Abstract
Background
Griffipavixanthone (GPX) is a compound extracted from Garcinia oblongifolia Champ. But, no research has yet been done about the effect of GPX on breast cancer.
Methods
We evaluated the proliferation of human breast cancer cells by CCK-8 assay and apoptosis by Annexin V (AV)-FITC and PI double staining. We used transwell assay to indicate the invasion and migration of MCF-7. To explore the molecular mechanism of GPX, we detected the mRNA and protein expression using qRT-PCR and Western blot.
Results
In this study, we evaluated if GPX could inhibit the proliferation of human breast cancer cell MCF-7 and T-47D with IC50 value of 9.64 ± 0.12 µM and 10.2 1 ± 0.38 µM at 48 h. And the IC50 value of MCF-10A is 32.11 ± 0.21 µM, which showed GPX had a tiny side effect for normal breast cells. Annexin V (AV)-FITC and PI double staining demonstrated firmly the apoptosis of MCF-7 resulting from GPX. Transwell assay indicated that GPX inhibited the invasion and migration of MCF-7. In addition, we found GPX cleaved caspase-8/9 and PARP, which play important roles in apoptotic pathway. Furthermore, through the Western blot assay, GPX increased the level of pro-apoptosis protein Bax and cytochrome C. On the contrary, GPX decreased the level of anti-apoptosis protein Bcl-2. Moreover, GPX increased the mRNA and protein expression level of p53 and its target genes, which indicated that GPX induced MCF-7 cell apoptosis by up-regulating p53 and Bax expression while suppressing Bcl-2 expression.
Conclusion
All the results showed that GPX induces MCF-7 cell apoptosis and could be considered as a potential drug for breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More and more wild plants are collected for medical purposes in China, as extracts from plants have been proved to play important roles. They are effective for various diseases, like inflammation, cancer, diabetes, heart disease and so on [1, 2]. Triptolide, an active chemical compound extracted from Chinese herb lei gong teng (Tripterygium wilfordii Hook F) [3], shows a broad spectrum of anti-cancer and anti-inflammation activities [4], especially its anti-tumor effect, against pancreatic cancer [5, 6], lung cancer [7], breast cancer [8] and renal cancer [9]. Resveratrol, which is extracted from grapes, red wine and tomatoes, is demonstrated to have anti-cancer effects in prostate, colon, lung and breast based on in vitro and animal model studies [10,11,12]. Chlorogenic acid, the extraction of Chinese herb Lonicerae, has been reported to have a potential role in diabetes [13]. Panax pseudoginseng is considered as an effective medicine in heart disease and hemostatic stasis [14]. In addition, the natural compounds are popular because of their low side effects [1]. Therefore, it is necessary to study the medical value of effective components in the plants.
Proliferation, apoptosis and metastasis of tumor cells are the key steps in the occurrence and development of cancer. The transcriptional factor p53 is widely recognized as an important tumor suppressor in cells, which could protect normal cell growth, initiate malignant cell death [15], results in the changes of different proteins’ expressions, including Bcl-2, Bax and caspase pathway, and finally suppress the proliferation of cells. In contrast, Bcl-2 gene acts in the opposite direction by encoding an anti-apoptotic protein. The p53, Bax and Bcl-2 genes are important regulators of apoptosis, and they have been found to play substantial roles in the pathogenesis of different types of cancers.
Breast cancer is the most commonly diagnosed malignancy among women [16] and one of the leading causes of cancer death in females in China [17]. It is difficult to be treated when metastasis occurs to bone and brain [18]. And the current medical care options of breast cancer, including surgery with adjunct radiotherapy, hormonal therapy and/or targeted biologic therapy have a lot of side effects. But, the compound from the Chinese herb may be effective.
Griffipavixanthone, a compound extracted from Garcinia oblongifolia Champ, has been reported to exhibit anti-proliferative effect on non-small cell lung cancer cell H520 [19] and esophageal cancer [20]. But, no research has yet been done about the effect of GPX on breast cancer. In this study, we examined the effect of GPX on human breast cancer cells and investigated the underlying mechanism of apoptosis induction by GPX. The results showed GPX could inhibit the proliferation, invasion and migration of human breast cancer cell MCF-7. Besides, GPX triggers the accumulation of p53 and Bax while it results in the decrease of Bcl-2.
Materials and methods
Reagents and siRNAs
GPX was received from Shixiu Feng as a gift [21]. Trypan blue and crystal violet were purchased from Sigma-Aldrich. 4% paraformaldehyde solution, annexin V-FITC Apoptosis Assay Kit (C1063), RIPA lysis buffer (p0013B) were purchased from Beyotime Company. The first antibodies for caspase-8 (AC056), caspase-9 (AC062), PARP (AP102) were obtained from Beyotime Company. P53 (SC-126) antibody was from Santa Cruz. Antibodies for Bax (2772) and Bcl-2 (2870) were from Cell Signaling Technology. The second antibodies (goat anti-rabbit and goat anti-mouse) and β-actin antibody were acquired from CWBIO company. The target sequences of si-p53 were 5′- GGUUCACUGAAGACCCAGTT-3′, 5′-CCACCAUCCACUACAACUATT-3′ and 5′-GACUCCAGUGGUAAUCUACTT-3′, while the sequence of control siRNA was 5′-UUCUCCGAACGUGUCACGUTT-3′.
Cell culture and transfection
MCF-7 human breast cancer cells were acquired from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The MCF-7 cells were cultured in DMEM medium (Gibco) with 10% fetal bovine serum, 100 units/ml of penicillin and streptomycin, which were incubated at 37 °C with 5% CO2. Transfections were carried out using Lipofectamine 2000 according to the manufacturer’s instructions.
Cell viability assay
5000 cells per well were plated at 96-well plate and treated with GPX at final concentrations of 0, 5, 10, 25, 50 and 100 µM until adherent (DMSO as negative control and culture medium as blank control). 10 µl CCK-8 solution was added into the well after incubated for 48 h. The 96-well plate was incubated at 37 °C for another 1 h and the absorbance was measured by the M200pro Multimode Plate Reader (Tecan, Switzerland) at 450 nm and 650 nm. IC50 was calculated by GraphPad Prism 6 (GraphPad software).
Cell proliferation assay
3 × 103 cells per well were plated at a 96-well plate. After adherence, various doses of GPX and DMSO (negative control) were added to the wells. 10 µl CCK-8 solution was added into the well after being incubated for 24 h. The 96-well plate was incubated at 37 °C for another 1 h and the absorbance was measured by the M200pro Multimode Plate Reader (Tecan, Switzerland) at 450 nm and 650 nm. The absorbance was measured every 24 h and the growth curve of breast cancer cells was plotted by GraphPad Prism 6 (GraphPad software).
Clonogenic assay
2000 cells were plated in two 60 mm dishes and cultured in medium with DMSO or 5 µM GPX. The cells were incubated at 37 °C incubator for 2–3 weeks. Then the cells in dishes were fixed by 4% paraformaldehyde solution and stained with 0.1% crystal violet for 10 min. Finally, the stained cell clones in dishes were pictured. Each treatment was repeated for triplicate.
Transwell for invasion and migration assay
50,000 cells treated with 0, 10 and 20 µM GPX were seeded into each well with serum-free medium at the upper compartment of the transwell plates (8 µm, Corning, Inc) coated with or without Matrigel Basement Membrane Matrix (BD). The medium with 10% FBS was added to the lower compartment of the chamber. After being cultured for 24 h, the cells going through the membrane were fixed with 4% formaldehyde and stained using 0.1% crystal violet and counted under a fluorescence microscope (Olympus IX-71, Tokyo, Japan). Each experiment was repeated for at least three times.
Apoptosis assay
About 50% cells were plated at 6-well plate and were treated with DMSO or various doses of GPX (5 µM, 10 µM, 20 µM) after adherence. The cells were incubated at 37 °C for 24 h and stained with Annexin V (AV)-FITC and propidium iodide (PI) according to the manufacturer’s instruction of Apoptosis Assay Kit. The stained cells were analyzed using Flow Cytometer (BD company, US). The experiment was repeated for three times.
RNA isolation and real-time PCR
MCF-7 cells were treated with various doses of GPX for 24 h. RNA was isolated using Trizol Reagent (Life Technologies) according to the manufacturer’s instructions. 1000 ng total RNA was reverse transcribed to cDNA using High-capacity cDNA Reverse Transcription Kits (TaKaRa). The real-time PCR was performed on the Bio-Rad CFX 96 Real-time PCR system using SYBR® PrimeScript™ RT-PCR Kit II (TaKaRa) and specific primers (Table 1). The primers were synthesized by Invitrogen Inc. The mRNA level of each gene was normalized to β-actin with the ΔΔCT method using Bio-Rad CFX Manager V1.1.308.1111 software. The experiment was repeated for three times.
Western blot
MCF-7 cells were treated with various doses of GPX for 48 h. Cells were collected and lysed with RIPA lysis buffer after the protein concentrations were determined using BCA assay. Proteins were denatured by boiling in 6× SDS sample buffer for 5 min at 95 °C and subjected to SDS–PAGE followed by Western blotting. Protein blots were probed with the indicated primary antibodies and appropriate secondary antibodies and protein bands were visualized using the ECL system. β-actin was used for the loading control.
Statistical analysis
All the data were expressed as mean ± SD. And all the experiments were repeated for at least three times. All the statistics were performed by GraphPad Prism 6 Program (GraphPad software, Inc.). A p value < 0.05 was considered as significant.
Results
GPX inhibits the proliferation, growth, invasion and migration of MCF-7 cells
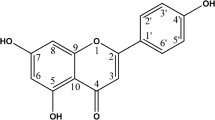
According to the published paper [21], the chemical structure of GPX was shown in Fig. 1a. First of all, we examined the IC50 value of breast cancer cells (Table 2). Besides, to investigate the effect of GPX on the breast cells’ proliferation, MCF-7, T-47D and MCF-10A cells were treated with four doses (0, 5, 10 and 20 µM) of GPX and incubated for 4 days to check the growth curve. At the same time, we compared the proliferation in breast cancer cells (T-47D, MCF-7) and normal cell MCF-10A. As shown in Fig. 1b, the proliferation of MCF-10A was much faster than T-47D and MCF-7, which indicated GPX had a slight side effect on normal cells compared with cancer cells. And the result showed that GPX inhibited the proliferation of MCF-7 and T-47D cells in a dose-dependent manner and the growth of cells was inhibited even at 5 µM. In addition, we carried out clonogenic assay to check the effect of GPX on the survivability of MCF-7 cells. The size and the number of colonies in GPX-treated group were much smaller and less compared with the control one (Fig. 1c), which indicated that GPX suppressed the proliferation of MCF-7 and T-47D cells significantly. Then, we examined the invasion and migration of MCF-7 with GPX (Fig. 1d). And the result showed that the invasion and migration of MCF-7 were suppressed obviously with the treatment of GPX. All the data above revealed that GPX could inhibit the proliferation, growth, invasion and migration of MCF-7 cells.
GPX inhibits the proliferation, growth and survivability of MCF-7 cells. a The chemical structure of GPX; b the growth curve of T-47D, MCF-7 and MCF-10A cells treated with four doses of GPX for 4 days; c the effect of GPX on the survivability of MCF-7 cells was evaluated by the size and quantity of cloning formation; d the invasion and migration of MCF-7 cells treated with three doses of GPX (scale bar: 200 µm); p < 0.05
GPX induced the apoptosis of MCF-7 cells
To examine the apoptosis of MCF-7 cells with or without GPX, the DNA content was measured by flow cytometry. As shown in Fig. 2, cells were treated with indicated doses of GPX and then stained with PI and Annexin V (AV)-FITC. The results suggested that GPX could induce the increasing percentage of both early (AV+/PI−) and late (AV+/PI+) apoptotic MCF-7 cells in a dose-dependent manner, which implied GPX could result in apoptosis of MCF-7.
Apoptotic analysis of MCF-7 treated with GPX by flow cytometry. The figure above showed the changes of MCF-7 cells treated with four doses of GPX in four stages: the intact cells (AV−/PI−), early apoptosis cells (AV+/PI−), later apoptosis cells (AV+/PI+) and the necrotic cells (AV−/PI+), and the figure below calculated the percentage of intact cells, necrotic cells and apoptotic cells, p < 0.05
Effect of GPX on p53 target genes and p53 protein
P53, as a tumor suppressor gene, has an important role in maintaining the growth of normal cells and inhibiting the growth of tumor cells. Being a transcription factor, p53 is closely connected with many cell pathways about apoptosis. To investigate the effect of GPX on p53 function, we examined p53 target genes’ expression using qRT-PCR. As shown in Fig. 3a, the mRNA level of these target genes (including Dr5, fas, p21, puma) were all up-regulated with doses of GPX. Meanwhile, we further examined the mRNA and protein level of p53. The results in Fig. 3b, c indicated that transcriptional activity of p53 gene and p53 protein level are enhanced by GPX in a dose-dependent manner. Moreover, we detected the cell viability and the apoptosis proteins by knock down p53, cells were transfected with p53 siRNA for 24 h and treated with 10 µM GPX for another 24 h, cell viability was detected by CCK-8 reagent. The result in Fig. 3d showed p53 knockdown groups promote the cell viability upon GPX, which indicated that p53 played a key role in the effect of GPX in MCF-7 cells. We furthermore investigated the effect of GPX on apoptosis proteins after p53 knockdown. The data in Fig. 3e showed that knockdown of p53 reduced Bax, PARP cleavage and increased Bcl-2. These results together suggested that triptolide may act in a p53-dependent manner.
Effect of GPX on p53 target genes and p53 protein. a The mRNA levels of p53 target genes were detected by qRT-PCR. β-actin was used as a control; the mRNA and protein levels of p53 were measured by qRT-PCR (b) and Western blot (c). β-actin was used as a control. p < 0.05. d Effect of p53 knockdown on cell toxicity of GPX. MCF-7 cells were transfected with p53 siRNA or negative siRNA for 24 h, and then treated with 10 µM GPX for another 24 h. Cell viability were normalized with the cells without GPX. e Effect of p53 knockdown on the induction of apoptosis proteins and PARP cleavage by GPX
Effect of GPX on caspases’ activities and Bax-Bcl-2 proteins
Caspases’ activities are considered as important regulators in cell apoptosis. In addition, the cleavage of PARP protein is also an induction of apoptosis. Because of caspase-3 deficiency in MCF-7 cells, we examined the protein levels of caspase-8, caspase-9 and PARP using Western blot. The results in Fig. 4a showed the caspase and PARP proteins were all cleaved in the cells treated with GPX compared to the protein without GPX. The cleaved protein bands of these proteins are increased, which the initial bands in the treatment without GPX disappear while the cleaved bands in the cells treated with GPX appear.
Bax and Bcl-2 are important proteins in cell apoptosis by releasing cytochrome C in mitochondria, which Bax induces apoptosis while Bcl-2 inhibits apoptosis. Here, to analyze the effects of GPX on Bax, Bcl-2 and cytochrome C, we examined the expression of these proteins using Western blot with or without GPX. As shown in Fig. 4b, Bax expression increased while Bcl-2 decreased in the treatment with GPX groups compared with control group. At the same time, cytochrome C in treatment groups was released. The results revealed that GPX could lead to MCF-7 cell apoptosis.
Discussion
GPX, extracted from Garcinia oblongifolia Champ, has been reported to exhibit an anti-proliferative effect on non-small cell lung cancer cell H520 [19] and esophageal cancer [20]. Dr. Shi’s research identified GPX and presented its anti-proliferative effect on NSCLC cell H520 with ROX generation. Dr. Ding showed that GPX could inhibit tumor metastasis and proliferation via down-regulating RAF–MEK–ERK pathway in esophageal cancer. However, there is no study on the effect of GPX on breast cancer. Because breast cancer is the most commonly diagnosed malignancy among women, it is necessary to examine the effect of GPX on breast cancer.
Apoptosis is a normal process in the growth of various cells [22]. The apoptosis of cells usually includes Bcl-2 expression and the elevation in Bcl-2 to Bax ratio prevents apoptosis and the cell survives [23]. On the other hand, apoptotic stimuli activates the expression of p53, resulting in a Bcl-2 suppression and Bax up-regulation [24]. In fact, the interaction of p53, Bcl-2 and Bax may cause a malignancy and p53–Bcl-2–Bax signaling pathway plays an important role in the axis of apoptosis in breast cancers [25].
In this study, we emphasize on the cell apoptotic detection of GPX on human breast cancer cell MCF-7. First of all, the growth curve, clonogenic assay and transwell assay (Fig. 1b–d) indicate that GPX could inhibit the proliferation, growth, invasion and migration of MCF-7 cells. Besides, AV-FITC/PI staining (Fig. 2) suggests GPX could induce cell apoptosis. Moreover, the growth curve of MCF-10A shows GPX has a minimal side effect to normal breast cell, which hints that GPX may be a potential drug for breast cancer. In the molecular part, we found that GPX increases the mRNA level of apoptosis related to gene p53 and its targeted genes in the treated cells (Fig. 3). Puma gene is a pro-apoptosis protein, combined with other Bcl-2 family proteins, resulting in the changes of the mitochondria membrane permeability, and accordingly caused cytochrome C release. In addition, we found that GPX increases the level of p53, pro-apoptosis protein Bax and cytochrome C in mitochondria while it decreases the level of anti-apoptosis protein Bcl-2 (Fig. 4b). And the releasing of cytochrome C usually result in the activation of caspases. Because of the deletion of caspase-3 in MCF-7, we then detected the activities of caspase-8/9 and PARP. The results of our study (Fig. 4a) show that PARP and caspase-8/9 are cleaved in GPX-treated cells, indicating that GPX induces MCF-7 apoptosis. All the results above demonstrate that GPX results in MCF-7 apoptosis and indicate GPX could be a potential drug for breast cancer.
In conclusion, our data showed GPX could increase the mRNA level of p53 gene and its target genes and change the protein level of p53–Bcl-2–Bax axis, which indicate GPX may induce MCF-7 apoptosis by regulating p53 and its association. However, the dependency of griffipavixanthone’s effects on p53 may be specific to MCF-7. T-47D cells possessed nearly the same IC50 with MCF-7 in spite of the fact that T-47D harbors L194F mutation in TP53 which impairs its function. And it still needs more investigation on the mechanisms of griffipavixanthone’s effects in T-47D. And the detailed mechanisms on how GPX affects other breast cancer cells like HER-2(+) also need to be further explored.
References
Zhu L, Ma W, Zhang M, Lee MM, Wong WY, Chan BD, et al. Scalable synthesis enabling multilevel bio-evaluations of natural products for discovery of lead compounds. Nat Commun. 2018;9(1):1283.
Yu J, Wang C, Kong Q, Wu X, Lu JJ, Chen X. Recent progress in doxorubicin-induced cardiotoxicity and protective potential of natural products. Phytomedicine. 2018;40:125–39.
Shamon LA, Pezzuto JM, Graves JM, Mehta RR, Wangcharoentrakul S, Sangsuwan R, et al. Evaluation of the mutagenic, cytotoxic, and antitumor potential of triptolide, a highly oxygenated diterpene isolated from Tripterygium wilfordii. Cancer Lett. 1997;112(1):113–7.
Chen SR, Dai Y, Zhao J, Lin L, Wang Y, Wang Y. A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front Pharmacol. 2018;9:104.
Zhang C, He XJ, Li L, Lu C, Lu AP. Effect of the natural product triptolide on pancreatic cancer: a systematic review of preclinical studies. Front Pharmacol. 2017;8:490.
Banerjee S, Sangwan V, McGinn O, Chugh R, Dudeja V, Vickers SM, et al. Triptolide-induced cell death in pancreatic cancer is mediated by O-GlcNAc modification of transcription factor Sp1. J Biol Chem. 2013;288(47):33927–38.
Song JM, Molla K, Anandharaj A, Cornax I, MG OS, Kirtane AR, et al. Triptolide suppresses the in vitro and in vivo growth of lung cancer cells by targeting hyaluronan-CD44/RHAMM signaling. Oncotarget. 2017;8(16):26927–40.
Cheng X, Shi W, Zhao C, Zhang D, Liang P, Wang G, et al. Triptolide sensitizes human breast cancer cells to tumor necrosis factor-alpha-induced apoptosis by inhibiting activation of the nuclear factor-kappaB pathway. Mol Med Rep. 2016;13(4):3257–64.
Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67(19):9407–16.
Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim Biophys Acta. 2015;1852(6):1178–85.
da Costa Araldi IC, Bordin FPR, Cadona FC, Barbisan F, Azzolin VF, Teixeira CF, et al. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem Biol Interact. 2018;282:85–92.
Aires V, Brassart B, Carlier A, Scagliarini A, Mandard S, Limagne E, et al. A role for peroxisome proliferator-activated receptor gamma in resveratrol-induced colon cancer cell apoptosis. Mol Nutr Food Res. 2014;58(9):1785–94.
Tousch D, Lajoix AD, Hosy E, Azay-Milhau J, Ferrare K, Jahannault C, et al. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem Biophys Res Commun. 2008;377(1):131–5.
Hong CY, Lai LJ, Yeh SF. Linoleate-rich triacylglycerol in Panax pseudo-ginseng improves erythrocyte deformability in vitro. Planta Med. 1993;59(4):323–5.
Zhao F, Huang W, Ousman T, Zhang B, Han Y, Clotaire DZ, et al. Triptolide induces growth inhibition and apoptosis of human laryngocarcinoma cells by enhancing p53 activities and suppressing E6-mediated p53 degradation. PLoS One. 2013;8(11):e80784.
Chen J, Wang X. MicroRNA-21 in breast cancer: diagnostic and prognostic potential. Clin Transl Oncol. 2014;16(3):225–33.
Chen L, Yang L, Yao L, Kuang XY, Zuo WJ, Li S, et al. Characterization of PIK3CA and PIK3R1 somatic mutations in Chinese breast cancer patients. Nat Commun. 2018;9(1):1357.
You MK, Kim HJ, Kook JH, Kim HA. St. John’s wort regulates proliferation and apoptosis in MCF-7 human breast cancer cells by inhibiting AMPK/mTOR and activating the mitochondrial pathway. Int J Mol Sci. 2018;19(4):966-80.
Shi JM, Huang HJ, Qiu SX, Feng SX, Li XE. Griffipavixanthone from Garcinia oblongifolia champ induces cell apoptosis in human non-small-cell lung cancer H520 cells in vitro. Molecules. 2014;19(2):1422–31.
Ding Z, Lao Y, Zhang H, Fu W, Zhu L, Tan H, et al. Griffipavixanthone, a dimeric xanthone extracted from edible plants, inhibits tumor metastasis and proliferation via downregulation of the RAF pathway in esophageal cancer. Oncotarget. 2016;7(2):1826–37.
Feng S, Jiang Y, Li J, Qiu S, Chen T. A new bixanthone derivative from the bark of Garcinia oblongifolia. Nat Prod Res. 2014;28(2):81–5.
Roca H, Jones JD, Purica MC, Weidner S, Koh AJ, Kuo R, et al. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J Clin Invest. 2018;128(1):248–66.
Zhu L, Hao J, Cheng M, Zhang C, Huo C, Liu Y, et al. Hyperglycemia-induced Bcl-2/Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp Cell Res. 2018.
Mirakhor Samani S, Ezazi Bojnordi T, Zarghampour M, Merat S, Fouladi DF. Expression of p53, Bcl-2 and Bax in endometrial carcinoma, endometrial hyperplasia and normal endometrium: a histopathological study. J Obstet Gynaecol. 2018;21:1–6.
Ren SX, Shen J, Cheng AS, Lu L, Chan RL, Li ZJ, et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One. 2013;8(5):e63641.
Acknowledgements
The authors thank Dr. Shixiu Feng for the gift (GPX). The authors are grateful for the help from Junmei Jia. The work was supported by the Startup Foundation for Doctors of Shanxi Medical University (BS03201645 to Y.M.) and the National Foundation of Shanxi Province (201601D021170 to B.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
Ma, Y., Wang, Y. & Song, B. Griffipavixanthone induces apoptosis of human breast cancer MCF-7 cells in vitro. Breast Cancer 26, 190–197 (2019). https://doi.org/10.1007/s12282-018-0912-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0912-2