Abstract
Background
For premenopausal women with breast cancer, information on the effects of chemotherapy and the risk of infertility is important. In this study, the effect of chemotherapy on the ovarian function in premenopausal women with hormone receptor-positive breast cancer was investigated, with an age-stratified analysis of the appearance of amenorrhea and the resumption of menstruation after the use of chemotherapy with anthracyclines or taxanes.
Patients and methods
Premenopausal women diagnosed with operable Stage I–III hormone receptor-positive breast cancer and underwent neoadjuvant or adjuvant chemotherapy with the standard regimen of anthracyclines and/or taxanes were included. The patients were classified into age groups in 5-year increments, and the rates of chemotherapy-induced amenorrhea (CIA), resumption of menstruation, and duration of CIA after chemotherapy were analyzed.
Results
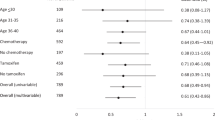
The subjects consisted of 101 patients (median age 45 years). CIA occurred in 97 (96%) patients and 40 patients resumed menstruation. In all patients aged ≤39 years menstruation restarted, whereas in all patients aged ≥50 years, menstruation did not restart. For the patients who resumed menstruation, the younger the patients, the sooner menstruation tended to restart. The resumption of menstruation occurred within 1 year for younger patients aged around 30 years, but for those aged ≥35 years, 60% of cases took around 2–3 years for resumption.
Conclusions
The incidence of CIA, the resumption of menstruation and duration of CIA after chemotherapy depend greatly on the patient’s age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of women diagnosed with early stage invasive breast cancer are candidates to receive systemic treatments to reduce the risk of recurrence and improve the prognosis [1]. Endocrine therapy is the most important therapeutic strategy for hormone receptor (HR)-positive breast cancer. However, even for patients with HR-positive breast cancer, chemotherapy is recommended when they are at high risk of recurrence [1]. Chemotherapy may cause various side effects, including chemotherapy-induced amenorrhea (CIA) and premature ovarian failure [2]. Prolonged ovarian failure and early menopause induce various adverse effects for women, such as hot flashes, depressive status, cardiovascular diseases, and osteoporosis [3]. For young women with breast cancer in particular, CIA and premature ovarian failure are a great concern, as these may cause infertility. The guidelines issued by the American Society of Clinical Oncology (ASCO) in 2013 indicate the risk levels for amenorrhea for different chemotherapy regimens and recommends that patients be asked whether or not they want children and be fully informed of the risk of infertility before undergoing chemotherapy [4].
Some previous studies have shown that premenopausal women in whom CIA developed had significantly better prognoses than women without CIA, particularly those whose tumors were estrogen receptor (ER)-positive [5, 6]; however, other studies showed no survival impact (review by Walshe et al.) [2]. The risk for CIA is associated with the patient’s age and the chemotherapeutic agents used, with age >40 years being the strongest predictor of ovarian failure. Use of tamoxifen (TAM) after chemotherapy induces a higher rate of prolonged CIA [2, 7, 8]. The effect of chemotherapy on the ovarian function must therefore be considered on an individual basis.
Information provided by an analysis of the effect of age would be particularly useful; however, there are few reports regarding the relationships between age and CIA and the resumption of menstruation for Japanese women with early breast cancer. For Japanese women, Okanami et al. reported that 48 of 66 patients (87.9%) experienced amenorrhea during chemotherapy, and 14 patients (21.2%) had persistent amenorrhea after chemotherapy [9]. The incidence and persistence of amenorrhea was higher in the patients older than 36 years of age [9].
In the preset study, we investigated the incidence of CIA and the resumption of menstruation in premenopausal women with HR-positive breast cancer according to their age.
Patients and methods
Patient population
The study subjects were premenopausal women diagnosed with Stage I–III HR-positive breast cancer who were treated with neoadjuvant or adjuvant chemotherapy in the Department of Breast Oncology, National Hospital Organization Kyushu Cancer Center between 2004 and 2009. Chemotherapy was administered according to the standard regimen of anthracyclines and/or taxanes. The clinical data were obtained from the patients’ medical records. The AJCC/UICC TNM classification and stage groupings were used.
Women who became amenorrheic during or after chemotherapy were defined as having developed CIA. In this study, CIA was defined as disappearing the periodical menstruation, not prolonged menstruation. If menstruation was not disappeared at the time of the last chemotherapy, it was defined as “No CIA”. When the menstruation was appeared periodically again, the state was defined as “a resumption of menstruation”. If menstruation had not restarted after 4 years’ follow-up, it was defined as “No resumption of menstruation”. Between 2004 and 2009, 229 premenopausal women with HR-positive breast cancer received neoadjuvant or adjuvant chemotherapy, and among them, the menstruation status after chemotherapy was confirmed in 114 patients. 30 patients were recurred and 24 of them were recurred within four years. Patients who received endocrine therapy with luteinizing hormone-releasing hormone agonist (LH-RHa) after chemotherapy were excluded. Patients in whom breast cancer recurred within four years after the completion of chemotherapy and who had not resumed menstruation at the time of recurrence were also excluded on the grounds of the possibility that their subsequent treatment might have affected the restoration of ovarian function. A total of 101 premenopausal women with HR-positive breast cancer were ultimately included in this study. All patients received 20 mg/day of TAM after completing chemotherapy. When menstruation was resumed after CIA, LH-RHa was added to TAM after discussing about the benefit of its sequential use in some patients. Patients were classified into age groups in 5-year increments (≤29, 30–34, 35–39, 40–44, 45–49, and ≥50 years), and the rates of CIA and resumption of menstruation after chemotherapy were analyzed.
Chemotherapy regimen
The chemotherapy regimen used comprised an anthracycline [5-FU + epirubicin + cyclophosphamide (FEC)/epirubicin + cyclophosphamide (EC)/adriamycin + cyclophosphamide (AC)] followed by a taxane [docetaxel (DTX) or paclitaxel (PTX)], anthracycline (FEC/EC) alone, or DTX and cyclophosphamide (TC). The doses of each agent in each regimen were as follows: FEC, 500/100/500 mg/m2; EC, 75/600 mg/m2; AC, 60/600 mg/m2; DTX, 75 mg/m2; PTX, 80 mg/m2 (weekly); and TC, 75/600 mg/m2.
Statistical analyses
The statistical analyses were performed using the JMP software package, version 9.0.2 (SAS Institute Inc., Cary, NC, USA). The associations between the duration of the chemotherapy and menstruation status was assessed using χ 2 tests. Differences were considered to be significant at p ≤ 0.05.
Results
Chemotherapy-induced amenorrhea (CIA), menopause, and resumption of menstruation
A median age of the 101 patients was 45 years (range 19–55 years), with 3 aged ≤29 years, 5 aged 30–34 years, 12 aged 35–39 years, 29 aged 40–44 years, 32 aged 45–49 years, and 20 aged ≥50 years. Table 1 shows the clinicopathological characteristics of the patients and the regimens used in each age group.
Table 2 shows the rates of CIA and subsequent resumption of menstruation in each age group. CIA occurred in 97 (96%) patients, and 40 patients resumed menstruation. One of the 3 (33.3%) patients aged ≤29 years, 2/5 (40%) of those aged 30–34 years, and 1/12 (3.4%) of those aged 40–44 years did not develop CIA (“No CIA”) despite of the chemotherapy. These four patients were given LH-RHa from the beginning of the endocrine therapy with TAM. For other 94 patients with CIA, endocrine therapy was started with TAM alone. In 41 of the 94 patients, their menstruation was resumed periodically and 13 of 41 patients were given LH-RHa sequentially. In 11 (38.1%) of those aged 40–44 years, 26 (81.3%) of those aged 45–49 years, and in all of those aged ≥50 years, menstruation was not resumed within 4 years after completing chemotherapy (“No resumption of menstruation”).
Duration to the resumption of menstruation
In all of the patients aged ≤39 years, menstruation was resumed; within 1 year for patients aged ≤29 years, within 2 years for those aged 30–34 years, and within 3 years for those aged 34–39 years. Focusing on those patients who did resume menstruation, the median time until it restarted was 2 months for patients aged ≤29 years, 5 months for those aged 30–34 years, 18 months for those aged 35–39 years, 16.5 months for those aged 40–44 years, and 27.5 months for those aged 45–49 years (Table 2). The younger the patient, the sooner menstruation tended to restart after the completion of chemotherapy.
Figure 1 shows the cumulative rate of resumption of menstruation in each age group. In the younger groups, menstruation tended to restart sooner after the completion of chemotherapy. In the older groups, menstruation tended to resume later, if it resumed at all, and a higher proportion of women underwent menopause. All of those aged ≥50 years underwent menopause without having restarted menstruation. Thus, the length of the time to the resumption of menstruation highly depended on the patient’s age.
Cumulative rate of the resumption of menstruation after CIA in each age group. The effect of age on the time to the resumption of menstruation in patients who developed CIA in each age group. In the younger groups, menstruation tended to restart sooner after the completion of chemotherapy. The rate of resumption of menstruation declined in the older age groups
Relation between duration of the chemotherapy and menstruation status
Table 3 shows the relationships between the duration of chemotherapy and menstruation status. The regimen of FEC/EC alone or TC takes 12 weeks, and the regimens of FEC/EC/AC followed by taxane (DTX or PTX) take 24 weeks. There was no statistical difference between 12 week and 24 week regimen groups in the age, the rate of CIA and the resumption of the menstruation, and the CIA duration (Table 3). In terms of the relationships between the chemotherapeutic regimens or the accumulative dose of cyclophosphamide and the CIA status, there was no statistical difference (data not shown).
Discussion
To our knowledge, this is the first report of the incidence of CIA and the resumption of menstruation after neoadjuvant or adjuvant chemotherapy, using current standard regimens composed of anthracyclines and/or taxanes, according to age groups in Japanese patients with HR-positive early breast cancer.
Amenorrhea is commonly induced among premenopausal women who receive neoadjuvant or adjuvant chemotherapy for early breast cancer. The definitions of amenorrhea vary among studies, from one missed period to a continuous lack of menses for more than 1 year [2]. We employed the definition of amenorrhea as at least one missed period during or after chemotherapy. Histologically, chemotherapy-induced ovarian dysfunction is believed to resemble the changes in the ovaries that occur due to aging, and the administration of anticancer agents decreases the number of stored ovarian follicles and causes ovarian follicular necrosis, the closure of primordial follicles, vascular insufficiency, and interstitial fibrosis, among other effects [7, 10]. The patients may experience temporary amenorrhea followed by the recovery of the ovarian function and the resumption of menstruation, or may enter menopause without resumption of menstruation.
Age is one of the greatest factors influencing CIA and resumption of menstruation after chemotherapy [2, 7, 8, 11,12,13]. In this study, the rate of resumption of menstruation also declined in older age groups. All patients aged ≤39 years resumed menstruation, compared with only 40% of those aged ≥40 years, whereas all patients aged ≥50 years underwent menopause without restarting menstruation. In terms of the length of time before menstruation restarted for those patients who resumed menstruation, more time was necessary in the older age group. Menstruation restarted within 1 year for younger patients aged around 30 years, but for those aged ≥35 years, resumption took around 2–3 years in 60% of cases.
The risk for CIA is associated with the chemotherapeutic agents used [2, 7, 8]. The accumulative dose of cyclophosphamide (CPA) is the well-known factor related to CIA and the classical CMF (CPA, methotrexate and 5-FU) is the representative regimen, which induce CIA. However, the total dose of CPA used for the patients of this study is lower than the classical CMF. In this study, there was no statistical difference between 12 week- and 24 week- regimen groups or among the chemotherapeutic regimens in the rate of resumption of the menstruation and the average of CIA duration.
The recommended endocrine therapy for premenopausal HR-positive breast cancer is TAM. As yet, however, there is no unified consensus on matters such as whether LH-RHa should be used as adjuvant endocrine therapy for all premenopausal HR-positive breast cancer patients. Previous clinical trials suggested the utility of ovarian function suppression using LH-RHas with TAM for the premenopausal patients with node-positive ER-positive breast cancer [14]. More recently, two trials investigating the addition of ovarian suppression to TAM did not show an overall clinical benefit for ovarian suppression by LH-RHas [15, 16]. Nonetheless, the addition of an LH-RHa to standard adjuvant therapy with TAM or aromatase inhibitor improved disease-free survival and improved freedom from breast cancer and distant recurrence compared with TAM alone among the subset of patients who were at sufficient risk for recurrence. Thus, the ASCO Panel recommends that high-risk patients receive ovarian suppression in addition to adjuvant endocrine therapy [17]. Based on the results in the present study, until around 40 years of age, almost all patients resume menstruation, most within 1–2 years, and the combined use of an LHRHa may thus be highly effective. On the other hand, beyond 45 years age, over 80% of patients undergo menopause, and even if they do restart menstruation, this is only after more than 2–3 years, meaning that LH-RHas are extremely unlikely to be effective.
For breast cancer patients who want to have children, restoring the ovarian function after chemotherapy is a very important issue. As is evident from the present results, the recovery of the ovarian function after chemotherapy depends greatly on the patient’s age. However, when providing information regarding fertility, it is important to take into account that fertility naturally declines between the ages of 35 and 40 years, making it more difficult to become pregnant [18]. In addition, it must not be forgotten that ensuring the health of the mother—that is, the breast cancer patient herself—is a necessary prerequisite for bearing and raising children. Post-chemotherapy fertility problems require insights from a number of different disciplines encompassing reproductive medicine, obstetrics and gynecology, and pediatrics, and both the cross-discipline exchange of information and further studies are needed.
Several limitations associated with the present study warrant mention. The number of patients included in this study is small, and the analysis was retrospective. However, there are few data of the incidence of CIA in Japanese breast cancer patients. Although Okanami et al. reported the incidence of CIA after adjuvant anthracyclin and taxane in 66 Japanese breast cancer patients, they compared it using only two age groups [9]. We included more patients and examined not only the incidence of CIA and premature menopause, but also the length of the time to recovery of the mensuration in more detail, which we think is an advantage of our study.
In conclusion, the incidence of CIA and subsequent menopause and the length of time until the resumption of menstruation after current standard adjuvant or neoadjuvant chemotherapy depend greatly on the patient’s age. These findings would be useful when providing information on the effects of chemotherapy, especially the risk of ovarian failure and infertility for premenopausal women with breast cancer.
References
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–46.
Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–79.
Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–29.
Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10.
Parulekar WR, Day AG, Ottaway JA, Shepherd LE, Trudeau ME, Bramwell V, et al. Incidence and prognostic impact of amenorrhea during adjuvant therapy in high-risk premenopausal breast cancer: analysis of a National Cancer Institute of Canada Clinical Trials Group Study–NCIC CTG MA.5. J Clin Oncol. 2005;23:6002–8.
Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–65.
Ben-Aharon I, Meizner I, Granot T, Uri S, Hasky N, Rizel S, et al. Chemotherapy-induced ovarian failure as a prototype for acute vascular toxicity. Oncologist. 2012;17:1386–93.
Swain SM, Land SR, Ritter MW, Costantino JP, Cecchini RS, Mamounas EP, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113:315–20.
Okanami Y, Ito Y, Watanabe C, Iijima K, Iwase T, Tokudome N, et al. Incidence of chemotherapy-induced amenorrhea in premenopausal patients with breast cancer following adjuvant anthracycline and taxane. Breast Cancer. 2011;18:182–8.
Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–39.
Hickey M, Peate M, Saunders CM, Friedlander M. Breast cancer in young women and its impact on reproductive function. Hum Reprod Update. 2009;15:323–39.
Jeon SJ, Lee JI, Jeon MJ, Lee M. Prognostic effects of adjuvant chemotherapy-induced amenorrhea and subsequent resumption of menstruation for premenopausal breast cancer patients. Medicine (Baltimore). 2016;95:e3301.
Sukumvanich P, Case LD, Van Zee K, Singletary SE, Paskett ED, Petrek JA, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer. 2010;116:3102–11.
Davidson NE, O’Neill AM, Vukov AM, Osborne CK, Martino S, White DR, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188). J Clin Oncol. 2005;23:5973–82.
Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–46.
Tevaarwerk AJ, Wang M, Zhao F, Fetting JH, Cella D, Wagner LI, et al. Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node-negative, hormone receptor-positive breast cancer (E-3193, INT-0142): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2014;32:3948–58.
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline update on ovarian suppression. J Clin Oncol. 2016;34:1689–701.
Reproductive Endocrinology and Infertility Committee, Family Physicians Advisory Committee, Maternal-Fetal Medicine Committee, Executive and Council of the Society of Obstetricians, et al. Advanced reproductive age and fertility. J Obstet Gynaecol Can. 2011;33:1165–75.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with this study.
About this article
Cite this article
Koga, C., Akiyoshi, S., Ishida, M. et al. Chemotherapy-induced amenorrhea and the resumption of menstruation in premenopausal women with hormone receptor-positive early breast cancer. Breast Cancer 24, 714–719 (2017). https://doi.org/10.1007/s12282-017-0764-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-017-0764-1