levels at the beginning of treatment decreased more after the treatment and had a higher infection rate [28]. In Greek study, IgG levels were normal during the course of treatment in those who were normal at baseline [19]. In our study, the cut off value of at the beginning of the ibrutinib treatment IgG level was 650 mg/dL in ROC- curve analysis (AUC:0.87 ± 0.6 CI 07–1.0, p:0.002) and its negative predictive value was 94.4%. For this reason, it seems more reasonable to start Ig replacement for patients with R/R CLL who have IgG values below 650 mg / dL and have recurrent infections when beginning of the treatment with ibrutinib.
There are some limitations in our study. Due to the retrospective nature of our study, NK, B and T cells, antibody production deficits and IgG subclass of all patients could not be examined. Some studies reported that the IgG subclass is better at showing the risk of infection than IgG. Although one study demonstrated that IgG1 and IgG3 were better at correlating recurrent infection [5], another study showed that IgG2 and IgG4 were better [29]. On the other hand, there was no correlation between IgG subclass and infections in some studies [30]. Another our limitation is that IGVH mutation can't be examined in our country. Also, the small number of patients is an our limitation. We think that our report is very promising. However, since our study is a single center experience, further large scale multicenter prospective studies are needed.
Conclusion
We reported a significant decrease in IgG values after ibrutinib monotherapy in patients with R/R CLL. This decrease occurs every month after the use of ibrutinib, but at most after 1 year. Other ibrutinib related AEs were more frequent in patients with SHG but response rate to treatment is higher in patients with SHG. There was no significant difference in OS and PFS between patients with and without SHG. In patients with R/R CLL, the most important factor in predicting SHG was the IgG level at the beginning of treatment. For this reason, it is more reasonable to initiate Ig replacement and follow up closely in patients with R/R CLL who have recurrent infections with IgG values below 650 mg/dL when starting ibrutinib treatment.
References
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H et al (2018) iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood J Am Soc Hematol 131(25):2745–2760
Hamblin A, Hamblin T (2008) The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull 87(1):49–62
Dhalla F, Lucas M, Schuh A, Bhole M, Jain R, Patel SY et al (2014) Antibody deficiency secondary to chronic lymphocytic leukemia: should patients be treated with prophylactic replacement immunoglobulin? J Clin Immunol 34(3):277–282
Mauro FR, Morabito F, Vincelli ID, Petrucci L, Campanelli M, Salaroli A et al (2017) Clinical relevance of hypogammaglobulinemia, clinical and biologic variables on the infection risk and outcome of patients with stage A chronic lymphocytic leukemia. Leuk Res 57:65–71
Freeman JA, Crassini KR, Best OG, Forsyth CJ, Mackinlay NJ, Han P et al (2013) Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leuk Lymphoma 54(1):99–104
Parikh SA, Leis JF, Chaffee KG, Call TG, Hanson CA, Ding W et al (2015) Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: natural history, clinical correlates, and outcomes. Cancer 121(17):2883–2891
Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC et al (2007) Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem Chem Enabl Drug Discov 2(1):58–61
Network NCC. Chronic lymphocytic leukemia, small lymphocytic leukemia 2019, December 20. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf
Eichhorst B, Robak T, Montserrat E, Ghia P, Hillmen P, Hallek M et al (2015) Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(5):v78–v84
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–656
Parmelee PA, Thuras PD, Katz IR, Lawton MP (1995) Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc 43(2):130–137
Sun C, Tian X, Lee YS, Gunti S, Lipsky A, Herman SE et al (2015) Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood J Am Soc Hematol 126(19):2213–2219
Shvidel L, Tadmor T, Braester A, Bairey O, Rahimi-Levene N, Herishanu Y et al (2014) Serum immunoglobulin levels at diagnosis have no prognostic significance in stage A chronic lymphocytic leukemia: a study of 1113 cases from the Israeli CLL Study Group. Eur J Haematol 93(1):29–33
Davey FR, Kurec AS, Tomar RH, Smith JR (1987) Serum immunoglobulins and lymphocyte subsets in chronic lymphocytic leukemia. Am J Clin Pathol 87(1):60–65
Rozman C, Montserrat E, Viñolas N (1988) Serum immunoglobulins in B-chronic lymphocytic leukemia. Nat Hist Prognost Sig Cancer 61(2):279–283
Griffiths H, Lea J, Bunch C, Lee M, Chapel H (1992) Predictors of infection in chronic lymphocytic leukaemia (CLL). Clin Exp Immunol 89(3):374–377
Doan A, Pulsipher MA (2018) Hypogammaglobulinemia due to CAR T-cell therapy. Pediatric Blood Cancer 65(4):e26914
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA et al (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369(1):32–42
Dimou M, Iliakis T, Pardalis V, Bitsani C, Vassilakopoulos TP, Angelopoulou M et al (2019) Safety and efficacy analysis of long-term follow up real-world data with ibrutinib monotherapy in 58 patients with CLL treated in a single-center in Greece. Leuk Lymphoma 60(12):2939–2945
Winqvist M, Asklid A, Andersson P-O, Karlsson K, Karlsson C, Lauri B et al (2016) Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica 101(12):1573–1580
Ysebaert L, Aurran-Schleinitz T, Dartigeas C, Dilhuydy MS, Feugier P, Michallet AS et al (2017) Real-world results of ibrutinib in relapsed/refractory CLL in France: early results on a large series of 428 patients. Am J Hematol 92(8):E166
O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA et al (2014) Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol 15(1):48–58
Agostini C, Blau I-W, Kimby E, Plesner T (2016) Prophylactic immunoglobulin therapy in secondary immune deficiency—an expert opinion. Expert Rev Clin Immunol 12(9):921–926
Lachance S, Christofides A, Lee J, Sehn L, Ritchie B, Shustik C et al (2016) A Canadian perspective on the use of immunoglobulin therapy to reduce infectious complications in chronic lymphocytic leukemia. Curr Oncol 23(1):42
Kaveri S, Maddur M, Hegde P, Lacroix-Desmazes S, Bayry J (2011) Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clin Exp Immunol 164:2–5
Boughton B, Jackson N, Lim S, Smith N (1995) Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clin Lab Haematol 17(1):75–80
Chapel H, Dicato M, Gamm H, Brennan V, Ries F, Bunch C et al (1994) Immunoglobulin replacement in patients with chronic lymphocytic leukaemia: a comparison of two dose regimes. Br J Haematol 88(1):209–212
De La Torre I, Leandro MJ, Valor L, Becerra E, Edwards JC, Cambridge G (2012) Total serum immunoglobulin levels in patients with RA after multiple B-cell depletion cycles based on rituximab: relationship with B-cell kinetics. Rheumatology 51(5):833–840
Aittoniemi J, Miettinen A, Lainf S, Sinisalo M, Laippala P, Vilpo L et al (1999) Opsonising immunoglobulins and mannan-binding lectin in chronic lymphocytic leukemia. Leuk Lymphoma 34(3–4):381–385
Fabbiani M, Mondi A, Colafigli M, D’Ettorre G, Paoletti F, D’Avino A et al (2014) Safety and efficacy of treatment switch to raltegravir plus tenofovir/emtricitabine or abacavir/lamivudine in patients with optimal virological control: 48-week results from a randomized pilot study (Raltegravir Switch for Toxicity or Adverse Events, RASTA Study). Scand J Infect Dis 46(1):34–45
Acknowledgements
The author would like to acknowledge the assistance of Fatih Demirkan, MD and Leylagül Kaynar, MD in the editing process and Ali Ünal,MD in the submission process.
Funding
The authors declared that this study received no financial support.
Author information
Authors and Affiliations
Contributions
Each author has contributed substantially to the research, preparation and production of the paper and approves of its submission to the Journal. All authors: Final approval of manuscript. Concept: SÇ, LK; Design: SÇ; Data Collection or Processing: SÇ, MK, MB, ZTG; Analysis or Interpretation: SÇ, AÜ, MÇ, LK, FD; Literature Search: SÇ, LK; Writing: SÇ.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Çelik, S., Kaynar, L., Güven, Z.T. et al. Secondary Hypogammaglobulinemia in Patients with Chronic Lymphocytic Leukemia Receiving Ibrutinib Therapy. Indian J Hematol Blood Transfus 38, 282–289 (2022). https://doi.org/10.1007/s12288-021-01466-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-021-01466-1