Abstract
Background
CD44 and aldehyde dehydrogenase 1 (ALDH1) has been reputed to be cancer stem cell (CSC) markers in breast cancer. Yet, the clinicopathologic and prognostic significance of these markers remain unclear. In this study, we have investigated the expression of these markers and their relation with conventional clinicopathologic tumor characteristic including molecular subtype.
Methods
CD44 and ALDH1 expression were investigated by immunohistochemistry in a series of 157 formalin-fixed paraffin-embedded breast cancer tissues.
Results
Overall, CD44 and ALDH1 are, respectively, detected in 33% (52 of 157) and 7% (10 of 157) of breast cancer cases. We also observed that CD44 expression was associated with histological grade (p = 0.005). For ALDH1, we found that its expression is more frequent with elderly women (> 50 years, p = 0.03). The investigation of relationship between the stem cell phenotype and breast cancer molecular subtype, revealed that CD44 and ALDH1 expression was more frequent in basal-like tumors (p = 0.005). Among the two cancer stem cell markers tested, ALDH1 showed a strong association with the basal marker EGFR (p = 0.05).
Conclusions
These findings suggest that CD44 and ALDH1 play a role in the clinical behavior in breast cancer and might be interesting biomarkers and therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the principal cause of cancer death among women, with more than 1,500,000 cases worldwide each year [1]. Breast cancer is a complex disease with a large heterogeneity, leading to highly variable clinical behavior and response to therapy [2]. However, the mechanisms resulting in this heterogeneity in breast cancer are not well-understood [3].
One possible explanation for the tumor heterogeneity is the cancer stem cells. These cell subpopulations have the capacity to self-renew and differentiate into multiple cell types, and may contribute to drug resistance that promotes tumor recurrence or metastasis [4]. In breast cancer, cancer stem cells are principally identifiable by the expression of CD44 and ALDH1 [5].
CD44 is a class I transmembrane glycoprotein that serves as the primary receptor for hyaluronan and binds other extra-cellular matrix components, such as collagen, laminin, and fibronectin [6]. This protein has been shown to promote growth, invasion, and metastatic dissemination in breast cancer cells [7, 8].
ALDH1, an enzyme responsible for the oxidation of intracellular aldehydes, has been a subject of research focus in recent years [9, 10]. Previous researches reported that ALDH1 contributed to normal and tumor stem cell differentiation as well as invasion and metastasis in breast cancer [11, 12].
Based on recent information, it is evident to support the use of the CD44 cell surface marker in combination with the ALDH1 activity as an accurate method to identify cancer stem cells within breast tumors [5].
Recently, many clinical studies have reported that tumors expressing cancer stem cell markers are associated with aggressiveness and with tumor progression [3, 13]. However, other researches do not confirm this observation [14, 15]. This discrepancy makes it important to further investigate the expression of these markers in breast cancer to assess their pathologic and clinical significance.
In the current study, we have examined the expression of the proposed breast cancer stem cell markers CD44 and ALDH1 in a series of breast cancer. In addition, we have investigated whether the expression of these markers is associated with conventional clinicopathologic tumor features.
Patients and methods
Patients
This study includes 157 invasive ductual breast carcinomas obtained from the archives of the Department of Pathology, Farhat Hached Hospital of Sousse (Tunisia). The cases were selected based on the availability of sufficient paraffin-embedded tissue, before any treatment.
The patients’ age at diagnosis ranged from 31 to 87 years, with a mean of 48.9 years and a median of 46 years. Tumors were graded according to the modified Scarff-Bloom-Richardson system. The clinical stage of the disease was determined according to the tumor-node-metastasis (TNM) classification of the International Union against Cancer (UICC). Table 1 lists clinical and pathological characteristics of the patients, including age, histological grade, tumor size; hormone receptors (estrogen (ER), progesterone (PR)) and HER2 are available for all the cases. Axillary lymph node status was available for 129 patients.
For all patients, the surgical procedure consisted of patey mastectomy in conjunction with post-operative irradiation, chemotherapy and/or hormonal therapy according to standard protocols.
Tumors were grouped according to their ER, PR, and HER2 immunohistochemical status into 4 intrinsic subtypes according to Goldhirsch et al. [16]: luminal A (ER+ and/or PR+, HER2−, low Ki67), luminal B (ER+ and/or PR+, HER2+ and/or high Ki67), HER2 overexpressing (ER−, PR−, HER2+) and triple negative (ER−, PR−, HER2−). Taking into account the expression of basal markers cytokeratin 5/6 and EGFR, we classified triple negative tumors into basal-like (CK5+/6 and/or EGFR+) and non basal-like tumors (CK5/6− and EGFR−) [16].
Immunohistochemical identification of CD44 and ALDH1
The expression of cancer stem cell markers CD44 (clone DF1485, 1:100, Leica, Newcasttle, UK) and ALDH1 (clone 400M-15, 1:100, Cell Marque, Rocklin, California, USA) was investigated by immunohistochemistry using the EnVision Flex system (DakoCytomation, Glostrup, Denmark) according to the manufacturer’s instructions.
Briefly, paraffin-embedded breast cancer tissues were cut at 5 µm, dried overnight at 60 °C and deparaffinized in Ottix Plus (Diapath, Martinengo, Italy). Subsequently, the sections were hydrated with Ottix Shapper (Diapath, Martinengo, Italy), and rehydrated in water.
For antigen retrieval, the sections were boiled in a water bath with citrate buffer (0.01 M, pH 6.0) for 40 min until the temperature reached 98 °C. The sections were then allowed to cool at room temperature for 20 min. Later, they were and placed in EnVision Flex Wash buffer (DakoCytomation, Glostrup, Denmark). The endogenous peroxidase activity was blocked with EnVision Flex Peroxidase-Blocking Reagent for 5 min. The sections were thoroughly washed with the Wash buffer. The samples were incubated at 4 °C overnight with the primary antibody. Subsequently, the sections were rinsed gently with Wash buffer.
Immunostaining was performed using the high sensitive polymer-based EnVision Flex /HRP system. After being rinsed in wash buffer, the sections were incubated in 3, 3 diaminobenzidine, a substrate–chromogen solution for 20 min. Finally, the slides were counterstained with Mayer hematoxylin, permanently mounted, and viewed with a standard light microscope.
Evaluation of immunostaining
In all the cases, immunostaining results were evaluated independently by two pathologists (M.T. and S.Z.). CD44 positive staining was evaluated in the cell membrane. For ALDH1, cytoplasmic staining was detected, whereas nuclear staining alone was considered nonspecific and was not included in the analysis. For the two antibodies, a case was considered positive if more than 10% of the cells exhibited immunostaining for this antigen, otherwise, it was negative [17].
Statistical analysis
Statistical analysis was carried out with the SPSS software package (version 20.0; SPSS, Chicago, IL, USA). The correlation between the patients’ clinicopathologic features, CD44 and ALDH1 expressions was investigated by the Chi square test or Fisher exact test, where appropriate. A p value ≤ 0.05 was considered to indicate statistical significance.
Results
CD44 and ALDH1 expression in breast cancer
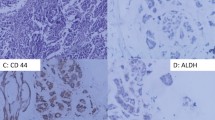
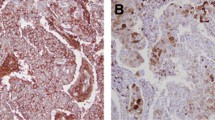
We analyzed CD44 and ALDH1 to identify the breast cancer cases with stem cell phenotype. Overall, 55 of the 157 (35%) breast cancer cases showed a stem cell phenotype with the expression of at least one of the stem cell markers. Indeed, 52 of the 157 (33%) breast cancer cases showed a strong and complete membranous CD44 expression in most tumor cells (Fig. 1). However, 10 of the 157 (7%) cases were classified as positive for ALDH1, showing a clear cytoplasmic expression in the tumor cells with a varying intensity and distribution (Fig. 2). Only 7 cases showed both CD44 and ALDH1 staining.
Examples of immunostaining for ALDH1 in breast cancer (original magnification, a, b × 100, c, f × 200, d, e × 400). Note the clear cytoplasmic expression of ALDH1 in the tumor cells (brown staining), with varying intensity and distribution, whereas the normal epithelial cells (arrows) remain negative
In the positive cases, the strong membranous expression of CD44 in the tumor cells contrasts with the absence of a detectable staining in the normal mammary epithelial cells. Furthermore, positive immunostaining for CD44 was also noted in the lymphocytes of the stroma in some cases (Fig. 1a). Regarding ALDH1, a focal staining was observed in some normal mammary lobules (Fig. 2b).
Correlation between CD44/ALDH1 expression and clinicopathologic parameters
As shown in Table 1, we found that CD44 expression was correlated with histological grade (p = 0.005). For ALDH1, we found that its expression is more frequently detected in women of advanced years (> 50 years, p = 0.03). In addition, a strong association was found between ALDH1 expression and the basal marker EGFR (p = 0.05).
Considering the expression of the two markers, we showed that breast tumors with stem cell phenotype (CD44+ and/or ALDH1+) were significantly correlated with high histological grade, as 48% (32/66) of the positive cases were grade III (p = 0.003), whereas 19% and 30% of the negative cases were, respectively, grade I and grade II. Moreover, a trend for correlation with progesterone receptor negativity was found (p = 0.06).
Association between intrinsic molecular subtypes and the expression of breast cancer stem cell markers
Among the 157 cases investigated in this study, 63 cases (23%) were luminal A, 21 cases (33%) were luminal B, 20 cases (30%) were HER2, and 53 cases (45%) were triple negative.
Taking into account the expression of basal markers cytokeratin5/6 and EGFR, we classified triple negative tumors into two sub-groups (basal-like and non basal-like).
The investigation of the relationship between the stem cell markers and breast cancer molecular subtype (Table 1) revealed a high prevalence of stem cell phenotype in triple negative tumors (45%) compared to luminal A (23%), luminal B (33%) and HER2 (30%) (p = 0.006).
In triple negative group, we found that tumors with stem cell phenotype were more frequent in basal-like than non basal-like tumors (p = 0.005).
Discussion
In the current study, we analyzed, through a large series of breast carcinomas, the expression of stem cell markers CD44 and ALDH1 to assess whether their expression is associated with a particular clinicopathologic feature.
We found that CD44 and ALDH1 were, respectively, expressed in 33% (52 of 157) and 7% (10 of 157) of breast cancer cases. Several studies have investigated those markers in breast cancer and they have reported variable rates of their expressions ranging from 20 to 55% for CD44 [3, 15] and 5 to 35% for ALDH1 [18,19,20,21,22,23,24,25,26].
Several factors have been involved to explain these differences in terms of prevalence of cancer stem cell markers expression in breast cancer. Those factors include the heterogeneity of the tested series due to differences in the inclusion criteria adopted in terms of the histological types [27] and the clinical stage [24, 26, 28, 29]. In addition, those differences might be the result of the differences in the experimental protocols used and the cutoff value adopted. In our study, we adopted a cutoff value of 10% as proposed by many other studies [3, 26, 28], whereas others used 5% [19,20,21] and 1% [11].
With regard to the clinicopathologic parameters, we found a strong correlation between CD44 expression and high histologic grade (p = 0.005). The last finding suggested that increasing CD44 expression may play a role in the tumor aggressiveness. This result was in agreement with several studies showing that CD44 expression was correlated with high histological grade, tumor growth, lymph node invasion and visceral metastases [3, 24].
Previous works have shown a significant association between ALDH1 expression and clinical aggressiveness parameters such as high tumor size, high histological grade and lymph node involvement [18,19,20,21,22,23,24,25,26]. In our data, we found no significant association between ALDH1 expression and any of the clinicopathologic parameters investigated. This might be due to the low number of ALDH1-positive cases in our series. It seems also the same in the work of Neumeister et al. [23], who found ALDH1 positivity in only 7% of their breast cancer cases.
On the other hand, we investigated whether an association existed between cancer stem cells and the molecular subtypes of breast cancers. We found more frequent cancer stem cell phenotype (ALDH1 and/or CD44 expression) in triple negative tumors (50%) than in HER2 (36%), luminal B (33%), or luminal A (24%) groups (p = 0.006). This finding is in accordance with many prior reports [10, 11, 30, 31]. It is well-documented that triple negative breast carcinomas are correlated with a worse prognosis than other molecular subtypes [30].
Taking into account the expression of basal markers, we showed a strong association between the expression of breast stem cell markers and basal-like breast cancer subtype (p = 0.005). This finding is consistent with several previous reports showing that basal-like tumors had more cancer stem cell phenotype than the other groups [10, 31]. The presence of basal-like trait was considered as an indicator of aggressive behavior and worse prognosis [31]. It has been hypothesized that those tumors derived from mammary luminal progenitor cells (estrogen receptor negative) are blocked at an early stage of differentiation and that such blockage was in relation with early inactivation of BRCA1 gene during the carcinogenesis [32]. In fact, breast cancer developed in BRCA1 germline mutation carriers were typically of basal-like subtype, possibly due to the crucial role BRCA1 in the differentiation of estrogen receptor-negative stem cells to estrogen receptor-positive luminal cells [33]. It has been also reported that sporadic breast cancer in which BRCA1 is inactivated by promoter hypermethylation or somatic mutations show histological features and clinical outcomes similar to those found in the tumors of BRCA1 germline mutated patients [34].
Current breast cancer treatment modalities target proliferating cells, but because the breast cancer stem cells are thought to be slowly cycling cells, they may escape the treatment when not actively proliferating [35]. This last fact may explain breast cancer treatment failures and relapse. Recent knowledge has proposed that therapies targeting CD44 may destroy the cancer stem cell population [27]. Indeed, promising pre-clinical studies focusing on CD44 targeting, including a monoclonal antibody directed against this antigen, have been highlighted [27]. Currently, new humanized anti-CD44 antibodies are under preclinical investigation for anti-cancer stem cell therapy to treat patients with metastatic or locally advanced malignant solid tumors expressing CD44 [36]. Two clinical trials, including a Phase III trial in metastatic colorectal cancer, have been very disappointing [27]. The therapeutic interest in this marker in breast cancer is not yet clear. In fact, targeting CD44 might hold a great promise for the cure of breast cancers particularly the triple negative/basal-like tumors.
Conclusions
In summary, we analyzed the expression of cancer stem cell markers CD44 and ALDH1 in a large series of breast cancer from Tunisian patients. We found that CD44 and ALDH1 are, respectively, expressed in 33 and 7% of cases. We also observed that breast tumors with stem cell phenotype were significantly correlated with the high histological grade and with the basal-like intrinsic molecular subtype. The results suggest that cancer stem cell markers especially CD44 might be interesting targets to develop new therapies particularly for triple negative/basal-like breast carcinomas.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; E359–E386.
Campbell LL, Polyak K. Breast tumors heterogeneity: cancer stem cells or clonal evolution. Cell Cycle. 2007;2332:6–8.
Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53.
Lawson JC, Blatch GL, Edkins AL. Cancer stem cells in Breast cancer and metastases. Breast Cancer Res. 2009;118:241 – 54.
Ricardo S, Vieira AF, Gerhard R, Leitao D, Pinto R, Cameselle-Teijeiro JF, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937 – 46.
Orian-Rousseau V. CD44, a therapeutic target for metastasin tumors. Eur J Cancer. 2010;46:1271–77.
Bourguignon LYW, Wong G, Earle C, Krueger K, Spevak CC. Hyaluronan–CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem. 2010;285:36721–35.
Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial–mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–74.
Zucchi I, Sanzone S, Astigiano S, Pelucchi P, Scotti M, Valsecchi V, et al. The properties of a mammary gland cancer stem cell. Proc Natl Acad Sci. 2007;104:10476–81.
Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci. 2006;103:11707–12.
Povsic TJ, Zavodni KL, Kelly FL, Zhu S, Goldschmidt-Clermont PJ, Dong C, et al. Circulating progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol. 2007;50:2243–48.
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cells lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13.
Buess M, Rajski M, Vogel-Durrer BM, Herrmann R, Rochlitz C. Tumor-endothelial interaction links the CD44(+)/CD24(–) phenotype with poor prognosis in early-stage breast cancer. Neoplasia. 2009;11:987–1002.
Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, et al. The clinicopathologic and prognostic significance of CD44+/CD24–/low and CD44–/CD24 + tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39:1096–102.
Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24–/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–59.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2013;24:2206 – 223.
Tan EY, Thike AA, Tan PH. ALDH1 expression is enriched in breast cancers arising in young women but does not predict outcome. Br J Cancer. 2013;109:109–13.
Neumeister V, Agarwal S, Bordeaux J, Camp RL, Rimm DL. In situ identification of putative cancer stem cells by multiplexing ALDH1, CD44, and cytokeratin identifies breast cancer patients with poor prognosis. Am J Pathol. 2010;176:2131–38.
Nogami T, Shien T, Tanaka T, Nishiyama K, Mizoo T, Iwamto T, et al. Expression of ALDH1 in axillary lymph node metastases is a prognostic factor of poor clinical outcome in breast cancer patients with 1–3 lymph node metastases. Breast Cancer. 2012;21:58–65.
Sakakibara M, Fujimori T, Miyoshi T, Nagashima T, Fujimoto H, Suzuki HT, et al. Aldehyde dehydrogenase 1-positive cells in axillary lymph node metastases after chemotherapy as a prognostic factor in patients with lymph node-positive breast cancer. Cancer. 2012;118:3899–910.
Dong Y, Bi LR, Xu N, Yang HM, Zhang HT, Ding Y, et al. The expression of aldehyde dehydrogenase 1 in invasive primary breast tumors and axillary lymph node metastases is associated with poor clinical prognosis. Pathol Res Pract. 2013;209:555–61.
Morimoto K, Kim SJ, Tanei T, Shimazu K, Tanji Y, Taguchi T, et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci. 2009;100:1062–68.
Kapucuoglu N, Bozkurt KK, Baspınar S, Kocer M, Eroglu HE, Akdeniz R, et al. The clinicopathological and prognostic significance of CD24, CD44, CD133, ALDH1 expressions in invasive ductal carcinoma of the breast CD44/CD24 expression in breast cancer. Pathol Res Pract. 2015;211:740–47.
Tsang JYS, Huang YH, Luo MH, Ni YB, Chan SK, Lui PCW, et al. Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;136:407–17.
Chekhun SV, Zadvorny TV, Tymovska YO, Anikusko MF, Novak OE, Polishchuk LZ. CD44+/CD24− markers of cancer stem cells in patients with Breast cancer of different molecular subtypes. Exp Oncol. 2015;37:58–63.
Uchoa DM, Graudenz MS, Callegari-Jacques SM, Hartmann CR, Ferreira BP, Fitarelli-Kiehl M, et al. Expression of cancer stem cell markers in basal and penta-negative breast carcinomas—a study of a series of triple-negative tumors. Pathol Res Pract. 2014;210:432 – 39.
Yan Y, Zuo X, Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4(9):1033–43.
Makki J, Myint O, Wynn AA, Samsudin AT, John DV. Expression distribution of cancer stem cells, epithelial to mesenchymal transition, and telomerase activity in breast cancer and their association with clinicopathologic characteristics. Clin Med Insights Pathol. 2015;8:1–16.
Kim YS, Jung MJ, Ryu DW, Lee CH. Clinicopathologic characteristics of breast cancer stem cells identified on the basis of aldehyde dehydrogenase 1 expression. J Breast Cancer. 2014;17:121 – 28.
Idowu MO, Kimeciak M, Dumu C, Burton RS, Grimes MM, Powers CN, et al. CD44+/CD24− cancer stem: progenitor cells are more abundant in triple-negative invasisve breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43:364 – 73.
De Beça FF, Caetano P, Gerhard R, Alvarenga CA, Gomes M, Paredes J, et al. Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J Clin Pathol. 2013;66:187 – 91.
Dontu G, El-Ashry D, Wisha MS. Breast cancer stem:progenitor cells and estrogen receptor. Trends Endocrinol Metab. 2004;15:193 – 97.
Liu S, Ginestier C, Charafe-Jauffret E, Focco H, Kleer CG, Merajver SD, et al. BRCA1 regulates human mammary stem:progenitor cell fate. Proc Natl Acad Sci USA. 2008;105:1680–85.
Turner N, Tutt A, Ashworth A. Hallmarks of “BRCAness” in sporadic cancers. Nat Rev Cancer. 2004;4:814–19.
Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–7.
Sahin IH, Klostergaard J. CD44 as a drug delivery target in human cancers: where are we now? Expert Opin Ther Targets. 2015;16:1–5.
Acknowledgements
The authors acknowledge Mr. Boukataya Samir (English teacher at the Faculty of Dental Medicine, Tunisia) for English editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
About this article
Cite this article
Louhichi, T., Ziadi, S., Saad, H. et al. Clinicopathological significance of cancer stem cell markers CD44 and ALDH1 expression in breast cancer. Breast Cancer 25, 698–705 (2018). https://doi.org/10.1007/s12282-018-0875-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0875-3