Abstract
The aim of this study is to evaluate the results of relapsed or refractory Hodgkin (HL) and non-Hodgkin Lymphomas (NHL) patients who underwent autologous stem cell transplantation supported high-dose chemotherapy (HDC–ASCT). Forty patients who received HDC–ASCT between November 2004 and February 2014 for relapsed or refractory HL and NHL were analysed retrospectively. There were 22 patients with HL and 18 patients with NHL. Thirty-eight patients could be evaluated after transplantation, as two of the patients died in the early post-transplantation period. We identified complete response in 24 patients (63%), partial response in 8 patients (21%), stable disease in 4 patients (11%) and progressive disease in 2 patients (5%). In all patient groups, 5-year overall survival (OS) and event free survival (EFS) were 43 and 40%, respectively; however there was no statistically significant survival difference between HL and NHL patients after ASCT, and 5-year OS and EFS were 47, 40 and 53%, 23%, respectively (p = 0.43, p = 0.76). Chemosensitive relapse had a positive impact on OS (p = 0.02). This study provides evidence for the effectiveness of HDC–ASCT as salvage therapy for patients with relapsed/refractory NHL and HL. Chemosensitive relapse is the most important prognostic factor determining the outcome of the ASCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) together represent a significant proportion of hematological malignancies. HL typically has a favorable outcome, but the prognosis is poor for patients with relapsed/refractory disease. The chemorefractory disease rate with first-line chemotherapy is 10–15% for HL [1], with relapse rates of 10–20% for stage I–II and 30–40% for advanced-stage disease [2]. Autologous stem cell transplantation-supported high-dose chemotherapy (HDC–ASCT) is the most commonly preferred option in patients with relapsed/refractory disease [3, 4]. The 5-year event-free survival (EFS) rate in patients administered high-dose platinum-based chemotherapy in HDC–ASCT is 59–70% [5, 6].
NHL includes numerous subtypes, of which diffuse large B cell lymphoma (DLBCL) is an aggressive subtype accounting for one-third of NHL cases [7]. Although rituximab-based immune-chemotherapy (R-CHOP) increases the overall survival (OS) of DLBCL patients, 4-year OS rates are 49–59% in those considered to be at moderate-high risk or high risk [8]. Among NHL patients, 10–15% are refractory to rituximab-based chemotherapy or suffer rapid disease progression soon after treatment. In one study, relapse within 2–3 years after first-line treatment occurred in one-third of patients who initially were responders [9]. In such patients, the results achieved with chemotherapy alone are not promising. In the group of chemosensitive NHL patients who respond to salvage chemotherapy and are therefore eligible for transplantation, ASCT is recommended [10, 11].
Salvage treatment consists of regimens such as etoposide, methylprednisolone, high-dose cytosine arabinoside, and cisplatin (ESHAP); dexamethasone, high-dose cytosine arabinoside, cisplatin (DHAP); and ifosfamide, carboplatin and etoposide (ICE) [12–14]. The most commonly used pre-ASCT conditioning chemotherapy regimen is the carmustine, etoposide, cytosine arabinoside and melphalan (BEAM) regimen. The long-term survival rate of these patients is 41–55% [15].
Factors such as chemosensitivity prior to ASCT, number of chemotherapy lines prior to ASCT, presence of B symptoms, and extranodal involvement at relapse are considered to be prognostic for lymphoma patients undergoing ASCT [13, 16]. The aim of this study was to evaluate the results of HDC–ASCT in patients with relapsed or refractory HL and NHL, and the importance of these prognostic factors in predicting outcome.
Patients and Methods
The 40 patients in this retrospective study had refractory or relapsed NHL or HL that was treated with HDC–ASCT between November 2004 and February 2014. All patients except the four who had T-cell lymphoma or Burkitt lymphoma received ASCT due to relapsed/refractory disease. Previous treatments, and the responses to them, were recorded for each patient. Induction therapy was defined as the first therapy administered after diagnosis. Complete response (CR) was defined as the absence of disease in clinical and radiological examinations, partial response (PR) was a ≥30% reduction in the greatest diameter at all sites of known disease, and stable disease (SD) was a < 30% reduction, or <20% increase, in the greatest diameter at all known sites of disease. Progressive disease (PD) was defined as a ≥ 20% increase in the size of known disease area and/or the development of new lesions [17]; bulky disease was any mass with a maximum diameter >10 cm. Patients were defined as having chemosensitive disease if they achieved CR or PR following salvage treatment; those with SD were defined as chemoresistant. Primary refractory disease was defined as non-CR to first-line therapy or disease progression during therapy. Disease progression after 1 month of non-existent radiological or clinical disease finding, determined on positron emission tomography-computed tomography (PET-CT) or CT scans, was regarded as relapsed disease, with late and early relapse defined as a CR lasting >12 and <12 months, respectively.
Cyclophosphamide/G-CSF (granulocyte colony stimulating factor) was the most commonly used mobilization regimen; other mobilization regimens were cyclophosphamide/etoposide/G-CSF, etoposide/carboplatin/ifosphamide/G-CSF, and gemcitabine/vinorelbine/G-CSF. The mobilization regimen was selected according to criteria such as disease burden, the intensity of previous regimens, and the performance status.
Two different therapy protocols were used in high-dose sequential chemotherapy. A BEAM conditioning regimen [300 mg intravenous (i.v.) BCNU/m2 on day −7, 800 mg i.v. etoposide/m2 on days −6 to −3, 1600 mg i.v. cytarabine/m2 on days −6 to −3, and 140 mg i.v. melphalan/m2 on day −2] was used to treat 24 patients, and an ICE conditioning regimen (1670 mg i.v. ifosfamide/m2 on days −1 to −3, 1670 mg i.v. mesna/m2 on days −1 to −3, i.v. carboplatin AUC 5 day −2, and 100 mg i.v. etoposide/m2 on days −1 to −3) in 16 patients. Because the BEAM regimen was not available at our center until 2009, patients enrolled prior to 2009 were treated with the ICE regimen, and thereafter with the BEAM regimen.
Patients received stem cell infusions 24 h after the last dose of HDC; this day was set as day 0. The acceptable stem cell dose was 2.5 × 106/kg. The day of neutrophil engraftment was defined as the first day of 3 consecutive days on which the absolute neutrophil count was ≥0.5 × 109/L. The day of thrombocyte engraftment was defined as the first of 3 consecutive days on which the thrombocyte count was ≥20 × 109/L without the requirement for thrombocyte transfusion. All patients were administered prophylactic oral antimicrobial therapy consisting of ciprofloxacin, fluconazole, and acyclovir. The necessary transfusions were administered according to the indications, and antibiotics according to the infectious agent. Treatment-related mortality (TRM) included any death related to a complication occurring in the absence of underlying disease within 100 days of the start of treatment.
Statistical Analysis
SPSS for Windows software (ver. 18.0; SPSS, Inc., Chicago, IL, USA) was used for the statistical analyses. A p value <0.05 was considered to indicate statistical significance. A χ2 test was used to compare the clinical features of the patients. Survival analysis was performed according to the Kaplan–Meier method, and log-rank statistics were used to compare subgroups. Parameters that affected OS were evaluated in a univariate analysis. EFS was defined as the time from ASCT to relapse, disease progression, or death from any cause, or until the last follow-up. OS was defined as the duration of patient survival beginning with the completion of ASCT until the day of death from any cause, or last follow-up.
Results
This retrospective study included 40 patients with relapsed or refractory NHL or HL who received HDC–ASCT. Their clinical characteristics are shown in Table 1. The median age of the 24 males and 16 females was 33 years (range 17–60 years); 22 had HL and 18 had NHL. Among the latter, 15 patients had a B-cell phenotype and 3 had a T-cell phenotype. The most common pathological subtypes were nodular sclerosis (n = 15) and mixed cellularity (n = 4) in HL patients, and DLBCL (n = 9) and T-cell lymphoma (n = 3) in NHL patients. There were 16 patients had stage II disease, 11 with stage III disease, and 13 with stage IV disease according to staging at the time of diagnosis. Most of the patients (n = 36) had nodal localized disease; 15 had bulky disease at the time of diagnosis. All patients with HL received ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy as the first-line treatment. Most of the NHL patients (n = 10, 55.8%) received R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisone). The median number of cycles was 6 (range 3–8). After first-line treatment, 32 (80.0%) patients had an objective response (CR: 16, PR: 16), and 8 had primary refractory disease. Radiotherapy was used to treat 23 patients (57.5%). The median time from diagnosis to first relapse was 11.36 months (range 2.7–58.4 months). In 19 patients, relapse was confirmed by biopsy.
All patients received ASCT due to relapsed/refractory disease, except three patients with T-cell lymphoma and one patient with Burkitt lymphoma (the latter patient had elevated LDH levels, stage 3 disease, a high İPİ (International Prognostic Index) score, and bulky disease). Among the patients who received ASCT, 25% (n = 9), 53% (n = 19), and 22% (n = 8) had late relapse, early relapse, and primary refractory disease, respectively.
As salvage therapy, 78% (n = 28) of the patients received ICE or R-ICE, 17% (n = 6) received DHAP or R-DHAP, and 5% (n = 2) received other regimens (COPP and ABVD, both n = 1) at the time of relapse. Among the 36 patients who received salvage chemotherapy, 28 showed an objective response, including 22 with a PR and 6 with a CR (NHL = 2, HL = 4). The remaining eight patients had refractory disease even after salvage therapy. In total, 9 patients had chemoresistant disease and 31 had chemosensitive disease. There was a trend toward a better response with ICE than with DHAP (p = 0.08) but the difference was not statistically significant, probably because of the small sample size.
The median time from diagnosis to ASCT was 19.6 months (range 8.7–209.1 months). For ASCT, autologous peripheral stem cells were used as the source in all patients. Except in seven patients, stem cells were harvested more than once. For the mobilization regimen, cyclophosphamide (2, 4 g/m2/day) plus G-CSF (57.5%) was most often used. Patients with liver dysfunction, poor performance status, and a history of a previous long-lasting neutropenic period received 2 g cyclofosfamide/m2, and all others received 4 g/m2. The median CD34 dose was 3.46 × 106/kg (1.5 × 9.0 × 106/kg). BEAM and ICE regimens served as the preparation regimens in 60% (n = 24) and 40% (n = 16) of the patients who received ASCT, respectively. The median time to reach neutrophil and thrombocyte engraftment was 11 days (range 8–15 days) and 12 days (range 8–19 days), respectively. The median duration of patient hospitalization was 29 days (range 15–72 days). Only one patient experienced mobilization failure; a second attempt at stem cell collection was successful after mobilization with G-CSF. Major toxicities of HDC were hematologic toxicity and febrile neutropenia. Grade 3–4 thrombocytopenia and neutropenia developed in all patients, and febrile neutropenia developed in 35% (n = 14). Non-hematologic toxicities that occurred in most patients consisted of grade 1–2 mucositis and nausea. Two patients died, due to sepsis and aspiration pneumonia, respectively, during hospitalization. The TRM rate was 5%. Among the 38 evaluable patients, 24 (63%) had a CR and 8 (21%) had a PR. Four patients (11%) had SD and two (5%) had PD 100 days after ASCT. Nine patients had disease chemoresistant to salvage chemotherapy and were evaluated separately; among this group, two patients achieved a CR, three showed a PR, two had SD, and two had PD.
The median follow-up duration after ASCT was 23.6 months (range 0.6–120.1 months). During follow-up, 24 patients suffered disease relapse and 20 died from their disease. Patients treated with the ICE regimen had more relapses than those treated with the BEAM regimen (81.3 vs. 45.8%, p = 0.025). Among the relapse group, 17 patients relapsed within 1 year, 5 within 1–2 years, and 2 within 2 years after treatment. Systemic treatment was administered to 15 patients after relapse. Two patients (one with T-cell lymphoma and one with DLBCL) received an allogenic stem cell transplant from a mismatched donor after they developed secondary acute lymphoblastic leukemia, but both patients died after transplantation. Twenty patients were alive at the time of evaluation.
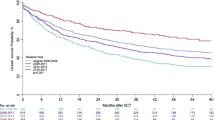
After ASCT, the median OS and EFS was 30.1 months (range 0.6–120.1 months) and 22.7 months (range 0.6–120.1 months), respectively. In HL and NHL patients, the 5-year OS and EFS were 43 and 40%, respectively. There was no statistically significant difference between the HL and NHL groups, in terms of survival, after ASCT; the respective 5-year OS and EFS were 47.0 and 40.0% (p = 0.43) versus 53.0 and 23.0% (p = 0.76), respectively (Fig. 1). The 6-year EFS was 18.0 and 23.0%, respectively.
An analysis of the patients according to response rate after salvage chemotherapy showed 5-year OS and EFS rates of 50.0 and 41.0% in chemosensitive (CR, PR) patients vs. 22.0 and 38.0% in chemoresistant (SD) patients (p = 0.02 and p = 0.10, respectively) (Fig. 2). An analysis of the patients in terms of CR and PR showed that the former had a trend toward a better EFS and OS but the difference was not statistically significant [Non-reached (NR) vs. 25.5 months, p = 0.06 for EFS; NR vs. 31.0 months, p = 0.18 for OS].
Both groups were also evaluated in terms of response to induction chemotherapy (Table 2). The 5-year OS and EFS were 51.0 and 58.0% in those who were responsive, compared to 38.0 and 28.0% in those who were non-responsive (both p = 0.07). Multivariate analysis was not performed due to the small sample size.
Discussion
In patients with relapsed lymphoma, and in those with refractory disease, cure is rarely achieved with conventional chemotherapy [18]. For both groups, HDC–ASCT has become a standard treatment. In randomized studies comparing salvage chemotherapy and ASCT in HL patients, EFS and progression-free survival (PFS) rates differed significantly and favored ASCT [19]. Compared with conventional chemotherapy, HDC–ASCT significantly increases EFS and OS in patients with chemosensitive NHL [20]. Based on these results, HDC–ASCT is considered to be more effective than conventional treatment in patients with relapsed/refractory lymphoma. In previous studies, the remission state before HDC and conventional salvage chemosensitivity were the most important factors that affected the results of HDC–ASCT [10, 21]. Thus, subsequent studies, but which were performed prior to the pre-ASCT period, focused on an optimal salvage regimen to achieve a maximum response. Regimens such as ESHAP and ICE are used as salvage treatment and their response rates are between 73 and 84% [12, 14]. There is no consensus regarding the optimum number of cycles of salvage chemotherapy before ASCT, but at most centers two or three cycles are administered. In our center, patients are also administered ICE and DHAP chemotherapy, as salvage therapy, over three cycles. The objective response rate of 78% was similar to that of other studies.
Retrospective studies of HL patients after ASCT have reported 5-year OS and EFS rates of 35–65 and 55–64%, respectively [22–25]. Our results were similar: 47 and 53%, respectively. Differences among studies may be due to the heterogeneity of the patient populations, different induction and salvage chemotherapies and cycles, and different HDC regimens. NHL is a heterogeneous disease with many subtypes. High response rates can be achieved with combined chemotherapies following the first diagnosis [26], but the prognosis of NHL patients with relapse or PD is not good. However, these patients may benefit from HDC–ASCT [27]. In previous studies of NHL patients treated with HDC–ASCT, the 5-year OS was 40% [28]. The 5-year OS of our HDC–ASCT-treated NHL patients was also 40%. In our study, DLBCL was the most frequent subtype of NHL. The difference in OS rates between NHL and HL patients was not significant (p = 0.43).
In HL patients who did not respond to induction chemotherapy and were thus treated with conventional chemotherapy, the OS rates were 18–26% [29, 30]. In the study of Lavoie et al. [16], the 15-year OS was 67% in patients who responded to induction chemotherapy but only 39% in those who did not. In retrospective studies carried out by Sweetenham et al. and Sucak et al. [31, 32], the prognostic factors were the response to induction therapy and the time from diagnosis to ASCT. A stratification of our first-line induction chemotherapy recipients resulted in a 5-year OS of 51% in the CR + PR group versus 38% in the SD + PD group. There was a trend toward statistical significance (p = 0.07). Thus, among patients who respond to induction therapy, ASCT will achieve more favorable results even if relapse occurs.
Chemosensitivity is the most important post-salvage prognostic factor in patients with relapsed/refractory lymphoma [13, 24]. In a study by Sirohi et al. [22], the 5-year OS after salvage chemotherapy was 79, 59, and 17% for patients with CR, PR, and PD, respectively, and chemosensitivity was a statistically significant predictor of OS. In the study of Sucak et al. [32], the 4-year PFS rates were 81, 43, and 15% in patients with CR, PR, and PD, respectively. In our study, which compared patients with chemosensitive (CR + PR) and chemoresistant (SD) disease after salvage chemotherapy, 5-year OS and EFS were 50.0 and 41.0% in the chemosensitive group (p = 0.02) and 22.0 and 38.0% in the chemoresistant group (p = 0.10), respectively. These results demonstrate the importance of salvage chemotherapy before ASCT and suggest that an increase in the response rate before ASCT contributes to an improved OS.
In patients who underwent ASCT following HDC, advanced age, poor performance at time of transplantation, the presence of bulky and extranodal disease, and two or more lines of therapy before HDC/ASCT were identified as risk factors for a poor prognosis [14, 25, 33–35]. In our study, female sex and chemoresistant disease were the risk factors. Determining treatment options according to the prognostic factors of the patient may be important for achieving better survival outcomes. However, our study was limited by the heterogeneity (both NHL and HL patients were included) and small size of the population, which may have influenced the findings.
In patients treated by HDC followed by ASCT, recent transplantation techniques and advances in support treatment have increased the safety of both, as evidenced by the low morbidity and mortality rates. In a 2005 study by Lavoie et al. [16], the TRM rate in the first 100 days after transplantation was 8%. Despite the development of grade 3–4 hematologic toxicities and grade 1–2 mucositis in most of the patients who received HDC–ASCT at our center, TRM was very low (5%) due to good support therapy. These results highlight the importance of more appropriate patient selection and better support therapies, which together can lower mortality rates.
Because long-term remission can be attained in patients with lymphoma after ASCT, treatment-related late complications may occur. In the study of Forrest et al. [33], the cumulative incidence of secondary malignancies was 9% in 15 years, whereas in our study it was 5%. Clinicians should be aware of the potential for secondary malignancies in post-transplantation patients and perform the appropriate medical workups when necessary. The high incidence of secondary malignancies in our patients can perhaps be partly explained by the long follow-up duration, as they were diagnosed between 1992 and 2012.
Previous cycles of chemotherapy strongly influence the results of ASCT, as shown by Sureda et al. [34]. In patients who received one previous line of chemotherapy, the 5-year OS was 79%, but in patients who received two or more lines of chemotherapy it was 49% (p = 0.0002). Majhail et al. [35] reported 5-year OS rates of 58 and 41% in patients who received less than three and more than three lines of chemotherapy, respectively (p = 0.04). In our study, except in four patients (three with T-cell lymphoma and one with Burkitt lymphoma), all patients received salvage treatment followed by ASCT because of relapsed/refractory disease after induction chemotherapy.
In addition to the above-mentioned limitations of a small, heterogeneous population, other limitations in our study were that it was performed at a single center and used a retrospective design. However, the clinical features and risk factors of our patients were similar to those in previously published multicenter studies.
In conclusion, this study provides further evidence for the effectiveness of HDC followed by ASCT as salvage therapy for patients with relapsed/refractory NHL and HD. It also highlights the importance of an acceptable pre-transplantation salvage chemotherapy response to achieve low rates of TRM.
References
Oza AM, Ganesan TS, Leahy M, Gregory W, Lim J, Dadiotis L, Barbounis V, Jones AE, Amess J, Stansfeld AG (1993) Patterns of survival in patients with Hodgkin’ s disease: long follow up in a single center. Ann Oncol 4:385–392
Viviani S, Bonadonna G, Santoro A, Bınfante V, Zanini M, Devizzi L, Soncini F, Valagussa P (1996) Alternating versus hybrid MOPP and ABVD combinations in advanced Hodgkin’ s disease: ten year results. J Clin Oncol 14:1421–1430
Ljungman P, Bregni M, Brune M, Cornelissen J, de Witte T, Dini G, Einsele H, Gaspar HB, Gratwohl A, Passweg J, Peters C, Rocha V, Saccardi R, Schouten H, Sureda A, Tichelli A, Velardi A, Niederwieser D (2010) Allogeneic and autologous transplantation for haematological diseases, solidtumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant 45:219–234
Ayala E, Tomblyn M (2011) Hematopoietic cell transplantation for lymphomas. Cancer Control 18(4):246–257
Josting A, Rudolph C, Mapara M, Glossmann JP, Sieniawski M, Sieber M, Kirchner HH, Dörken B, Hossfeld DK, Kisro J, Metzner B, Berdel WE, Diehl V, Engert A (2005) Cologne high dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: results of a large multicenter study of the German Hodgkin Lymphoma Study Group(GHSG). Ann Oncol 16:116–123
Evens AM, Altman JK, Mittal BB, Hou N, Rademaker A, Patton D, Kaminer L, Williams S, Duffey S, Variakojis D, Singhal S, Tallman MS, Mehta J, Winter JN, Gordon LI (2007) Phase I/II trial of total lymphoid irradiation and high-dose chemotherapy with autologous stem-cell transplantation for relapsed and refractory Hodgkin’s lymphoma. Ann Oncol 18:679–688
Morton LM, Wang SS, Devesa SS, Hardge P, Weisenburger DD, Linet MS (2006) Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 107:265–276
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, Gascoyne RD, Connors JM (2007) The Revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with RCHOP. Blood 109:1957–1961
Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trümper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M (2008) Six versus eight cycles of biweekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20 + B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 9:105–116
Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Frevtes CO, Pavlovsky S, Keating A, Yanes B, van Besien K, Armitage JO, Horowitz MM (2001) Autologus transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol 19:406–413
Kewalramani T, Zelenetz AD, Hedrick EE, Donnelly GB, Hunte S, Priovolos AC, Qin J, Lyons NC, Yahalom J, Nimer SD, Moskowitz CH (2000) High-dose chemoradiotherapy and autologous stem cell transplantation for patients with primary refractory aggressive non-Hodgkin lymphoma: an intention-to-treat analysis. Blood 96:2399–2404
Aparicio J, Segura A, Garcera S, Oltra A, Santaballa A, Yuste A, Pastor M (1999) ESHAP is an active regimen for relapsing Hodgkin’s disease. Ann Oncol 10:593–595
Moskowitz CH, Kewalramani T, Nimer SD, Gonzalez M, Zelenetz AD, Yahalom J (2004) Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin’s disease. Br J Haematol 124:645–652
Ferme C, Mounier N, Divine M, Brice P, Stamatoullas Reman O, Voillat L, Jaubert J, Lederlin P, Colin P, Berger F, Salles G (2002) Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: results of the Groupe d’Etudes des Lymphomes de I’Adulte H89 Trial. J Clin Oncol 20:467–475
Wang EH, Chen YA, Corringham S, Bashey A, Holman P, Ball ED, Carrier E (2004) High-dose CEB vs BEAM with autologous stem cell transplant in lymphoma. Bone Marrow Transplant 34:581–587
Lavoie JC, Connors JM, Phillips GL, Reece DE, Barnett MJ, Forrest DL, Gascoyne RD, Hogge DE, Nantel SH, Shepherd JD, Smith CA, Song KW, Sutherland HJ, Toze CL, Voss NJ, Nevill TJ (2005) High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood 106:1473–1478
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Longo DL, Duffey PL, Young RC, Hubbard SM, Ihde DC, Glatstein E, Phares JC, Jaffe ES, Urba WJ, DeVita VT Jr (1992) Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin’s disease after combination chemotherapy: the low probability for cure. J Clin Oncol 10:210–218
Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, Chopra R, Milligan D, Hudson GV (1993) Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet 341:1051–1054
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL (1995) Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 333:1540–1545
Crump M, Smith AM, Brandwein J, Couture F, Sherret H, Sutton DM, Scott JG, McCrae J, Murray C, Pantalony D (1993) High-dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin’s disease: importance of disease status at transplant. J Clin Oncol 11:704–711
Sirohi B, Cunningham D, Powles R, Murphy F, Arkenau T, Norman A, Oates J, Wotherspoon A, Horwich A (2008) Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma. Ann Oncol 19:1312–1319
Viviani S, Di Nicola M, Bonfante V, Di Stasi A, Carlo-Stella C, Matteucci P, Magni M, Devizzi L, Valagussa P, Gianni AM (2010) Long-term results of high-dose chemotherapy with autologous bone marrow or peripheral stem cell transplant as first salvage treatment for relapsed or refractory Hodgkin lymphoma: a single institution experience. Leuk Lymphoma 51:1251–1259
Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ, Petit J, López A, Lahuerta JJ, Carreras E, García-Conde J, García-Laraña J, Cabrera R, Jarque I, Carrera D, García-Ruiz JC, Pascual MJ, Rifón J, Moraleda JM, Pérez-Equiza K, Albó C, Díaz-Mediavilla J, Torres A, Torres P, Besalduch J, Marín J, Mateos MV, Fernández-Rañada JM, Sierra J, Conde E (2005) Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol 16:625–633
Czyz J, Dziadziuszko R, Knopinska-Postuszuy W, Hellmann A, Kachel L, Holowiecki J, Gozdzik J, Hansz J, Avigdor A, Nagler A, Osowiecki M, Walewski J, Mensah P, Jurczak W, Skotnicki A, Sedzimirska M, Lange A, Sawicki W, Sulek K, Wach M, Dmoszynska A, Kus A, Robak T, Warzocha K (2004) Outcome and prognostic factors in advanced Hodgkin’s disease treated with high-dose chemotherapy and autologous stem cell transplantation: a study of 341 patients. Ann Oncol 15:1222–1230
Kaiser U, Uebelacker I, Abel U (2002) Randomised study to evaluate the use of high-dose therapy as part of primary treatment for ‘aggressive’ lymphoma. J Clin Oncol 2:4413–4419
Josting A, Reiser M, Rueffler U, Salzberger B, Diehl V, Engert A (2000) Treatment of primary progressive Hodgkin’s and aggressive non-Hodgkin’s lymphoma: is there a chance for cure? J Clin Oncol 18:332–339
Chang H, Cheong JW, Hahn JS (2006) High dose chemotherapy and autologous stem cell transplantation in non-Hodgkin’s Lymphoma: an eight-year experience. Yonsei Med J 47:604–613
Radman I, Basic N, Labar B, Kovacevic J, Aurer I, Bogdanic V, Zupancić-Salek S, Nemet D, Jakić-Razumović J, Mrsić M, Santek F, Grgić-Markulin L, Boban D (2002) Long-term results of conventional-dose salvage chemotherapy in patients with refractory and relapsed Hodgkin’s disease (Croatian experience). Ann Oncol 13:1650–1655
Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A (2000) Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood 96:1280–1286
Sweetenham JW, Carella AM, Taghipour G, Cunninqham D, Marcus R, Della Volpe A, Linch DC, Schmitz N, Goldstone AH (1999) High-dose therapy and autologous stem-cell transplantation for adult patients with Hodgkin’s disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. Lymphoma Working Party. J Clin Oncol 17:3101–3109
Sucak GT, Çakar MK, Suyanı E, Akı Z, Altındal Ş, Acar K (2013) Outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin lymphoma patients in a centre from Turkey. Hematology 18:269–276
Forrest DL, Hogge DE, Nevill TJ, Nantel SH, Barnett MJ, Shepherd JD, Sutherland HJ, Toze CL, Smith CA, Lavoie JC, Song KW, Voss NJ, Gascoyne RD, Connors JM (2005) High-dose therapy and autologous hematopoietic stem cell transplantation does not increase the risk of second neoplasms for patients with Hodgkin’s lymphoma: a comparison of conventional therapy alone versus conventional therapy followed by autologous hematopoietic stem-cell transplantation. J Clin Oncol 23:7994–8002
Sureda A, Arranz R, Iriondo A, Carreras E, Lahuerta JJ, Garcia-Conde J, Jarque I, Caballero MD, Ferrà C, López A, García-Laraña J, Cabrera R, Carrera D, Ruiz-Romero MD, León A, Rifón J, Díaz-Mediavilla J, Mataix R, Morey M, Moraleda JM, Altés A, López-Guillermo A, de la Serna J, Fernández-Rañada JM, Sierra J, Conde E (2001) Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autólogo de Médula Osea Spanish Cooperative Group. J Clin Oncol 19:1395–1404
Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, Arora M, Ramsay NK, Orchard PJ, MacMillan ML, Burns LJ (2006) Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant 12:1065–1072
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethical approval was obtained.
Rights and permissions
About this article
Cite this article
Bozkaya, Y., Uncu, D., Dağdaş, S. et al. Evaluation of Lymphoma Patients Receiving High-Dose Therapy and Autologous Stem Cell Transplantation: Experience of a Single Center. Indian J Hematol Blood Transfus 33, 361–369 (2017). https://doi.org/10.1007/s12288-016-0756-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-016-0756-x