Abstract
Background
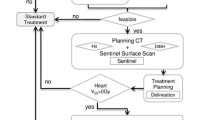
We evaluated the effectiveness of visual feedback (VF) on radiotherapy with deep inspiration breath-holding (DIBH), and reviewed the dose for organs at risk (OARs).
Methods
Respiratory motions during DIBH for 15 s were monitored during planning computed tomography (CT)-scanning and simulation for 40 patients after breast-conserving surgery from June 2007 to February 2008. For 22 of 40 patients, a goggle-type liquid crystal display monitor was used for VF. The opposing tangential field was planned. The prescribed dose was 50.0 Gy in 25 fractions.
Results
The mean differences of the chest wall respiratory movement in DIBH between planning CT-scanning and simulation were 4.7 ± 2.6 mm for the patients without VF and 1.0 ± 0.9 mm for those with VF (p < 0.01). The mean chest wall excursion as a whole in DIBH using VF (2.0 ± 1.0 mm) was smaller than in those without VF (4.1 ± 2.4 mm) (p < 0.01). According to reproducibility and stability parameters, 4 mm was added as a posterior margin to the clinical target volume for RT with VF, and 10 mm for those without VF. The mean heart doses were 1.3 ± 0.5 Gy with VF and 2.4 ± 1.1 Gy without VF (p < 0.01). Mean dose and max dose of right breast were significantly reduced in procedures with VF use vs. in those without VF (p < 0.01 and < 0.01, respectively).
Conclusions
VF increases the accuracy of postoperative radiotherapy with DIBH, and also helps reduce the dose for OARs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative radiotherapy (RT) after breast-conserving surgery helps in achieving local control and in improving survival [1]. However, it has been reported that the cardiac-related mortality and morbidity increased 10 years or later after postoperative RT [2, 3]. For reducing the irradiated heart volume, the technique of RT using breath-adapted radiation therapy (BART) was considered [4]. In Japan, from April 2018, BART is covered by health insurance for left-sided breast cancer patients receiving postoperative RT. We previously reported that RT on deep inspiration breath-holding (DIBH) could reduce the irradiated heart volume compared to that on free breath (FB) and end-inspiration gating (EIG) [5]. For administering RT with DIBH, it is necessary to keep the chest wall positions on the same level as during planning computed tomography (CT) for approximately 15 s, if RT was administered using by a linear accelerator delivering 600 monitor units/min [6]. However, the margin from the clinical target volume (CTV) to the planning target volume (PTV) in RT planning with DIBH tends to enlarge compared to that with FB and EIG [4, 7], which may increase the dose to the contralateral breast. Recent studies showed that visual feedback (VF) resulted in better amplitude control [8], although there have been few studies evaluating dose-volume histograms (DVHs) of RT with DIBH using VF compared to that without VF. This study evaluated the feasibility and reproducibility of RT during DIBH with and without VF. In our previous study, we planned the tangential field anatomically, while the breast as a CTV was contoured according to European Society for Radiation Oncology (ESTRO) guidelines [9, 10], and the posterior margins, which were calculated on the basis of reproducibility, were added in this study. Furthermore, we investigated whether the improvement in reproducibility led to reduced dose to organs at risk (OARs).
Methods and materials
Patient characteristics
Between June 2007 and February 2008, 233 breast cancer patients received postoperative RT after breast-conserving surgery. We included patients as per following criteria: (1) left-sided breast cancer; (2) receiving breast-conserving surgery; (3) aged ≤ 65 years; (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; (5) no history of cardiac or pulmonary surgery; and (6) provided informed consent.
This study has been approved by the appropriate ethics committee (the institutional review board approved study number; 2017–1063) and has therefore been performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki and all subsequent revisions.
A series of 40 patients were candidates for this study. Informed consents were obtained according to the institutional review board. Respiratory motion without VF was obtained for 18 of 40 patients between June and September 2007, and that with VF for 22 patients, between October 2007 and February 2008.
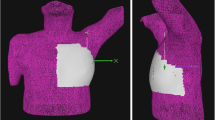
We previously reported the details of a respiratory-gating RT system [5]. Before the planning CT-scanning, all 40 patients were coached on how to maintain their breathing at a constant volume and rate by expert radiation therapy technologists. From October 2007, a goggle-type liquid crystal display (LCD) monitor was used to guide patients’ breathing and chest wall position for 22 patients (Fig. 1a, b). The patients could look own chest wall position projected on LCD monitor in real time (Fig. 1b). Yellow band reflected chest wall position, and changed to green when the yellow band was within blue band. The height of blue band was 4 mm, and superior position of blue band was set same as the patient’s maximum level of deep inspiration measured in the DIBH training. The chest wall respiratory motion during the FB phase and the DIBH phase with 15 s of breath-holding was monitored in planning CT-scanning and simulation using a RPM system (Varian Medical System Inc.). Chest wall respiratory movements were monitored by a RPM marker (an infrared reflecting marker) placed on the right chest, to determine differences in chest wall vertical position between inspiration and end-expiration. The chest wall respiratory movement for FB was defined as the height between end-expiration and end-inspiration, and shown as asterisk (*) in Fig. 2a, and was calculated for respiration curves during 30 s. Chest wall positions of respiratory movement for DIBH were obtained after reiterated training (5–10 min of individual coaching by expert radiation therapy technologists) on the treatment position just before planning CT-scanning. The chest wall movement for DIBH was defined as the vertical position between end-expiration and the maximum level of deep-inspiration, and shown as asterisk (*) in Fig. 2b. For each patient, the difference in chest wall movement for DIBH between planning CT-scanning and simulation was calculated based on the movement of a RPM marker. When planning CT-scanning was performed, we mark the area where a RPM marker was placed. A RPM marker was correctly placed on the same area as the time of planning CT-scanning and simulation. In the DIBH phase, we measured the chest wall excursion which was defined as the vertical position of the chest wall during 15 s of breath-holding and shown as dagger (†) in Fig. 2b.
Chest wall position of CT-scanning and simulation with or without visual feedback. A goggle type LCD monitor (Fig. 1a) and anteroposterior movement of a RPM marker is projected in real time on LCD monitor (Fig. 1b). Yellow band reflects the chest wall position in real time and moves to upward when the patient breathed in. When the band was within blue band, the yellow band changed to green
Respiratory function test
Respiratory function test was performed for all patients before surgery. No patients had history of pulmonary surgery, and experienced pulmonary complication after surgery.
Planning study
The CT slice thickness was 5.0 mm. For each patient, left-sided breast as the clinical target volume (CTV) and right-sided breast were delineated according to the ESTRO guidelines [9, 10]. The planning target volume (PTV) was constructed by adding a 5 mm margin to the CTV. However, a posterior margin was created according to the chest wall excursion and the difference in chest wall movement during DIBH between planning CT-scanning and simulation with or without visual feedback. Based on the difference in chest wall movement between planning CT-scanning and simulation, we calculated the margin including an organ motion and a setup error in DIBH, and also calculated the margin as an organ motion in DIBH based on chest wall excursion. Finally, a PTV margin, which included an organ motion and a setup error, was calculated by using the following formulas reported from Van Herk [11]:
Σ and σ were calculated for both chest wall excursion (Σexcursion and σexcursion) and differences in chest wall movements between planning CT-scanning and simulation (Σdifference and σdifference), as per the following formula:
N number of patients; xi chest wall excursion or difference in chest wall movements between planning CT-scanning and simulation for each patient; \(\overline {x}\) an average of chest wall excursions or differences in chest wall movements between planning CT-scanning and simulation for all patients.
\(\sigma\) standard deviation of chest wall excursions or differences in chest wall movements between planning CT-scanning and simulation.
Finally, to the CTV, 4 mm was added as a posterior margin in procedures with VF, and 10 mm was added in procedures without VF. The following normal organs were contoured: right-sided breast, lung, and heart. Whole lung were contoured using pulmonary windows, and hilar, trachea and main bronchus were excluded. Whole heart was contoured based on previously published guideline [12]. The prescribed dose was 50.0 Gy in 25 fractions and PTV coverage of the dose distribution was maintained between 95 and 107% of the prescribed dose. The appropriate opposing tangential 6-MV photon field with field-within-field technique was planned using the Varian Medical Eclipse software ver. 13.6.
Statistical analysis
We compared the following parameters between patients with and without VF use, by employing a Mann–Whitney U test: age, lung function, measurement values of respiratory curves during FB and DIBH, and dose-volume parameters for OARs. The chest wall movements for DIBH between planning CT-scanning and simulation were compared by using Wilcoxon signed-rank test. Surgery, tumor location and clinical staging were analyzed using the Fisher’s exact test. Statistical significance was set to < 0.05. Statistical analyses were performed by using the SPSS Base System software program version 24.0.0.0 (SPSS, Chicago, IL) and the SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Patients’ baseline characteristics
The median age of all patients was 50 years. Eight patients were in clinical stage 0, 19 in IA, and 13 in stage IIA. All patients were treated with partial mastectomy as an initial treatment. Tumor location was classified as inner or outer quadrant based on where the tumor was mainly located. There was no significant difference in the tumor location between the groups with and without VF (p = 0.64). All 40 patients were diagnosed as having favorable cardio-pulmonary function using a cardiac and respiratory function test performed before surgery. Patient characteristics are summarized in Table 1.
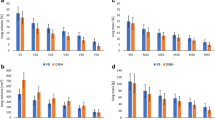
The mean chest wall movement for FB was: without VF: 4.7 ± 2.7 mm, and with VF: 5.3 ± 1.9 mm (p = 0.06) (Table 2). The mean chest wall movements for DIBH in planning CT-scanning and simulation were 22.1 ± 6.1 mm and 21.6 ± 5.7 mm without VF, and 18.6 ± 4.0 mm and 18.4 ± 3.5 mm with VF, respectively (p = 0.74 and 0.53, respectively) (Table 2; Fig. 3). The mean differences in chest wall movements during DIBH between planning CT-scanning and simulation were 4.7 ± 2.6 mm and 1.0 ± 0.9 mm, respectively (p < 0.01) (Table 2; Fig. 3). The mean chest wall excursion as a whole in DIBH using VF (2.0 ± 1.0 mm) was smaller than in those without VF (4.1 ± 2.4 mm) (p < 0.01). VF significantly helped patients maintain chest wall position during DIBH (for 15 s-breath holding).
Table 3 shows the dose-volume parameters for OARs such as lung, heart, and the right-sided breast. VF significantly reduces the lung volume receiving 20 Gy (lung V20) (p < 0.01). Furthermore, heart volume receiving 25 Gy or more than (Heart V25) was significantly reduced by using VF (p < 0.01). The mean heart doses were 1.3 ± 0.5 Gy using VF and 2.4 ± 1.1 Gy without using VF (p < 0.01). The mean max doses of the right-sided breast were 4.2 ± 4.0 Gy using VF and 17.1 ± 15.2 Gy without using VF (p < 0.01). The mean right-sided breast doses were also significantly reduced by the use of VF (p < 0.01). In 3 of 18 patients scanned without VF, the mean dose of the right-sided breast exceeded 3.0 Gy, while that all patients scanned with VF was less than 3.0 Gy.
Discussion
We have previously reported that the irradiated left ventricular volumes with EIG and DIBH were significantly smaller than that with FB [5]. In other reports, DVH with DIBH led to reduce irradiated heart and lung volumes compared to that with EIG and FB [4, 13], although the margin in RT planning with DIBH was larger than that with FB and EIG [4]. By using VF, a better reproducibility and stability of the patients’ chest wall position may be obtained [13, 14], and Cerviño et al. reported that the average reproducibility changed from 2.1 mm without VF to 0.5 mm with VF [8]. Furthermore, in other reports, the chest wall excursion using VF and audio coaching was within 2.0 mm [15]. In this study, by using VF, we could significantly improve the differences between the chest wall movements during planning CT-scanning and simulation, and the chest wall excursion with DIBH for 15 s. Korreman et al. reported that 12 mm was added as the margin from CTV to the edge of fields in RT planning with DIBH without VF [4]. On the other hand, in RT planning DIBH with VF, 5 mm was added as a PTV margin [15]. We calculated a posterior margin to the CTV on the basis of reproducibility, and added 4 mm for cases with VF and 10 mm for those without VF in this study.
The incidence of cardiac-related mortality 10 years or more after postoperative RT was significantly higher in patients with left-sided breast cancer vs. that in patients with right-sided breast cancer [2, 3]. Darby et al. reported that the incident rates of the major coronary events increased linearly with the mean heart dose by 7.4% per Gy without an apparent threshold in breast cancer patients who received adjuvant RT [16]. In the NSABP B-51/RTOG1304 trial, the mean heart dose was proposed to be 4.0 Gy or less for left-sided whole breast or chest wall-irradiated patients [17]. In this study, the mean heart doses both with and without VF were < 4 Gy, although the mean heart dose with VF (1.3 Gy) was significantly reduced compared to that without VF (2.4 Gy) (p < 0.01). Henson et al. reported that cardiac morbidity was significantly higher in left-sided vs. right-sided breast cancer patients treated with postoperative RT during 1973–1982, but was not different in those treated during 1983–1992 [18]. This may have been due to modified RT protocols in the United States since the 1980s, with reduced irradiation of the internal mammary chain (IMC). In recent reports, the mean heart dose while irradiating the whole breast by using opposing tangential fields was about 2.0 Gy [19, 20], and about 3.8 Gy while irradiating the whole breast and regional lymph nodes [21]. DIBH using VF may help to reduce the dose to the heart even further in whole breast and regional lymph node-irradiated patients. The chest wall position of the matching line in the half-beam technique should be estimated by using a four dimensional CT, if the regional lymph node is included in RT with DIBH using VF. One limitation of this study is that four dimensional CT was not used. Further study with the use of four dimensional CT is necessary to more accurately evaluate the DVH parameters of the OARs on considering the reproducibility of the chest wall for DIBH.
The incidence of second malignancies after postoperative RT in breast cancer patients significantly increased compared to that in the general population [22]. Furthermore, patients treated with adjuvant RT, in particular those aged 45 years or younger, experienced a significantly higher risk of contralateral breast cancer [22,23,24]. Boice et al. reported that the average dose to the contralateral breast was 2.82 Gy in patients receiving radiation doses to the chest wall and regional lymph nodes including the IMC [24]. In this study, the mean doses to the right-sided breast in both cases with and without VF were lower than 1 Gy. However, in 3 of 18 patients scanned without VF, the mean dose to the right-sided breast exceeded 3.0 Gy, while all patients scanned with VF received less than 3.0 Gy. The dose to the contralateral breast probably depends on a posterior margin, not BART, because the relationship between the position of the ipsilateral and contralateral breasts do not change under any breathing conditions. Smaller posterior margins, which were obtained by using VF, led to reduced dose to the contralateral breast.
VF could improve the reproducibility and stability of the chest wall position, and this led to reduce doses to OARs such as the heart and the contralateral breast.
In conclusion, VF helps to significantly reduce scatter and excursions of the respiratory chest wall motion. This increases the accuracy of postoperative RT with DIBH, and also leads to reduced doses to the heart and the contralateral breast.
References
Clarke M, Collins R, Darby A, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet 2005; 366:2087–106.
Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study about 300000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65.
McGale P, Darby SC, Hall P, Adolfsson J, Benqtsson NO, Bennet AM, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–75.
Korreman SS, Pederson AN, Nottrup TJ, Specht L, Nyström H. Breathing adapted radiotherapy for breast cancer: comparison of free breathing gating with the breath-hold technique. Radiother Oncol. 2005;76:311–18.
Nemoto K, Oguchi M, Nakajima M, Kozuka T, Nose T, Yamashita T. Cardiac-sparing radiotherapy for the left breast cancer with deep breath-hold. Jpn J Radiol. 2009;27:259–63.
Lee JW, Hong S, Choi KS, Kim YL, Park BM, Chung JB, et al. Performance evaluation of field-in-field technique for tangential breast irradiation. Jpn J Clin Oncol. 2008;38:158–63.
Pederson AN, Korreman SS, Nyström H, Specht L. Breathing adapted radiotherapy of breast cancer: reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol 2004;72:53–60.
Cerviño LI, Gupta S, Rose MA, Yashar C, Jiang SB. Using surface imaging and visual coaching to improve the reproducibility and stability of deep-inspiration breath hold for left breast-cancer radiotherapy. Phys Med Biol. 2009;54:6853–65.
Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10.
Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol. 2016;118:205–8.
van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–35.
Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–8.
Damkjær SM, Aznar MC, Pedersen AN, Vogelius IR, Bangsgaard JP, Josipovic M. Reduced lung dose and improved inspiration level reproducibility in visually guided DIBH compared to audio coached EIG radiotherapy for breast cancer patients. Acta Oncol. 2013;52:1458–63.
Kini VR, Vedam SS, Keall PJ, Patil S, Chen C, Mohan R. Patient training in respiratory-gated radiotherapy. Med Dosim. 2003;28:7–11.
Vikstrom J, Hjelstuen MH, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol. 2011;50:42–50.
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Eng J Med. 2013;368:987–98.
Breast NSA, Project BNSABP., Protocol B-51: A randomized phase III clinical trial evaluating post-mastectomy chest wall and regional nodal xrt and post-lumpectomy regional nodal xrt in patients with positive axillary nodes before neoadjuvant chemotherapy who convert to pathologically negative axillary nodes after neoadjuvant chemotherapy. NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines), Breast Cancer (version 1. 2014). 2014. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 28 Jan 2014.
Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179–82.
Schubert LK, Gondi V, Sengbusch E, Westerly DC, Soisson ET, Paliwal BR, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol. 2011;100:241–6.
Jagsi R, Ben-David MA, Moran JM, Marsh RB, Griffith KA, Hayman JA, et al. Evaluation of four techniques using intensity-modulated radiation therapy for comprehensive locoregional irradiation of breast cancer. Int J Radiat Oncol Biol Phys. 2010;78:1594–603.
Yeung R, Conroy L, Long K, Walrath D, Li H, Smith W, et al. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiat Oncol. 2015;10:200.
Hamilton SN, Tyldesley S, Li D, Olson R, McBride M. Second malignancies after adjuvant radiation therapy for early stage breast cancer: is there increased risk with addition of regional radiation to local radiation? Int J Radiat Oncol Biol Phys. 2015;91:977–85.
Hooning MJ, Aleman BM, Hauptmann M, Baaijens MH, Klijn JG, Noyon R, et al. Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J Clin Oncol. 2008;26:5561–8.
Boice JD, Harvey EB, Blettner M, Stovall M, Flannery JT. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781–5.
Acknowledgements
This work was supported by University of Tsukuba.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest for all authors.
About this article
Cite this article
Murofushi, K.N., Nakajima, M., Tomita, T. et al. Impact of visual feedback on dose-volume histograms for organs at risk in postoperative radiotherapy with deep inspiration breath-holding for patients treated with breast-conserving therapy: a planning study. Breast Cancer 25, 656–662 (2018). https://doi.org/10.1007/s12282-018-0870-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0870-8