Abstract
There is lack of data on iron metabolism in critically ill sepsis children from population with high prevalence of iron deficiency (ID). The study was designed to study impact of sepsis on iron parameters in children with ID. Sepsis patients (age 6–59 months) and their apparently healthy sibling/cousin as controls were enrolled in this case–control pilot study. Serum iron, TIBC, transferrin saturation, ferritin and sTfR were measured in the two groups. sTfR-Ferritin index was calculated. Patients (n = 134) were significantly underweight compared to controls (n = 54) (WAZ score < − 2; 58% vs. 28%; p < 0.001). Serum iron and sTfR (mg/L) were lower [71.5 (51.0, 115.0) vs. 87.0 (64.5, 130.5), p = 0.068; 3.1 (2.1, 4.5) vs. 3.5 (2.8, 4.8), p = 0.026 respectively] while serum ferritin was higher [229 (94, 484.5) vs. 22 (9.2, 51); p < 0.001] in patients compared to controls. sTfR-Ferritin index was lower in patients [1.3 (0.8, 2.3) vs. 2.5 (1.8, 4.5); p < 0.001]. ROC AUC (patients vs. controls) were 0.89 (95% CI 0.83–0.95) and 0.76 (95% CI 0.68–0.85) for ferritin and sTfR-ferritin index respectively. Survivors and non-survivors were similar in terms of iron parameters. Sepsis-induced alterations in iron parameters among ID children are complex. Qualitatively it is similar (with quantitative differences) to non-ID adult population. Lack of correlation of iron parameters with mortality may be due to ID-associated immune dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory cytokines alter iron metabolism to create a state of ‘stress hypoferremia’ characterized by low serum iron, total iron binding capacity (TIBC) and high serum ferritin, [1]. It is advantageous during sepsis as microorganisms are deprived of free iron for growth and multiplication [2]. Iron is also essential for optimal functioning of innate and adaptive immunity, and thus iron deficiency (ID) may affect host immune response against microorganisms as well [3, 4]. Iron-supplementation led to higher infection-related deaths in community-based trials [5,6,7] and guidelines recommend avoiding iron for first 2 weeks in management of malnourished children [8, 9]. However, evidence is mounting to support iron therapy in the select group of critically ill patients [10], even in a population with low prevalence (9%) of absolute ID [2, 10]. Iron therapy is likely to be more effective in critically ill patients from communities with high prevalence of pre-existing nutritional ID. Prevalence of iron deficiency anemia (IDA) in Indian children (age, 6–35 months) is 60–70% [11]. It may translate to almost universal ID as individuals begin to suffer well before development of anemia [12].
Available studies on effect of sepsis (and other inflammatory conditions) on iron parameters are from adult patients from population with low prevalence of ID [2, 13, 14]. Data are scanty on effect of sepsis on iron parameters in children [15] and patients from communities with high prevalence of ID [16]. Understanding the pathophysiology of iron metabolism in sepsis children from a population with the high prevalence of malnutrition and ID is urgently needed [17]. This study was designed to know the effect of acute infections on iron parameters in Indian children, with intention to provide baseline data, and monitoring tools for interventional trials on iron supplementation in critically ill sepsis children in low- and middle- income countries.
Materials and Methods
Clinical Setting and Patients
This cross-sectional case–control pilot study was performed in pediatric emergency room (PER) and pediatric intensive care unit (PICU) of a tertiary care urban teaching hospital of a developing country over 13 months’ period (November 2009–November 2010). Inclusion criteria included patients with > 6 months and < 59 months with sepsis [defined by systemic inflammatory response syndrome (SIRS) caused by clinically suspected infection] [18]. Consequent patients were planned to be enrolled within 24 h of getting intubated and ventilated. Apparently healthy sibling/cousin of patients (age > 6–59 months; coming from the same family background and sharing the same kitchen) were taken as controls. Patients with parents/guardians not willing to give written consent for enrollment in the study, patient/sibling having received iron supplementation or blood transfusion during the last 6 months, having hemolytic anemia or a chronic ailment were excluded from the study. The Institutional Ethics Committee approved the study protocol, and written informed consent was obtained from parents or legal guardians. The manuscript was approved by the Departmental Review Board.
Data Collection and Clinical Care of Patients
Demographic, nutritional, clinical and hematological parameters [using an automated blood cell counter (SF 3000 Sysmex or LH 750 Beckman Coulter)] and peripheral blood smear examination data were recorded at enrolment for patients and controls. Blood samples (4–5 ml) were drawn within 2 days of enrolment in morning hours (08.00–09.00 a.m.) and collected in an iron-free tube. Serum was separated via centrifugation and immediately stored at − 20 °C. Serum iron and TIBC were measured by a modification of method recommended by the International Committee for Standardization in Haematology (ICSH) [19] and are based on the development of a colored complex when ferrous iron is treated with a chromogen solution. Percent of transferrin saturation [20] was calculated using these two parameters. Serum Ferritin was measured by Immunometric Enzyme Immunoassay for the quantitative determination of ferritin in serum by ELISA Kit (ORGENTEC Diagnostika GmbH, Germany). Serum for soluble Transferrin Receptor (sTfR) was stored at − 80 °C and measured by Quantikine® IVD® ELISA kit (R&D Systems, USA) at end of the study. As per manufacturer, the kit’s assay range is 3–80 nmol/L (= 0.26–6.80 mg/L), while median for non-blacks living within 300 m of sea-level is 18.4 (95% CI 8.7–28.1) nmol/L [= 1.564 (95% CI 0.74–2.39) mg/L]. The sTfR-Ferritin index was calculated as the ratio of sTfR (mg/L) to the log10 of serum ferritin (ng/mL) [21]. Iron deficiency was defined as serum ferritin less than 30 ng/mL [22]. Patients were managed as per unit protocol in PER followed by transfer to PICU as and when the beds became available. Subsequently, patients were shifted to pediatric wards from where they got discharged.
Statistical Analysis
Systematic sample size calculation was not done for the lack of literature on effect of sepsis on iron parameters in children from a region with high prevalence of iron deficiency. We planned for a sample size of convenience. Continuous and categorical variables were described by median with interquartile range (IQR) and number (percentage). Inter-group comparisons were performed by Mann–Whitney U test and Chi square test/Fisher exact test respectively. Correlations between variables were examined by Spearman’s correlation test and shown by multi-correlation plots. Receiver operating characteristic (ROC) curves were drawn to define sensitivity and specificity for different cut-offs of iron parameters in differentiating patients from controls, and survivors from non-survivors among patients. The area under curve (AUC) was computed from ROC curves and DeLong method was used to define 95% CI. The optimum cut-off was determined based on Youden’s J statistic. A p value of < 0.05 indicated statistical significance. R statistical software (version 3.5.2) [23] with pROC [24], ggplot2 [25], readxl [26], Ggally [27] and gtsummary [28] were used for statistical analysis.
Results
A total of 134 sepsis patients, and one sibling each of 54 patients were enrolled as controls. Remaining 80 patients either did not have any sibling/cousin with defined criteria, not accompanying the patient during hospital admission, parents did not give consent for inclusion of sibling or their parents could not bring the sibling/cousin despite repeated requests. Patients were symptomatic for a median duration of 5 days (IQR 3, 10) days prior to admission to our hospital. Pneumonia was the most common diagnosis (35.6%, n = 47) followed by pyogenic sepsis with/without shock (34.1%, n = 45), acute central nervous system (CNS) infection (19.7%, n = 26) and sepsis without focus (9.1%, n = 12). Their critical care needs included oxygen therapy only (22%, n = 30), shock management (10%, n = 14), assisted ventilation (15%, n = 20) and management of raised intracranial pressure (22%, n = 29). Thirteen (9.7%) patients died. Demographic characteristics, nutritional status, blood counts and red cell indices of patients and control are shown in Table 1. Patients were significantly more malnourished compared to controls. More than half of the patients were underweight compared to a-fourth among the controls (58% vs. 28%, p < 0.001), while median height for age Z-score was significantly lower among patients (p = 0.017). Similarly, hematological parameters suggested higher incidence as well as severity of microcytic hypochromic anemia among patients compared to controls (Table 1). Macrocytes were seen in significantly more patients compared to controls in the peripheral blood smear (23% vs. 7.5%, p = 0.029). Mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) were similar in the two groups. However, significantly lower mean corpuscular hemoglobin concentration (MCHC) in patients suggest more severe ID.
Iron parameters are shown in Table 2. Controls had median serum ferritin of 22 ng/ml (IQR 9.2, 51.0). Thirty-three (61%) children had iron deficiency (serum ferritin < 30 ng/ml). Fourteen children (27%) had depleted iron stores (serum ferritin < 13 ng/ml), 11 of whom had serum ferritin less than 3 ng/ml. Almost all had TIBC higher than the upper normal reference level, while transferrin saturation lower than the lower normal reference level. Median sTfR was also substantially elevated compared to the normal upper reference range given by the manufacturer (median, 18.4 nmol/L; 95% CI 8.7–28.1). Median TfR-Ferritin index in controls was 2.5 (IQR 1.8, 4.5). Surprisingly, serum iron was within normal range in the majority of controls (90.7%) despite being significantly iron deficient. sTfR and sTfR-Ferritin index were negatively correlated with hemoglobin (rho = − 0.44, p = 0.005; r = − 0.34, p = 0.04, respectively), but not the ferritin (rho = 0.20, p = 0.174). Patients had significantly higher ferritin (median, 229 ng/mL vs. 22 ng/mL, p < 0.001), lower TIBC (median, 424.5 mcg/dL vs. 536 mcg/dL; p < 0.001) but similar transferrin saturation compared to controls. Serum iron was lower in patients compared to controls but it could not reach statistical significance (p = 0.068). sTfR was elevated in both groups compared to the upper normal reference level. Patients had significantly lower sTfR (p = 0.026) and sTfR-Ferritin index (p < 0.001) compared to controls. Among patients, none of ferritin, sTfR and sTfR-Ferritin index were correlated with hemoglobin (rho = 0.06, p = 0.51; rho = − 0.05, p = 0.59; rho = − 0.06, p = 0.55 respectively).
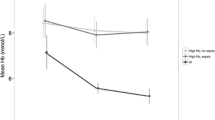
Ferritin has ROC AUC of 0.89 (95% CI 0.83–0.95) in differentiating patients from controls (Fig. 1). Ferritin cut off of 61 ng/mL has sensitivity of 0.86 and specificity of 0.90 in picking up patients from an iron deficient population. sTfR and serum iron were close to the diagonal line of neutrality [AUC (95% CI), 0.63 (0.53–0.71) and 0.59 (0.50–0.68) respectively). The sTfR-ferritin index performed poorer than ferritin in differentiating patients from controls (ROC AUC 0.76; 95% CI 0.68–0.85). sTfR-Ferritin index cut off of 1.40 has sensitivity of 0.55 and specificity of 0.95 in picking up patients from an ID population. Serum ferritin was positively correlated with total leucocyte count (TLC) and absolute neutrophil count (ANC) (rho = 0.25, p = 0.005; rho = 0.02, p = 0.20 respectively) while sTfR and sTfR-ferritin index were negatively correlated [(TLC; rho = − 0.15, p = 0.117; rho = − 0.20, p = 0.03 respectively) (ANC; rho = − 0.20, p = 0.04; r = − 0.23, p = 0.02 respectively)].
There were differences between iron parameters among survivors (n = 120) and non-survivors (n = 14), though none were statistically significant probably due to small numbers in the latter group. Serum iron [71 (51.0–114.0) vs. 88 (51.8–115.8), p = 0.9], transferrin saturation [17.0 (12.0–27.8) vs. 20.0 (16.0–24.5), p = 0.7] and ferritin [228 ng/ml (97–459) vs. 368 ng/ml (78–832); p = 0.6] were lower in survivors, while TIBC was higher [428.5 (347.0–540.8) vs. 407.0 (364.2–525.2), p = 0.7]. Ferritin > 500 ng/ml was associated with higher (though statistically not significant) mortality [5/31 (16.1%) vs. 8/95 (8.4%), RR 1.92; 95% CI 0.6705.43; p = 0.22)]. Higher sTfR among non-survivors [35.5 (24.1–52.1) vs. 48.9 (29.4–67.5); p = 0.3] may indicate more severe iron deficiency compared to survivors. sTfR-ferritin index was higher among non-survivors [1.3 (0.8, 2.2) vs. 1.6 (0.9, 2.4), p = 0.6)]. None of the iron parameters could predict mortality in patients of the study cohort (Fig. 1). Correlations between various iron parameters in the two groups is shown in the Supplemental Figure.
Discussion
The study provides insight into response of hematological and iron parameters to sepsis in a pediatric population with high prevalence of ID. Controls were largely ID, as 60% of them had serum ferritin < 30 ng/mL, while a fourth had serum ferritin < 13 ng/mL. Majority of patients had microcytic hypochromic anemia, and was significantly worse compared to controls in terms of hemoglobin, peripheral smear and red cell indices similar to our previous observation [29]. Children with more severe ID in a family may be more predisposed to infections and sepsis, as majority of cases were ill just for a week which is unlikely to affect hematological parameters. Macrocytes on peripheral blood smear in significant number of patients and controls support concurrent deficiency of vitamin B12 and folate. These concurrent deficiencies may worsen ID-induced fall in erythropoiesis and complicate iron metabolism further.
Patients had elevated ferritin, decreased TIBC and serum iron compared to controls similar to observations in critically ill adults [2], Transferrin saturation remained unaltered. sTfR and sTfR-Ferritin index were significantly lower in patients. Preserved serum iron level is surprising, and is difficult to explain considering the prevalence and degree of IDA in the study cohorts. TIBC was significantly lower in patients, more so among non-survivors. TIBC values were higher than normal reference level in majority of patients (75/124, 60%) suggesting ID to be a stronger factor to affect (in bringing up) TIBC compared to sepsis (in bringing it down). Though transferrin saturation usually decreases during inflammation [1, 30, 31], significant fall in TIBC and relatively preserved serum iron was probably responsible for lack of change in transferrin saturation in our patients. Similar finding was observed in a study on critically ill adult patients [2].
Despite having ID, serum ferritin increased significantly during sepsis especially among non-survivors. Inflammatory cytokines seem to be a stronger stimulus in affecting serum ferritin than ID. Ferritin could predict presence of sepsis in the studied cohort of the malnourished and ID population with excellent discrimination (AUC 0.89). Serum ferritin was shown to correlate positively with inflammatory markers while serum iron and transferrin to correlate negatively [2, 29]. We also demonstrated serum ferritin to be positively correlated with TLC and ANC in patients. C-reactive protein (CRP) and procalcitonin were not available in our hospital during the study. Higher ferritin level (> 500 ng/mL) was associated with increased mortality as observed in a previous study [29].
Significantly elevated sTfR, in both the groups, indicates completely depleted iron stores [12, 21]. With such depleted iron stores, serum ferritin usually gets plateaued, while sTfR continues to be a sensitive indicator of iron status [12]. sTfR is considered to largely remain unaffected by inflammation [32]; however available in vitro data are contradictory [33]. In two clinical studies, reduction in sTfR (mean ± SD) was documented in ID patients with and without inflammatory diseases—5.1 ± 2.0 vs. 6.2 ± 3.5 mg/L [21] and 3.13 ± 1.11 vs. 4.36 ± 1.23 mg/L [34]. Acute malaria was also shown to decrease sTfR [35, 36]. In the current study, sTfR was not only significantly reduced in patients but also was negatively correlated with TLC and ANC. Infection induced suppression of erythropoiesis may be an explanation for this fall in sTfR; significantly lesser hemoglobin compared to controls may support the hypothesis. However, the prediction of sepsis (AUC 0.62) and death (AUC 0.61) among children with ID was low which may argue against it being affected by inflammation. These contradictions need further exploration with larger studies. Higher sTfR among non-survivors may suggest contributory role of severe ID associated immune dysfunction in the clinical outcome.
Considering the reciprocal relationship between sTfR and serum ferritin, the sTfR-Ferritin index seems to summarize the iron metabolism during all stages of depleting iron stores with a linear correlation; the higher value indicates more deficiency [12, 37]. Due to minimal interference of inflammation on sTfR [32], sTfR-Ferritin index is used to identify absolute ID in anemic patients with chronic inflammatory diseases; higher value supports the former [13, 14, 38]. Lower sTfR-Ferritin index in ID patients with inflammatory diseases compared to those without in two previous studies [21, 34] were similar to our findings. sTfR-Ferritin index may be useful in identifying subclinical infection/inflammation in tropical countries with a high prevalence of nutritional ID; lower values may support presence of inflammation.
Higher serum iron [2], ferritin [2, 29] and transferrin saturation [2] and lower transferrin/TIBC [2] are associated with higher mortality as seen in our study as well. It is probably due to failure of sepsis-induced ‘stress hypoferremia’ [2]. Lack of statistical significance may be due to small sample size, lesser level of sickness and lesser mortality compared to other studies [2, 29]. High prevalence of severe ID and associated vitamin B12 and folate deficiency [11] might have precluded the role of iron parameters in predicting the mortality. Further, dysfunctional innate and adaptive immunity due to long-term multiple nutritional deficiencies [39] might also have blunted the impact of failed iron homeostasis on mortality.
Recruiting apparently healthy siblings as control is a strength, as it has provided information about socio-economic, cultural and nutritional backgrounds of patients. Though data were collected about a decade back, nutritional and hematological parameters of enrolled children are similar to those reported in a recently conducted study from the same center [29]. Measuring inflammatory markers (e.g., CRP and procalcitonin) and hepcidin and correlating them with iron parameters would have provided better understanding of iron metabolism in the studied population. However, these were not available to us during the study period. Enrolling children with normal iron status as another control and excluding children with vitamin B12 and folate deficiencies would have added more value and provided more specific information. Imprecision and assay drift with sTfR ELISA kit used in this study [32] may limit the internal validity while small sample size may limit the external validity.
Conclusions
Majority of sepsis patients were malnourished and had ID. Sepsis-induced alterations in iron parameters were complex and seems to be qualitatively similar (with quantitative differences) to that in non-ID adult population. sTfR seems to be affected by (degree of) inflammation. Ferritin performed better in identifying sepsis compared to sTfR-Ferritin index. There was no correlation between iron parameters and mortality. More data on larger sample is required to validate these findings.
References
Darveau M, Denault AY, Blais N et al (2004) Bench-to-bedside review: iron metabolism in critically ill patients. Crit Care 8:356–362
Tacke F, Nuraldeen R, Koch A et al (2016) Iron parameters determine the prognosis of critically ill patients. Crit Care Med 44:1049–1058
Piagnerelli M, Boudjeltia KZ, Gulbis B, Vanhaeverbeek M, Vincent JL (2007) Anemia in sepsis: the importance of red blood cell membrane changes. Transfus Altern Transfus Med 9:143–149
Cherayil BJ (2011) The role of iron in the immune response to bacterial infection. Immunol Res 50:1–9
Sazawal S, Black RE, Ramsan M et al (2006) Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367:133–143
Veenemans J, Milligan P, Prentice AM et al (2011) Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med 8:e1001125
Soofi S, Cousens S, Iqbal SP et al (2013) Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 382:29–40
Bhatnagar S, Lodha R, Choudhury P et al (2007) IAP guidelines 2006 on hospital based management of severely malnourished children (adapted from the WHO Guidelines). Indian Pediatr 44:443–461
Ashworth A, Khanum S, Jackson A, Schofield C. Guidelines for the inpatient treatment of severely malnourished children. 1. Guidelines for the inpatient treatment of severely malnourished children. World Health Organization 2003. Available at https://www.who.int/nutrition/publications/guide_inpatient_text.pdf. Accessed on April 1, 2020
Shah A, Roy NB, McKechnie S et al (2016) Iron supplementation to treat anaemia in adult critical care patients: a systematic review and meta-analysis. Crit Care 20:306
Gonmei Z, Toteja GS (2018) Micronutrient status of Indian population. Indian J Med Res 148:511–521
Skikne BS, Flowers CH, Cook JD (1990) Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 75:1870–1876
Shin DH, Kim HS, Park MJ et al (2015) Utility of access soluble transferrin receptor (sTfR) and sTfR/log ferritin index in diagnosing iron deficiency anemia. Ann Clin Lab Sci 45:396–402
Oustamanolakis P, Koutroubakis IE, Messaritakis I et al (2011) Soluble transferrin receptor-ferritin index in the evaluation of anemia in inflammatory bowel disease: a case–control study. Ann Gastroenterol 24:108–114
Krawiec P, Pac-Kożuchowska E (2019) Soluble transferrin receptor and soluble transferrin receptor/log ferritin index in diagnosis of iron deficiency anemia in pediatric inflammatory bowel disease. Dig Liver Dis 51:352–357
Goyal R, Das R, Bambery P et al (2008) Serum transferrin receptor-ferritin index shows concomitant iron deficiency anemia and anemia of chronic disease is common in patients with rheumatoid arthritis in North India. Indian J Pathol Microbiol 51:102–104
Murthy S, Kissoon N (2018) Hyperferritinemia in sepsis in children: ironing out the global details. Pediatr Crit Care Med 19:692–693
Goldstein B, Giroir B, Randolph A (2005) International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8
Revised recommendations for the measurements of the serum iron in human blood (1990) Iron panel of the international committee for standardization in haematology. Br J Haematol 75:615–616
Saarinen UM, Siimes MA (1977) Developmental changes in serum iron, total iron-binding capacity, and transferrin saturation in infancy. J Pediatr 91:875–877
Punnonen K, Irjala K, Rajamäki A (1997) Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 89:1052–1057
Lopez A, Cacoub P, Macdougall PC, Peyrin-Biroulet L (2016) Iron deficiency anaemia. Lancet 387:907–916
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Robin X, Turck N, Hainard A et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform 12:77
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wickham H, Bryan J (2019) readxl: Read Excel Files. R package version 1.3.1.2019. Available at https://CRAN.R-project.org/package=readxl
Schloerke B, Crowley J, Cook D, Briatte F, Marbach M, Thoen E, Elberg A, Larmarange J (2020) GGally: Extension to ‘ggplot2’. R package version 1.5.0. 2020. Available at https://CRAN.R-project.org/package=GGally
Sjoberg DD, Hannum M, Whiting K, Zabor EC (2019) gtsummary: presentation-ready data summary and analytic result tables. R package version 1.2.4. 2019. Available at https://CRAN.R-project.org/package=gtsummary
Ghosh S, Baranwal AK, Bhatia P et al (2018) Suspecting hyperferritinemic sepsis in iron-deficient population: do we need a lower plasma ferritin threshold? Pediatr Crit Care Med 19:e367–e373
Heming N, Montravers P, Lasocki S (2011) Iron deficiency in critically ill patients: highlighting the role of hepcidin. Crit Care 15:210
Finch CA, Huebers H (1982) Perspectives in iron metabolism. N Engl J Med 306:1520–1528
Worwood M (2002) Serum transferrin receptor assays and their application. Ann Clin Biochem 39:221–230
Fahmey M, Young SP (1993) Modulation of iron metabolism in monocyte cell line U937 by inflammatory cytokines: changes in tranferrin uptake, iron handling and ferritin mRNA. Biochem J 296:175
Marković M, Majkić-Singh N, Ignjatović S, et al (2007) Reticulocyte haemoglobin content vs. soluble transferrin receptor and ferritin index in iron deficiency anaemia accompanied with inflammation. Int J Lab Hematol 29:341–346
Beesley R, Filteau S, Tomkins A et al (2000) Impact of acute malaria on plasma concentrations of transferrin receptors. Trans R Soc Trop Med Hyg 94:295–298
Williams TN, Maitland K, Rees DC et al (1999) Reduced soluble transferrin receptor concentrations in acute malaria in Vanuatu. Am J Trop Med Hyg 60:875–878
Olivares M, Walter T, Cook JD et al (2000) Usefulness of serum transferrin receptor and serum ferritin in diagnosis of iron deficiency in infancy. Am J Clin Nutr 72:1191–1195
Skikne BS, Punnonen K, Caldron PH et al (2011) Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: a prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am J Hematol 86:923–927
Maggini S, Pierre A, Calder PC (2018) Immune function and micronutrient requirements change over the life course. Nutrients 10:1531. https://doi.org/10.3390/nu10101531
Acknowledgements
This study was performed at Division of Pediatric Critical Care, Advanced Pediatrics Center, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Author information
Authors and Affiliations
Contributions
Arun Kumar Baranwal: Conceptualization, Study design, Fund Acquisition, Data curation, Literature search, Original and Final draft preparation, Supervision of Statistical analysis, Guarantor. Reena Das: Conceptualization, Methodology, Laboratory analysis, Supervision, Critical review and Editing. Ramachandran Rameshkumar: Data collection, Data curation, Review and Editing. Praveen Kumar-M: Statistical analysis, Data curation, Software. Prateek Bhatia: Literature search, Review and Editing. Ashwini Nair: Literature search, Data curation, Statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary Figure 1
Multi-correlation plot between different iron parameters for patients and control. Spearman correlation coefficient [rho (ρ)] and associated p values are mentioned for each comparison. Density plots are depicted in the diagonal line.(JPEG 2888 kb)
Rights and permissions
About this article
Cite this article
Baranwal, A.K., Das, R., Rameshkumar, R. et al. Effect of Sepsis on Iron Parameters in a Population with High Prevalence of Malnutrition and Iron Deficiency: A Cross-Sectional Case–Control Pilot Study. Indian J Hematol Blood Transfus 37, 609–615 (2021). https://doi.org/10.1007/s12288-020-01393-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-020-01393-7