Abstract
Transfusion of blood and blood products is a double edged sword, so it should be used judiciously. The primary aim of the centralized Haemovigilance Program is to improve transfusion safety. To determine the incidence of adverse transfusion reactions (ATRs) in recipients of blood and blood components. Prospective study from January 2014 till April 2015 was done. ATRs reported to the Department of Transfusion Medicine were recorded and analyzed on the basis of their clinical features and lab tests. During the study period 25,099 units of blood and blood components were transfused and 100 ATRs (0.40 %) were reported. The incidence of febrile nonhemolytic transfusion reactions (FNHTR) was maximum (73 %) followed by allergic reactions (24 %), bacterial sepsis (1 %), hypotension due to ACE inhibitors (1 %) and acute hemolytic transfusion reaction (AHTR) (1 %). Of all the reported ATRs, 76 % occurred with packed red cells, 15 % occurred with whole blood, while platelets and Fresh Frozen Plasma transfusions were responsible for 8 % and 1 %, respectively. The majority of the reactions were FNHTRs followed by allergic reactions. Reporting of all adverse events and continuous medical education to medical and paramedical staff will help in strengthening hemovigilance system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transfusion of blood and blood components is not without risks and it can lead to complications. Though blood transfusion can be life-saving, it can also lead to certain adverse reactions which can be fatal. Knowledge about various types of adverse transfusion reactions (ATRs) will help not only in their early identification and management, but also in taking adequate measures to prevent the same [1]. Haemovigilance is a continuous process of data collection and analysis of transfusion-related adverse reactions in order to investigate their causes and outcomes, and prevent their occurrence or recurrence. It includes the identification, reporting, investigation and analysis of adverse reactions and events in recipients and blood donors as well as incidents in manufacturing processes and, eventually errors and “near-misses”. A haemovigilance system is also an integral part of quality management in a blood system, triggering corrective and preventive actions, and for the continual improvement of the quality and safety of blood products and the transfusion process [2].
Aim
To determine the incidence of ATRs in recipients of blood and blood components.
Materials and Methods
ATRs reported to the Department of Transfusion Medicine between January 2014 to April 2015 were included in the study. During issue of blood/blood component a Transfusion Reaction Reporting Form (TRRF) was provided. The clinicians were trained about the transfusion protocol i.e. the Whole Blood (WB) and Packed Red Blood Cells (PRBC) transfusion should be started within half an hour and completed within 4 h after issue while Platelet concentrate (PC) and Fresh Frozen Plasma (FFP) should be transfused immediately after issue and completed within 15–20 min. There was no premedication protocol before the start of transfusion. Clinicians from department of Medicine, Surgery, Gynaecology and Obstetrics, Paediatrics and Orthopaedics were trained to have a uniform reporting of data to the Department of Transfusion Medicine (Fig. 1). The duly filled TRRF along with used blood bag and attached blood transfusion (BT) set, 2 blood samples (EDTA and plain vial) taken from the opposite limb and 1st post transfusion urine specimen after reaction were received. On the basis of reported signs and symptoms by the treating physician, Transfusion Medicine workup and the reports of various investigations, the reactions were classified (Table 1) [3]. All the reactions were evaluated according to the transfusion reaction work up form issued by National Institute of Biologicals(NIB), Ministry of Health and Family Welfare 2012 [2]. Reporting of serious adverse transfusion reactions was done to Haemovigilance Center, NIB (Fig. 1).
Flow chart for reporting serious adverse reactions in blood transfusion [2]
The patient’s name and identification number (Central registration number i.e. C.R. No.) both on the pre transfusion sample vial and post transfusion sample vials and requisition form was rechecked to rule out the possibility of wrong sampling or bedside transposition. Most recent results of blood typing were compared with the patient’s previous transfusion records if patient was transfused previously and results written in the blood requisition form. Clinical signs and symptoms i.e., fever, chills, hypotension, rigors, rashes, respiratory discomfort or any other untoward events developed during the course or following transfusion were used in classification of the transfusion reactions.
Laboratory investigations Blood bag and transfusion set were examined grossly for any abnormal findings namely discoloration, clot, haemolysis or foul smell. Reconfirmation of ABO Rh typing of the patient and implicated blood/component was done and Compatibility testing was repeated on pre and post-transfusion sample. Microbiological examination was done from blood bag along with attached BT set and patient’s blood in a blood culture bottle from the bedside. Patient’s pre and post transfusion samples were checked for haemolysis. In case of suspected hemolytic reaction: Estimation of raised plasma hemoglobin, Direct Antiglobulin test/Indirect Antiglobulin test (DAT/IAT), Urine for hemoglobinuria, Serum bilirubin, Renal Function Test (RFT), Liver Function Test (LFT), Peripheral blood smear examination were done.
Circumstantial evidences for thermal, oncotic, and osmotic injury was looked for by reviewing the mode of storage and storage conditions of the issued unit after it was released from the Department of Transfusion Medicine. The history of any medication given to the patient along with blood transfusion especially through the same blood transfusion set was taken. In non hemolytic transfusion reactions, investigations were done according to their clinical presentations.
Results
Prospective study was conducted from January 2014 till April 2015. ATRs reported to the Department of Transfusion Medicine were recorded, analyzed on the basis of their clinical features and lab tests. During the study period 26,059 units of blood and blood components were issued. 25,099 units were transfused to patients admitted in various clinical specialties while 960 units were returned back unused. Out of 25,099 units transfused, 100 patients (0.40 %) had ATRs during or after transfusion.
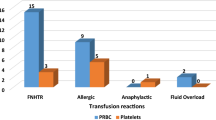
The incidence of Febrile Non Hemolytic Transfusion Reactions (FNHTR) was maximum, 73 % followed by Allergic Reactions, 24 %; Bacterial sepsis, 1 %; Hypotension due to ACE inhibitors, 1 %; Acute Hemolytic Transfusion Reactions (AHTR), 1 %; (Table 2). All patients were managed successfully and no casualty due to transfusion reactions was reported. Of all the reported ATRs, 76 % occurred with PRBC, 15 % occurred with WB, while platelets and FFP transfusions were responsible for 8 % and 1 %, respectively (Table 2). The age of the patients ranged from new born to 80 years. Maximum reactions (41 %) occurred in the age group of 21–30 years. Maximum cases of ATRs were from Department of Gynaecology and Obstetrics (Fig. 2). Of the 100 patients who had ATRs, 20 were males and 80 were females. 71 % of females were in the age group of 21–40 years (child bearing age group). History of previous transfusion was present in 53 % of patients. The mean volume of blood or its component transfused, when reactions were noted was 120 ml (range 10–320 ml).
WB/PRBCs Out of 21,047 WB/PRBC transfused, 91 (0.43 %) patients had ATRs. The time interval between issue and transfusion of the implicated unit ranged from 15 min to 7 h. During this period units were kept in unmonitored conditions in wards. PC Out of 1584 PC transfused, 8 patients (0.50 %) had ATRs. The time interval between issue of the PC and the beginning of the transfusion ranged from 15 to 25 min. During this period, the PC were kept at the patient’s bedside. FFP A single ATR (FNHTR) out of 2468 FFP transfusions was reported (0.04 %). The time interval between issue and the reaction was 20 min.
Categorization of ATRs
Transfusion related adverse events were classified as acute (within 24 h) and delayed (onset after 24 h). All the cases in our study were acute transfusion reactions.
FNHTR
A total of 73 patients had signs and symptoms of FNHTR. 61 were females and 12 were males. Age ranged from 10 days to 80 years. Of 73 patients, 66 patients had WB/PRBCs transfusion, 6 had been transfused with PC and 1 had FFP transfusion. Fever was the most common presenting symptom (50.9 %) followed by chills and rigors in 40.9 % of patients.
Allergic Reactions
Allergic reactions were observed in 24 patients (18 females and 6 males). Age ranged from 8 months to 70 years. Of the 24 patients, 22 patients had WB/PRBCs transfusion and 2 were transfused with PC. Most common presentation of allergic reaction was urticaria (17.2 %), followed by rash (13.6 %).
AHTR
A single case of AHTR was observed. The patient was a 65 year old female admitted in Department of Medicine. She was transfused 15 ml of PRBCs when she developed symptoms of reaction but was managed successfully. On investigating it was found to be a case of AHTR (ABO incompatibility due to clerical error). DAT was positive in this case. There was slight increase in S. Bilirubin. Other parameters like RFT & LFT were normal.
Bacterial Sepsis
A single case of bacterial sepsis was observed. The patient was a 34 year old female admitted in Department of Obstetrics and Gynaecology. The patient while receiving PRBC transfusion developed symptoms of reaction in the form of high grade fever. The sepsis was confirmed by positive blood cultures for Klebsiella pneumonia of both blood bag and patient’s blood. The donor could not be traced out in this case.
Hypotensive Reaction
Hypotensive reaction was observed with PRBCs in a 45 years old male who was already on ACE inhibitors admitted in Department of Medicine.
Discussion
Clinical reporting is the only source of information about the incidence of transfusion reactions. In the present study all the reactions reported were acute. No case of delayed transfusion reactions was reported possibly due to under reporting and under diagnosis of the cases though all the recipients of blood and blood components were advised to visit the clinician after every 15 days for 6 months.
Data on the incidence of FNHTR varies greatly in the literature. Possible reasons for this variation include differences in recording of symptoms by the bedside staff, case ascertainment, and use of pre transfusion medications to control fever. In our study the highest percentage of reactions was constituted by FNHTR (73 %). The incidence of FNHTR is high because PRBCs were not leucodepleted. Prestorage WBC reduction significantly reduced the rate of FNHTRs to PCs and PRBCs [4]. All the subjects that had transfusion reaction presented with fever with an average highest temperature of 39.3 ± 0.6 °C Fever was associated with rigor and chills. The present study correlated well with the study done by Chowdhury et al. [5], Khalid et al. [6] and Bhattacharya et al. [7] which also showed highest incidence of FNHTR in their studies (Table 3). Febrile reactions result from the interaction of the recipient antibodies with the antigens on donor leucocytes and can be reduced by transfusion of leuco-reduced blood products.
Incidence of allergic reactions was found to be the second highest, constituting 24 % in the present study. Some amount of plasma kept in PRBCs to reduce the viscosity may be responsible for enhanced allergic reactions. Majority of the allergic reactions presented with skin manifestations such as urticaria, rashes and pruritis. Incidence of allergic reactions varied greatly in literature (25–55.1 %) [1, 5]. The present study correlated well with the literature [5–7] which also showed the second highest prevalence of allergic reactions in their studies (Table 3).
Bacterial sepsis remains an important cause of transfusion-related morbidity and mortality. Sources of bacteria are believed to arise from donor either from venepuncture site, from unsuspected bacteremia or during component preparation [11]. It was found in one patient. The pathogen isolated was klebsiella pneumonia. The case occurred in summer season which suggests that either sweating might be a factor for bacterial proliferation in the donor skin flora or lack of appropriate disinfection of the phlebotomy site. Acute hemolytic reaction was observed in one case, that was due to ABO incompatibility. This correlated well with the study done by Khalid et al. [6] (Table 3).
One case of hypotensive reaction in patient on ACE inhibitor was seen. Similar case was reported by Kalra et al. [12].
Only a single ATR was reported due to FFP i.e. FNHTR. No allergic reaction was reported with FFP as the incidence of ATRs with FFP was quite low. Red cells and whole blood were most commonly associated with transfusion reactions (Table 4). PRBCs were the blood components most frequently involved in immediate ATRs compared to other components. This is probably due to the high consumption of PRBCs, especially by obstetric cases, trauma center with patients of multiple injuries, acute hemorrhages and those requiring surgical procedures. Maximum cases of ATRs were reported from Department of Gynaecology and Obstetrics whereas lesser incidence of ATRs in other departments may be due to under reporting of cases.
Patients who had previous history of transfusion are more susceptible to have ATRs as against those who had no previous history of blood transfusion.
Conclusions
The majority of ATRs were FNHTRs followed by allergic reactions. Leucodepleted PRBCs help in reduction of FNHTR. Complete removal of plasma and its replacement by additives will reduce allergic reactions. Proper disinfection of phlebotomy site and adoption of diversion technique will decrease the risk of bacterial sepsis. Improper storage in unmonitored conditions outside the blood banks lead to deterioration of red cell units. Hence strict adherence to protocol in blood bank and clinical ward will reduce ATRs. Reporting of all ATRs and continuous medical education to medical and paramedical staff will help in strengthening hemovigilance system.
References
Kumar P, Thapliyal R, Coshic P, Chatterjee K (2013) Retrospective evaluation of adverse transfusion reactions following blood product transfusion from a tertiary care hospital: a preliminary step towards hemovigilance. Asian J Transfus Sci 7(2):109–115
IPC-NIB Guidance Document for reporting serious adverse reactions in blood transfusion service. National Institute of Biological, Ministry of Health and Family Welfare, Government of India 2012. 30(1):1–31
Mazzezi CA, Popovsky MA, Kopko PM (2014) Noninfectious complications of blood transfusion. Technical manual, 18th edn. American Association of Blood Banks, USA, pp 685–695
Yazer Mark H, Podlosky Linda, Clarke Gwen, Nahirniak Susan M (2004) The effect of prestorage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion 44(1):10–15
Chowdhury FS, Biswas J, Siddiqui MAE, Hoque MM, Adnan SK (2008) Transfusion reaction among the blood recipient:a study of 120 cases. J Dhaka Med Coll 17(2):67–71
Khalid S, Usman M, Khurshid M (2010) Acute transfusion reactions encountered in patients at a tertiary care center. J Pak Med Assoc 60(10):832–836
Bhattacharya P, Marwaha N, Dhawan HK, Roy P, Sharma RR (2011) Transfusion-related adverse events at the tertiary care center in North India: an institutional hemovigilance effort. Asian J Transfus Sci 5(2):164–170
Venkatachalapathy TS (2012) A prospective audit of blood transfusion reactions in tertiary care hospital for the use of blood and blood components. J Blood Disord Transfus 3:118
Payandeh M, Zare ME, Kansestani AN, Pakdel SF, Jahanpour F, Yousefi H et al (2013) Descriptions of acute transfusion reactions in the teaching hospitals of Kermanshah university of medical sciences Iran. Int J Hematol Oncol Stem Cell Res 7(2):11–16
Haslina MNN, Fakhri MAM, Saw TH, Salamah AS (2012) An audit on acute transfusion reaction in North Eastern Malaysia. Sch J Med 2(5):60–62
Sazarma K (1994) Bacteria in blood for transfusion: a review. Arch Pathol Lab Med 118:35065
Kalra A, Palaniswamy C, Patel R, Kalra A, Selvaraj DR (2012) Acute hypotensive transfusion reaction with concomitant use of angiotensin-converting enzyme inhibitors: a case report and review of the literature. Am J Ther 19(2):e90–e94
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There was no conflict of interest among authors.
Rights and permissions
About this article
Cite this article
Bassi, R., Aggarwal, S., Bhardwaj, K. et al. Patterns of Adverse Transfusion Reactions in a Tertiary Care Centre of North India: A Step Towards Hemovigilance. Indian J Hematol Blood Transfus 33, 248–253 (2017). https://doi.org/10.1007/s12288-016-0684-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-016-0684-9