Abstract
Background
Invasive lobular carcinoma (ILC) is known to be the second most common histological type following invasive ductal carcinoma (IDC). Definitive clinical features of ILC are controversial.
Methods
We retrospectively analyzed a cohort of 330 patients with metastatic breast cancer, 303 of IDC, 19 of ILC, and 8 of others. We compared the patient age and tumor–node–metastasis factors, disease-free survival (DFS), estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2) expression at the primary site between ILC and IDC. We then selected the patients in the ER+ or PR+/HER2− subtype specifically and compared sites of recurrence, and the survival curve starting from the point of development of metastatic disease.
Results
The clinical stage was significantly higher in the ILC patients than in the IDC (p = 0.001). The mean (±SD) of DFS for the ILC and IDC patients was 2.6 ± 0.6 and 2.4 ± 0.3 years, respectively, with no significant difference (p = 0.18). However, the hormone receptor status was same between both groups; the rate of HER2 positivity was significantly lower in the ILC group (0%) than in the IDC group (16.2%) (p = 0.05). In ER+ or PR+/HER2− subtype, the mean DFS for the ILC and IDC was 2.9 ± 0.6 and 3.1 ± 0.3 years, and the median survival time after the recurrence for ILC and IDC patients was 4.2 ± 0.7 and 5.6 ± 0.7 years, respectively, with no significant difference (p = 0.77). The frequency of lung metastases was significantly lower in the ILC group (6.3%) than in the IDC group (53.7%) (p < 0.01), while the frequency of peritoneal metastases was significantly higher in the ILC group (68.8%) than in the IDC group (1%) (p = 0.00). Of note, the prognosis after the diagnosis of peritoneal metastases was poor, with a median survival time of 19 ± 9 months and resistance to hormone therapy.
Conclusions
The extremely high rate (68.8%) of peritoneal metastases was observed in long-term follow-up for the metastatic breast cancer patients with ILC. We need to reveal the definitive feature of ILC and develop new therapeutic strategies to prevent the dissemination of ILCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a heterogeneous disease traditionally subdivided into distinct histological subtypes. Invasive lobular carcinoma (ILC) is known to be the second most common histological type of invasive cancer, representing 5–15% of all breast cancer cases, after invasive ductal carcinoma (IDC), which accounts for 65–75% of all cases [1]. Ethnic differences in incidence are observed 10–15% in European countries [2, 3] and 3–5% in Asian countries [4, 5]. Epidemiological data have indicated that the incidence of ILC is increasing, especially among postmenopausal woman [6], due in part to the use of postmenopausal hormone replacement therapy [7].
ILC is tends to occur in older patients, and to be relatively large and difficult to diagnose by palpation or mammography, due to the unclear margins of ILC [8]. Other distinguishing features of ILC are controversial, including its relationship with hormone dependence [9] and whether or not the patterns of recurrence and overall prognosis differ from IDC [10–12].
Several case reports which demonstrated the peritoneal metastases from ILC are available [13, 14], and high incident rate 60–90% of peritoneal metastases was reported in autopsy cases [15, 16]. However, the clinical significance of peritoneal metastases for the patient with metastatic breast cancer was not elucidated well. Therefore, we retrospectively analyzed the cohort of patients with metastatic breast cancer which were surveyed for long periods. The special features of ILC concerning the developing peritoneal metastases were presented in this article.
Patients and methods
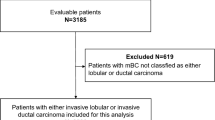
Our analyzed cohort comprised 330 patients with metastatic breast cancer treated in our hospital between April 1999 and December 2015. The number of patients according to histological type was 303 of IDC, 19 of ILC, and 8 of others (3 of mucinous, 1 of apocrine, 2 of metaplastic, 1 of adenoid cystic carcinoma and 1 of unknown histology).
The median follow-up period from the diagnosis of breast cancer was 9.3 ± 12 (range 0.3–55) years. We compared the patient age and tumor–node–metastasis (TNM) factors, categorized using the pTNM pathological classification system, by histological type. Tumor diameter was set as T1 if <2 cm, T2 if 2–<5 cm, T3 if ≥5 cm, and T4 for a tumor of any size with skin or thoracic wall invasion. The pN factor was set as N0 if there were no lymph nodes involved, N1 for involvement of 1–3, N2 for involvement of 4–9 or extranodal invasion, and N3 for involvement of more than 10. M0 indicated no metastases, while M1 indicated the presence of metastases [17]. The disease-free survival (DFS) in the patients with IDC and ILC was compared using the Kaplan–Meier method. The 72 patients (66 of IDC, 6 of ILC) with M1 at initial therapy, whose DFS was defined as 0 year, were included in this analysis.
We also evaluated the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2) expression at the primary site. ER and PR expressions were judged according to the Allred score [18], and scores exceeding 3 indicated positivity. HER2 expression was judged according to the ASCO/CAP guidelines [19], with positivity indicated by a score of 3+ in an immunohistochemistry (IHC) analysis or an HER2/CEP17 score >2.0 using fluorescence in situ hybridization (FISH). We then divided the patients into four groups based on their expression of ER, PR, and HER2, as follows: ER+ or PR+/HER2−, ER+ or PR+/HER2+, ER− and PR−/HER2+, and ER− and PR−/HER2−. The patients who did not undergo biomarker testing were categorized as an unknown subtype.
We then selected the patients in the ER+ or PR+/HER2− subtype specifically and compared the characteristics described above, DFS, sites of recurrence, and the survival curve starting from the point of development of metastatic disease. The sites of recurrence were confirmed by reviewing the clinical record and radiographic findings on CT and bone scintigraphy. The sites of recurrence were the brain (including leptomeningeal metastases), lung (including pleural metastases), liver, local (including the lymph nodes, chest wall, and breast), skin, bone, peritoneum (including the gastrointestinal system, colon, and ovaries), and others.
Statistical analysis
The characteristics between ILC and IDC were compared using the Chi-square test and Pearson’s correlation coefficient analysis, as appropriate. The survival curve was constructed via the Kaplan–Meier method. The statistical significance was examined using the log-rank test with the IBM SPSS statistics package, ver. 2.1.
Results
Characteristics of patients with ILC and IDC
The clinical characteristic of the 19 patients with ILC and 303 with IDC are shown in Table 1. The mean [±standard deviation (SD)] ages of the ILC and IDC patients were 56 ± 12 and 55 ± 13 years, and the proportion of patients over 50 years old was roughly the same between both groups (approximately 59.7%; p = 0.95). The clinical stage was significantly higher in the ILC patients than in those with IDC (p = 0.001), likely due to the pN factors, as markedly more patients had an N score of 2–3 in the ILC group than in the IDC group, while no significant differences were noted in T or M factors.
The mean (±SD) of DFS for the ILC and IDC patients was 2.6 ± 0.6 and 2.4 ± 0.3 years, respectively, with no significant difference (p = 0.18). However, while the hormone receptor status was largely same between both groups, the rate of HER2 positivity was significantly lower in the ILC group (0%) than in the IDC group (16.2%) (p = 0.05).
Survival of patients with ILC and IDC in the ER+ or PR+/HER2− subtype
In ER+ or PR+/HER2− subtype, the mean DFS for the ILC (n = 16) and IDC (n = 203) was 2.9 ± 0.6 and 3.1 ± 0.3 years (Table 1), and the median survival time after the recurrence for ILC and IDC patients was 4.2 ± 0.7 and 5.6 ± 0.7 years, respectively, with no significant difference (p = 0.77) (Fig. 1).
Metastatic sites of ILC and IDC
No significant differences were noted between the ILC and IDC patients in the frequency of developing metastases in the brain, liver, local sites, skin, or bone. The frequency of lung metastases was significantly lower in the ILC group (6.3%) than in the IDC group (53.7%) (p = 0.00), while the frequency of peritoneal metastases was significantly higher in the ILC group (68.8%) than in the IDC group (1%) (p = 0.00) (Table 2).
Survival time after the diagnosis of peritoneal metastasis of 11 ILC patients was 19 ± 9 months (Table 3). Most of all patients had been treated by hormone therapy and/or chemotherapy for long period until the peritoneal metastases was diagnosed. Only one patient who was diagnosed with breast cancer due to metastatic ovarian cancer was able to be treated with hormone therapy for long period (27 months). The other ten patients with peritoneal metastases had become resistant to hormone therapy at the diagnosis of peritoneal metastasis. They were treated by hormone therapy only for 0–8 months. mTor inhibitor was administered to one patients only for 3 months. The chemotherapies were administered to all patients for 10 ± 5 months, among them six patients could be treated by chemotherapy (selected agents were capecitabine, S1 and bevacizumab + paclitaxel; data were not shown) for more than 10 months.
Discussion
Invasive lobular carcinoma is the second most common type of invasive breast cancer after IDC [1], but its frequency differs by country, due to ethnic variations [4, 5]. The proportion of ILC increased from 5% in 1981–1984 to 9% in 1989–1992, due in part to an increased acceptance of the histologic diagnostic criteria [6]. The observed increasing frequency of ILC among postmenopausal patients has suggested a relationship with serum estrogen levels [7].
ILC develops in the milk-producing lobules or glands of the breast. Morphologically, ILC is characterized by small, round cells that are bland in appearance and have scant cytoplasm. These cells infiltrate the surrounding breast tissue in either a single-file or targeted manner [20]. ILC can be difficult to diagnose, as its distinctive growth pattern and biology often prevents the cells from forming any distinct masses that can be easily diagnosed by palpation or mammography [21].
Thus far, the patients with ILC have been treated the same as those with IDC, according to the subtype, and the prognoses have been reported to be similar between the two [8, 10, 22]. Most ILC patients have positive expression of hormone receptors and negative expression of HER2 [8, 10], a feature which we also noted in our cohort, as 84.1% of our patients were positive for both ER and PR while none were HER2-positive. However, most studies have analyzed a large number patients with primary breast cancer [8, 10–12], and a few reports have examined a cohort of patients with metastatic breast cancer.
In our study focusing on patients with metastatic breast cancer, we noted no marked differences in the tumor size between the ILC and IDC patients, despite tumor size being reported as a definitive feature of ILC. However, the ILC patients had a significantly higher rate of lymph node metastasis than the IDC patients. We also noted no marked differences between types in the DFS or survival curve after recurrence (Fig. 1).
ER+ or PR+/HER2− subtype of IDC can be divided into two categories: Luminal A and Luminal B HER2 negative; however, we were unable to distinguish between these due to a lack of data on the Ki67 labeling index for this cohort. The range of DFS and survival time in IDC varied, hampering comparison of the IDC and ILC patients of the ER+ or PR+/HER2− subtype.
Several studies have compared the metastatic patterns between ILC and of IDC. A lower incidence of spreading to the lung or pleura and a higher incidence of spreading to the bone, gastrointestinal, or gynecologic tracts have been observed in ILC compared to IDC [8]. Although our study was a single institute cohort study, our long-term follow-up for all metastatic lesions revealed markedly high incidence (68.8%) of peritoneal metastases among the ILC patients, including the gastrointestinal tract (n = 3), colon (n = 1), and ovary (n = 4). Of note, the survival time was depending on the peritoneal metastases, with a median survival time of 19 ± 9 months. It is explained by the reason that peritoneal metastases were difficult to be detected in the early stage and become obvious after acquiring hormone-refractoriness. Therefore, we should know the fact that overcoming the peritoneal metastases is an important target in the treatment for ILC.
The causes of these definitive features of ILC are unclear. The loss of expression of the cell–cell adhesion molecule E-cadherin in ILC, which is not observed in ductal cancers [23, 24], might account in part for the different metastatic patterns observed in these types of tumors. An association has been reported between mutations in the cadherin (CDH1) gene and the development of ILC [23, 25]. Indeed, ILC has been shown to occur in 20–54% of women with family members who have had hereditary diffuse gastric cancer and who carry germline mutations in the CDH1 gene [26, 27].
Regarding the treatment for ILC, there are no targeted therapies specialized for ILC. Our experience suggested some chemotherapies might contribute to prolong the survival of the patients with peritoneal metastasis, that is almost same as the treatment for the patients with metastasis of IDC. Further investigation will be needed to clarify the mechanisms by which the definitive features of ILC are expressed. We hope that new therapeutic strategies will be developed to prevent the dissemination of ILCs.
References
Ashikari R, Huvos AG, Urban JA, Robbins GF. Infiltrating lobular carcinoma of the breast. Cancer. 1973;31:110–6.
Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996;77:113–20.
Martinez V, Azzopardi JG. Invasive lobular carcinoma of the breast: incidence and variants. Histopathology. 1979;3:467–88.
Fu L, Tsuchiya S, Matsuyama I, Ishii K. Clinicopathologic features and incidence of invasive lobular carcinoma in Japanese women. Pathol Int. 1998;48:348–54.
Jung SY, Jeong J, Shin SH, Kwon Y, Kim EA, Ko KL, et al. The invasive lobular carcinoma as a prototype luminal A breast cancer: a retrospective cohort study. BMC Cancer. 2010;10:664.
Li CI, Anderson BO, Porter P, Holt SK, Daling JR, Moe RE. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer. 2000;88:2561–9.
Chen CL, Weiss NS, Newcomb P, Barlow W, White E. Hormone replacement therapy in relation to breast cancer. JAMA. 2002;287:734–41.
Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149–56.
Horsfall DJ, Tilley WD, Orell SR, Marshall VR, Cant EL. Relationship between ploidy and steroid hormone receptors in primary invasive breast cancer. Br J Cancer. 1986;53:23–8.
Garcia-Fernandez A, Lain JM, Chabrera C, Garcia Font M, Fraile M, Barco I, et al. Comparative long-term study of a large series of patients with invasive ductal carcinoma and invasive lobular carcinoma. Loco-regional recurrence, metastasis, and survival. Breast J. 2015;21:533–7.
Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26:3006–14.
Mersin H, Yildirim E, Gulben K, Berberoglu U. Is invasive lobular carcinoma different from invasive ductal carcinoma? Eur J Surg Oncol. 2003;29:390–5.
Yagi Y, Sasaki S, Yoshikawa A, Tsukioka Y, Fukushima W, Fujimura T, et al. Metastatic gastric carcinoma from breast cancer mimicking primary linitis plastica: a case report. Oncol Lett. 2015;10:3483–7.
Jones GE, Strauss DC, Forshaw MJ, Deere H, Mahedeva U, Mason RC. Breast cancer metastasis to the stomach may mimic primary gastric cancer: report of two cases and review of literature. World J Surg Oncol. 2007;5:75.
Harris M, Howell A, Chrissohou M, Swindell RI, Hudson M, Sellwood RA. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer. 1984;50:23–30.
Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol. 1991;48:28–33.
Veronesi U, Viale G, Rotmensz N, Goldhirsch A. Rethinking TNM: breast cancer TNM classification for treatment decision-making and research. Breast. 2006;15:3–8.
Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
Fisher ER, Gregorio RM, Fisher B, Redmond C, Vellios F, Sommers SC. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975;36:1–85.
Krecke KN, Gisvold JJ. Invasive lobular carcinoma of the breast: mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am J Roentgenol. 1993;161:957–60.
Molland JG, Donnellan M, Janu NC, Carmalt HL, Kennedy CW, Gillett DJ. Infiltrating lobular carcinoma—a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast. 2004;13:389–96.
Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, van Roy F, et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13:1919–25.
De Leeuw WJ, Berx G, Vos CB, Peterse JL, Van de Vijver MJ, Litvinov S, et al. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997;183:404–11.
Gruel N, Lucchesi C, Raynal V, Rodrigues MJ, Pierron G, Goudefroye R, et al. Lobular invasive carcinoma of the breast is a molecular entity distinct from luminal invasive ductal carcinoma. Eur J Cancer. 2010;46:2399–407.
Keller G, Vogelsang H, Becker I, Hutter J, Ott K, Candidus S, et al. Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol. 1999;155:337–42.
Pharoah PD, Guilford P, Caldas C, International Gastric Cancer Linkage Consortium. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
About this article
Cite this article
Inoue, M., Nakagomi, H., Nakada, H. et al. Specific sites of metastases in invasive lobular carcinoma: a retrospective cohort study of metastatic breast cancer. Breast Cancer 24, 667–672 (2017). https://doi.org/10.1007/s12282-017-0753-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-017-0753-4