Abstract
Cancer is a group of cells which grow in an uncontrolled manner and invades to the adjacent organs to form malignant tumors. Tumor hypoxia results due to contrast between the cellular oxygen expenditure and oxygen supply to the cells. Hypoxia inducible factor (HIF) is a heterodimeric transcription factor encompass of oxygen sensitive α subunit and constitutively expressed β subunit both of which are basic helix-loop-helix protein. The stability of HIF is primarily regulated by post translational prolyl hydroxylation, catalyzed by prolyl hydroxylase 2 (Phd-2). Phd-2 is a group of enzymes that acts as an oxygen sensor. Cancer cells have altered metabolism as they fulfil their energy needs through glycolysis and lipid biogenesis. HIF-1α is known to upregulate glycolysis by activating the transcription of enzymes on the glycolytic pathway and through lipogenesis. Cancer cells have over expressed fatty acid synthase owing to altered glycolytic pathway. Considering the above, it is hypothesized that chemical activation of Phd-2 can curtail down HIF-1α and subsequently fatty acid synthase expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A body is made up of many living cells, which grow, multiply and generate new cells and die after some time in an accordingly manner called apoptosis. In case of cancer, body cells start growing out of control. They cannot die and grow deliberately to form new cells and form their own blood vessels through angiogenesis. Subsequently, cancer cells metastasise into the nearby organs, when their growth is not diversified or arrested. At this stage, cancer cells replace normal tissues and form new mass termed as tumor. There are eight endorsements of cancer participating in the physiology of cancer: self sufficient pro growth signalling, loss of sensitivity to antigrowth signals, resistance to cell death, replicating without limit, angiogenesis, invasion and metastasis, reprogrammed metabolism and evading immune surveillance [1, 2].
Cells manage a wide range of functions including growth, movement, housekeeping and many more which requires energy. The energy is liberated from the chemical bond in food molecules and serves as fuel for the cells through a string of oxidation reduction process. Sugar molecule plays a very paramount role, as they are oxidized in small steps to carbon dioxide and water. A large array of mechanisms are involved in the breakdown/catabolism of sugars to produce adinosine tri phosphate (ATP), nicotinamide adenine di phosphate (NADPH) and other molecules [3].
The normal cells and cancer cells are peculiar from each other in their intermediatry metabolism, which helps the cancerous cells to survive in hypoxic microenvironment. Cancer cells get energy from the process of glycolysis rather any other process, as more ATP is generated via glycolysis (Warburg effect) [4]. During glycolysis, two molecules of pyruvate with a net gain of two molecules of ATP are generated from the metabolism of one molecule of glucose. Under normoxic condition, the end product of glycolysis, i.e., pyruvate get converted into acetyl CoA which works as the starting material for citric acid cycle and oxidative phosphorylation, yielding about 34 ATP molecules. During tumor hypoxia (low oxygen level), cells goes through shift to glycolytic pathway for the stipulation of energy (Fig. 1) [5]. Thereby, tumor hypoxia and hypoxia inducible factors (HIFs) are the important pathway for tumorigenesis and angiogenesis. HIF can also get triggered under normal condition of oxygen (normoxia) by the loss of Von Hippel Lindau tumor suppressor protein (pVHL) [6].
Energy requirement for normal cell and for cancer cell. Metabolic differences between normal and cancer cells. Normal cells, primarily metabolizes glucose into pyruvate(end product of glycolysis) for survival and growth followed by citric acid cycle and as a result thirty-four ATP molecules are generated. In cancer cells, there is increased glucose uptake but there is inefficient utilization of glycolytic end product and pyruvate is converted into lactate which may leads to biomass incorporation and cancer cell proliferation. This effect is termed as “Warburg effect”
Hypoxic microenvironment in tumor cell is a result of high oxygen consumption within the tumor vasculature by rapidly procreating tumor cells. To support the unceasing growth in the hypoxic microenvironment, cancer cells are known to stimulate alternate metabolic pathways including anaerobic metabolism, increased signalling of growth factors, diversified regulation of cell cycle, cellular proliferation and protein catabolism. During hypoxia, cancer cell derive energy first and foremost through glycolysis as oxygen is not available to derive acetyl CoA from pyruvate. This change in metabolic pathway during normoxic condition is conventionally termed as “Warburg effect.”
HIF activation also vitiates mitochondrial respiration by making HIF a key regulator of cancer cell metabolism along with stimulation of glycolysis [7, 8]. It has been reported that there are alterations in lipid metabolism and high rates of de novo fatty acid biosynthesis in tumor cells [9]. Fatty acid synthase (FASN) was identified as the breast tumor associated protein and there are diversified studies to show that FASN is overexpressed in tumor cells including mammary gland carcinomas. According to various clinical and preclinical studies, it is clear that human cancer cells have the capacity to synthesize their own fatty acid which is autonomous of the regulatory signals. The mechanism which is culpable for FASN up regulation in cancer is unknown [10–13]. Nonetheless, FASN appears to be an attractive target in the management of lipoidal tumors and thereby has aimed increased response. Notwithstanding, the present review was ventured to elucidate the various aspects of tumor hypoxia arbitrated regulation of FASN along with the present and future aspects of modulating FASN overexpression through prolyl hydroxylase (Phds).
Tumor hypoxia
Tumor hypoxia is a situation where the level of oxygen is decreased in tumor cells. Oxygen is the primitive requirement for a cell to grow [14–16] and most of the solid tumors are hypoxic in nature due to the limited amount of vasculature, poor tumor vessels with chaotic architecture [17]. In normal cells, hypoxia is associated with decreased proliferation and differentiation. Hypoxia decreases the production of energy and cell viability, it arrests cell growth and induces cell death [18, 19]. Hypoxic environment is also responsible for the activation of anaerobic metabolism, signalling of growth factors, regulation of cell cycle, cell proliferation and protein catabolism. These processes enable tumor cells to survive in a poorly oxygenated conditions [20, 21]. Therefore hypoxia enhances the expansion of tumor cells with diminished apoptotic potential [22]. HIF is the main effecter of oxygen homeostasis, allocating various genes involved in biological processes like angiogenesis [23, 24]. HIF also binds to the vascular endothelial growth factor gene which is obligatory for transactivation of vascular endothelial growth factor (VEGF) in response to hypoxia. This type of binding promotes the proliferation, which accompany the formation of blood vessels. HIF-1α plays a very imperative role in metabolic angiogenesis and is proclaimed to be highly expressed in a wide variety of human cancers [25].
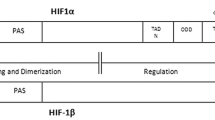
Under normoxic condition, prolyl hydroxylation allows the interplay of HIF-1α to pVHL and acts as recognition component of E3 ubiquitin ligase and subsequently HIF-1α goes through proteosomal degradation [26–28]. In hypoxic condition, HIF-1α fails to interact with pVHL mediated degradation and relocates into the nucleus to form a complex with HIF-1β (Fig. 2). This complex can actuate the transcription of target genes with the help of core hypoxia response element (HRE) [29, 30]. HIF-1α also interacts with p300/CBP co-activator complex which is regulated by oxygen and Asn 803 (human HIF-1α). The HIF C terminal transactivation domain is also catalyzed by another 2-OG oxygenase named as factor inhibiting HIF (FIH), who blocks the HIF p300 interaction [31, 32]. Henceforth, two factors that can control the HIF activity: Phds and FIH [27, 33, 34]. HIF-1α is forthwith perceived as a major molecular target for anti-cancer therapy [35]. Notwithstanding, the Phds and FIH are foreseen as the factors, who can control the HIF activity, making HIF-1α as a major molecular target for anticancer activity.
Effect of normoxic and hypoxic condition on cellular proliferation. In the presence of oxygen, Phd-2 oxidize HIF-1α which is recognized by Von Hippel Linadu protein (pVHL) and then HIF-1α get ubiqutinized followed by proteosomal degradation of HIF-1α. Conversely, when Phd-2 is not active in hypoxic condition then it is not recognized by pVHL and HIF-1α get translocates to the nucleus and forming a complex with HIF-1β and stimulates a number of processes like vascular endothelial growth factor (VEGF) induced angiogenesis
Prolyl hydroxylase
Phds domain proteins are the group of enzymes, who can hydroxylate HIF-1α and supervise its activity. Phds belongs to a 2-oxoglutarate (2OG)-dependent dioxygenase superfamily [36, 37]. Along with 2OG, these enzymes require Fe2+, oxygen and ascorbate to modify the functions of HIF-1/2α. There are four isoforms of this enzyme, named as Phd-1, Phd-2, Phd-3 and Phd-4 [38]. In relation to HIF-1α, Phd-1 and Phd-2 are found to be very comparable (407 and 426 amino acid residue proportionately) while Phd-3 is found to be short (only 236 amino acid residues). Phd-4 is recorded to be the larger one with 502 amino acids [39, 40]. Phd-4 has been found to be appended with membrane and its active site is located into the lumen of endoplasmic reticulum [41]. Phds possess a double stranded β-helix core fold in which Fe2+ is bound with catalytic centre [42]. Phd-2 is ubiquitously expressed, while Phd-1 is expressed in placenta and Phd-3 in the heart. Role of Phd-2 in tumor angiogenesis is controversial [43, 44]. Some authors have reported that, tumor vasculature is regulated by Phd-2. Whereas others observed that Phd-2 expression is reduced and lead to the normalization of blood vessels with reduced tumor metastasis [45, 46]. A modest set of researchers have also divulged that Phd-2 deficiency is associated with cell invasion and metastasis in pancreatic tumor cells, thereby suggesting its tumor promoting nature [46, 47]. By and large, Phd-2 is perceived to reduce tumor metastasis and few of the activators of Phd-2 have been reported to manifest anticancer activity [48, 49].
Fatty acid synthase (FASN)
FASN is a major enzyme for lipogenesis. It is a complex multienzyme protein that contains seven catalytic domains [50–54]. The major function of FASN is to catalyze the condensation of acetyl CoA and malonyl CoA to produce palmitic acid in the presence of NADPH. FASN is allocated in two classes: type I and type II. FASN I is a multifunctional polypeptide and very common in mammals and fungi. FASN I produces palmitic acid and cooperate with FASN II for the production of lipid products [55]. FASN II is commonly found in archaea and bacteria and it is symbolized to be a multifunctional enzyme [56]. In highly lipogenic tissues like liver, lactating breast and adipose tissues, FASN have three major functions:
-
I.
Storage of profound excess energy in the form of fat.
-
II.
If the diet is low in fat then the synthesis of fat from carbohydrate or protein.
-
III.
Synthesis of fat for lactation.
Analogues to normal cells, the majority of fatty acid in cancer cells are derived from de novo synthesis rather than dietary fats [57, 58]. Upsurge in lipogenesis is reflected as an inflated activity of lipogenic enzymes such as ATP citrate lyase and FASN [59, 60]. The FASN level is overexpressed in lipoidal tumor cells and is one of the most common molecular changes which occur in cancerous cell [60–64]. Generally, glycolysis increases the uptake of oxygen in cancer cells away from blood vessels and consumes glucose which results in accumulation of pyurvate and lactate. This exhaustion of glucose and agglomeration of lactate is the main cause of hypoxia induced apoptosis [65]. A decent set of FASN inhibitors like cerulenin, C75 and orlistat [66] are reported to urge cell death in a variety of tumors mainly lipiodal tumors [60–64]. FASN inhibitors can cause cell death and are considered to be a particularised target for the treatment of cancer. It also has been proclaimed that the essential parts of FASN act as energy substrates for the cells. Thereby inhibition of FASN induces decrease in the production of lipids in the tumor cells by which the tumor cells cannot get the energy for their propagation [67, 68].
FASN and mammary gland carcinoma
A unique pathophysiological microenvironment including hypoxia, low pH and nutrient starvation implicates in the mammary gland tumors [69]. This type of environment triggers several intracellular signalling pathways and can induce the level of FASN [66]. It was reported that hypoxic microenvironment can upregulate sterol regulatory element-binding protein (SREBP), which is a major transcriptional regulator of FASN gene through Akt phosphorylation [70]. FASN overexpression is a well reported phenomenon in the preclinical and clinical cases of mammary gland carcinoma [60].
Researchers have reported increased expression of FASN in MNU and DMBA induced mammary gland carcinoma in mouse model and thereby considered it as a viable target for chemoprevention [71–73]. Inhibition of FASN has been reported to be associated with reduced synthesis of some essential fatty acids requisite for the cell growth [74, 75]. In line to the animal studies, FASN is reported to be highly expressed in hormone independent SKBR3 and hormone dependent MCF-7 and ZR-75-1 cell lines as well [75–77]. Over expression of FASN has been linked with poor prognosis and reduced disease free survival in many cancer types including mammary gland carcinoma [13, 78, 79]. Infact, several cohort studies have linked the FASN overexpression with poor patients survival and chemoresistance [79–81]. Authors would also like to mention that chemoresistance in cancer patients have been linked with FASN overexpression mediated palmitate overproduction [82]. Clinical studies revealed that the stage 1 of breast cancer patients with high levels of FASN expression have fourfold increased risk for death [59]. Together with previous findings for FASN as a poor prognostic marker for breast cancer patients, we suggest that FASN has a key role to play in drug resistance and can be considered as an ideal target for chemosensitization in breast cancer chemotherapy.
HIF and tumor microenvironment
Hypoxia plays a paramount role in the embryonic development and human physiology [83–85]. Almost all cancerous cells are reported to be hypoxic in nature and their proliferation is resolved through HIF-1 activation [86, 87]. Henceforth, HIF-1α plays a momentous role in tumor proliferation [88]. Energy is implemented to the cells with the help of angiogenesis which fulfil the oxygen supply [89]. This energetic shift in cancerous cell coordinates with the variety of enzymes, which are diversified in the process of glycolysis for example glucose transporters (aldolase A and pyruvate kinase) [90]. These transporters help the cells to produce energy in the hypoxic micrenvironment [89]. This constitutive activation of anaerobic metabolism in tumor cells provides a relationship of cell response to the oxygen deficiency [91, 92]. Thus HIF-1α function in hypoxic environment is involved in VEGF arbitrated angiogenesis, increased glycolysis and other steps in tumor progression [93].
Regulating Phd-2 to counteract tumor hypoxia and FASN over expression
Initially it was found that Phd-2 is the most critical hydroxylase in a variety of cultured cells and could be attributed to the fact that Phd-2 is more profoundly expressed than other Phds [38, 94]. The activity of Phds can be restrained by post translational mechanisms including proteosomal degradation and modulation of Phds activity by collaborating proteins. It has been reported that all Phds can downregulate HIF-1α in vitro, however the specific role of Phds in cancer is still inconclusive [17]. In tumor hypoxia, there is a shift to glycolytic mechanism as respiration is not feasible without oxygen. Tumor hypoxia and activation of HIF-1α is an important pathway that contributes to so many processes like tumorigenesis, angiogenesis, up surged glycolysis and tumor cell survival. HIF-1α is a key regulator of cancer metabolism [6, 7] and glycolysis is a major target of cancer cells for the stipulation of energy. The end product of glycolysis is pyruvate which can be persuaded into cytosolic lactate which is secreted or converted into mitochondrial acetyl CoA and which is mediated into citrate within the mitochondria. Citrate can be processed by the citric acid cycle or exported to the cytoplasm where it is cleaved by ATP citrate lyase and generate cytosolic acetyl CoA which is a building block for fatty acid biosynthesis.
Hypothesis
Notwithstanding, herewith it is hypothesized that the chemical activators of Phd-2 would cause accelerated deterioration of HIF-1α through proteosomal degradation which can leads to the favourable outcomes against cancer. With the help of Phd-2 activators, the activity of HIF-1α will be curtailed and this will cause decrease in glycolytic pathway and subsequent diminution in the FASN level which is recorded to be over expressed in tumor cells (Fig. 3). On the account of curtailed glycolysis, one can expect decreased pyruvate and subsequently lactate formation. As mentioned earlier, the lactate formation significantly participates in the biomass formation in the cancerous cells. FASN is a multifunctional enzyme participating in citric acid cycle and is overexpressed in the fastly growing cancerous cells to meet the energy requirements. Pyruvate (end product of glycolysis) is converted into citric acid (starting material of citric acid cycle). Diminshed glycolytic activity due to HIF-1α proteosomal degradation (due to Phd-2 activation) can lead to scarcity in the pyruvate formation subsequently citric acid synthesis in cells would be curtailed leading to abatement in the citric acid cycle and FASN overexpression. All in all, we hypothesize that activation of Phd-2 can affect cancerous cells in a multidirectional way. Firstly, by inhibiting the activation of HIF-1α (a well established target in cancer chemoprevention); secondly, decreased production of lactate may help to cut down the biomass formation and lastly, by downregulating the FASN overexpression through curtailing the citric acid cycle (Fig. 4).
Regulatory pathway for FASN and its modulation by HIF-1α and Phd-2. In normoxic condition, HIF-1α interacts with pVHL followed by proteosomal degradation. In hypoxic condition HIF-1α cannot recognize by pVHL and translocates into the nucleus and complex with HIF-1β. HIF-1α is regulated by iron and 2-oxoglutarate dependent dioxygenase family termed as Phd-2. In nucleus the glycolysis takes place along with Pentose Phosphate Pathway (PPP) which results in pentose sugar and Nicotinamide adenine dinucleotide phosphate (NADPH). The end product of glycolytic pathway is pyruvate which gets converts into citrate. The citrate participates in the citric acid cycle and the resultant product is 16 C polyunsaturated palmitic acid in the presence of fatty acid synthase (FASN)
Hypothesized outcomes of activation of Phd-2. In hypoxic condition, HIF-1α escapes proteosomal degradation and translocates into nucleus where targets the glycolytic pathway and fatty acids for the requirement of energy and the resultant is overexpression of FASN. Whereas in normoxic condition, Phd-2 gets activated and HIF-1α is degraded so decrease in glycolytic activity and decrease in pyruvate and lactate formation and the resultant is downregulation of FASN expression. The hypothesized outcomes of activation of Phd-2 is inhibition of VEGF mediated angiogenesis, decrease in biomass production and decrease in lipid production and palmitate mediated chemoresistance so tumor cells cannot propagate
Present scenario and future directions
As elaborated above, modifying the Phd-2 could be a viable target for cancer prognosis, which can be contemplated to have a momentous effect on the glycolytic pathway and thereby FASN expression in lipoidal cancers. It is to be noted that only three activators of Phd-2 namely KRH102053, KRH102140 and R59949 has been reported till date, with KRH102053 and KRH102140 having a conspicuous effect on angiogenesis [95, 96]. On the contrary, the R59949 (diacyl glycerol kinase II inhibitor) was also recorded with Phd-2 activating potential without much affecting the tumor progression [97]. It would be important to pen down that apart from activating the Phd-2, other physiological effects of KRH102053 and KRH102140 are unelucidated till date. In short, the underlying mechanism of these compounds is poorly understood, which we are in opinion could be transmutations in pathways of energy production and subsequent alteration in FASN over expression. We would also like to mention that the effect of KRH102053 and KRH102140 on glycolytic pathway and subsequent FASN overexpression could be a research question for future endeavours.
Down regulating the FASN over expression in lipoidal cancer cells to customize tumor growth is a well studied and established phenomena. In fact, a sufficient number of FASN inhibitors (e.g., cerulenin-3-derivative C75, β-lactone orlistat, green tea polyphenol epigallocatechin-3-gallate (EGCG) and other naturally occurring flavonoids as well as antibiotic triclosan) are proclaimed to have a profound effect on cell proliferation, angiogenesis, metastasis and apoptosis [98].
Most of the reported compounds are broad spectrum FASN inhibitors and mediate their action through ameliorating β-ketoacyl synthase. The compounds are well documented for their anti-cancer activity owing to their FASN inhibiting potential [10]. However, repercussion of FASN inhibition on glycolytic pathway, regulation of HIF-1α and consequent effect on Phd-2 needs to be elucidated to full and stands still as a research question.
Additionally, most of the FASN inhibitors have several metabolic and pharmacological limitations which restrict their transformations from preclinical to clinical phase of drug discovery. Authors are in opinion that these impediments are the repercussion to the fact that FASN overexpression is the later consequence of hypoxic situation in cancerous cells and merely prohibiting the FASN, will not cater compelling detrimental effects on the tumor progression. In fact, we are not hesitant to put on records that activation of Phd-2 appears to be a more viable and constructive targets in terms of overseeing the hypoxic microenvironment and FASN overexpression in expeditiously dividing tumor cells.
With all above, authors are in strong accredit that activating Phd-2 could be a generous target to look after cancer progression and is hypothesized to be effectuated through down regulation of FASN over expression.
Abbreviations
- ATP:
-
Adinosine tri phosphate
- NADPH:
-
Nicotinamide adenine di phosphate
- HIF:
-
Hypoxia inducible factor
- pVHL:
-
Von Hippel Lindau tumor suppressor protein
- FASN:
-
Fatty acid synthase
- VEGF:
-
Vascular Endothelial Growth Factor
- HRE:
-
Hypoxia response element
- Phd-2:
-
Prolyl hydroxylase 2
- 2OG:
-
2-oxoglutarate
- EGCG:
-
Epigallocatechin-3-gallate
- SREBP:
-
Sterol regulatory element binding protein
References
Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE. Increased cell division as a cause of human cancer. Cancer Res. 1990;50(23):7415–21.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Alberts B, Johnson A, Lewis J, et al. How Cells Obtain Energy from Food. In: Molecular biology of the cell. 4th ed. New York: Garland Science; 2002. http://www.ncbi.nlm.nih.gov/books/NBK26882/.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–9.
Young CD, Anderson SM. Sugar and fat-that’s where it’s at: metabolic changes in tumors. Breast Cancer Res. 2008;10(1):202.
Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11(5):407–20.
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187–97.
Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–85.
Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;13(1):27–9.
Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202–8.
Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci. 1994;91(14):6379–83.
Swinnen JV, Vanderhoydonc F, Elgamal AA, Eelen M, Vercaeren I, Joniau S, et al. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer. 2000;88(2):176–9.
Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, et al. Fatty Acid Synthase Expression Defines Distinct Molecular Signatures in Prostate Cancer1 1 NCI (Director’s Challenge CA84995-04, SPORE in Prostate Cancer CA90381-01A1, and PO1 CA89021-02), Novartis Investigator, and CaPCURE awards. Mol Cancer Res. 2003;1(10):707–15.
Clanton TL, Hogan MC, Gladden LB. Regulation of cellular gas exchange, oxygen sensing, and metabolic control. Compr Physiol. 2013;3:1135–90. doi:10.1002/cphy.c120030.
Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17(21):2614–23.
Zepeda AB, Pessoa A, Castillo RL, Figueroa CA, Pulgar VM, Farías JG. Cellular and molecular mechanisms in the hypoxic tissue: role of HIF-1 and ROS. Cell Biochem Funct. 2013;31(6):451–9.
Jokilehto T, Jaakkola PM. The role of HIF prolyl hydroxylases in tumour growth. J Cell Mol Med. 2010;14(4):758–70.
Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225–39.
Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47.
Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–84.
Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402.
Welsh R, Jensen F, Cooper N, Oldstone M, Banapour B, Sernatiriger J, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:4.
Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5(5):343–54.
Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res. 2006;71(4):642–51.
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 2000;14(1):34–44.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5.
Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13(2):167–71.
Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci. 1995;92(12):5510–4.
Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW, et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277(29):26351–5.
Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295(5556):858–61.
Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O2 levels. J Cell Sci. 2003;116(15):3041–9.
Safran M, Kaelin WG Jr. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Investig. 2003;111(6):779.
Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–54.
Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337–40.
Myllyharju J. Prolyl 4-hydroxylases, master regulators of the hypoxia response. Acta Physiol. 2013;208(2):148–65.
Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279(37):38458–65.
Koivunen P, Tiainen P, Hyvärinen J, Williams KE, Sormunen R, Klaus SJ, et al. An endoplasmic reticulum transmembrane prolyl 4-hydroxylase is induced by hypoxia and acts on hypoxia-inducible factor α. J Biol Chem. 2007;282(42):30544–52.
McDonough MA, Li V, Flashman E, Chowdhury R, Mohr C, Liénard BM, et al. Cellular oxygen sensing: crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2). Proc Natl Acad Sci. 2006;103(26):9814–9.
Oehme F, Ellinghaus P, Kolkhof P, Smith TJ, Ramakrishnan S, Hütter J, et al. Overexpression of PH-4, a novel putative proline 4-hydroxylase, modulates activity of hypoxia-inducible transcription factors. Biochem Biophys Res Commun. 2002;296(2):343–9.
Chowdhury R, McDonough MA, Mecinović J, Loenarz C, Flashman E, Hewitson KS, et al. Structural basis for binding of hypoxia-inducible factor to the oxygen-sensing prolyl hydroxylases. Structure. 2009;17(7):981–9.
Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci USA. 2006;103(3):654–9.
Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359(25):2685–92.
Chan DA, Kawahara TL, Sutphin PD, Chang HY, Chi JT, Giaccia AJ. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell. 2009;15(6):527–38.
Mazzone M, Dettori D, de Oliveira RL, Loges S, Schmidt T, Jonckx B, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136(5):839–51.
Klotzsche-von Ameln A, Muschter A, Mamlouk S, Kalucka J, Prade I, Franke K, et al. Inhibition of HIF prolyl hydroxylase-2 blocks tumor growth in mice through the antiproliferative activity of TGFβ. Cancer Res. 2011;71(9):3306–16.
Couvelard A, Deschamps L, Rebours V, Sauvanet A, Gatter K, Pezzella F, et al. Overexpression of the oxygen sensors PHD-1, PHD-2, PHD-3, and FIH Is associated with tumor aggressiveness in pancreatic endocrine tumors. Clin Cancer Res. 2008;14(20):6634–9.
Gossage L, Zaitoun A, Fareed KR, Turley H, Aloysius M, Lobo DN, et al. Expression of key hypoxia sensing prolyl-hydroxylases PHD1,-2 and-3 in pancreaticobiliary cancer. Histopathology. 2010;56(7):908–20.
Chirala SS, Jayakumar A, Gu ZW, Wakil SJ. Human fatty acid synthase: role of interdomain in the formation of catalytically active synthase dimer. Proc Natl Acad Sci. 2001;98(6):3104–8.
Mattick JS, Tsukamoto Y, Nickless J, Wakil S. The architecture of the animal fatty acid synthetase. I. Proteolytic dissection and peptide mapping. J Biol Chem. 1983;258(24):15291–9.
Nemoto T, Terashima S, Kogure M, Hoshino Y, Kusakabe T, Suzuki T, et al. Overexpression of fatty acid synthase in oesophageal squamous cell dysplasia and carcinoma. Pathobiology. 2000;69(6):297–303.
Rangan VS, Joshi AK, Smith S. Mapping the functional topology of the animal fatty acid synthase by mutant complementation in vitro. Biochemistry. 2001;40(36):10792–9.
Tsukamoto Y, Wong H, Mattick J, Wakil S. The architecture of the animal fatty acid synthetase complex. IV. Mapping of active centers and model for the mechanism of action. J Biol Chem. 1983;258(24):15312–22.
Jenke-Kodama H, Sandmann A, Müller R, Dittmann E. Evolutionary implications of bacterial polyketide synthases. Mol Biol Evol. 2005;22(10):2027–39.
Fulmer T. Not so FAS. SciBX. 2009;2(11). doi:10.1038/scibx.2009.430.
Menendez JA, Lupu R. Fatty acid synthase-catalyzed de novo fatty acid biosynthesis: from anabolic-energy-storage pathway in normal tissues to jack-of-all-trades in cancer cells. Arch Immunol Ther Exp (Warsz). 2004;52(6):414–26.
Menendez JA, Colomer R, Lupu R. Why does tumor-associated fatty acid synthase (oncogenic antigen-519) ignore dietary fatty acids? Med Hypotheses. 2005;64(2):342–9.
Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66(12):5977–80.
Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–77.
Alo PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77(3):474–82.
Milgraum LZ, Witters LA. Pasternack Ga, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3(11):2115–20.
Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, et al. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150(1):201.
Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98(1):19–22.
Kasinskas RW, Venkatasubramanian R, Forbes NS. Rapid uptake of glucose and lactate, and not hypoxia, induces apoptosis in three-dimensional tumor tissue culture. Integr Biol. 2014;6(4):399–410.
Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68(4):1003–11.
Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64(6):2070–5.
De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63(13):3799–804.
Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005;94(1):1–4.
Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100(9):1369–72.
Moral R, Solanas M, Manzanares EM, Haro D, Escrich E. Influence of DMBA-induced mammary cancer on the liver CPT I, mit HMG-CoA synthase and PPARα mRNA expression in rats fed a low or high corn oil diet. Int J Mol Med. 2004;14(2):283–7.
Lin CY, Smith S, Abraham S. Fatty acid synthetase from a mouse mammary adenocarcinoma. Cancer Res. 1975;35(11):3094–9.
Lu S, Archer MC. Fatty acid synthase is a potential molecular target for the chemoprevention of breast cancer. Carcinogenesis. 2005;26(1):153–7.
Jayakumar A, Tai MH, Huang WY, Al-Feel W, Hsu M, Abu-Elheiga L, et al. Human fatty acid synthase: properties and molecular cloning. Proc Natl Acad Sci. 1995;92(19):8695–9.
Chuang HY, Chang YF, Hwang JJ. Antitumor effect of orlistat, a fatty acid synthase inhibitor, is via activation of caspase-3 on human colorectal carcinoma-bearing animal. Biomed Pharmacother. 2011;65(4):286–92.
Kuhajda F. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obes. 2008;32:S36–41.
Wang ZY, Wang DM, Loo TY, Cheng Y, Chen LL, Shen JG, et al. Spatholobus suberectus inhibits cancer cell growth by inducing apoptosis and arresting cell cycle at G2/M checkpoint. J Ethnopharmacol. 2011;133(2):751–8.
Wang YY, Kuhajda FP, Li JN, Pizer ES, Han WF, Sokoll LJ, et al. Fatty acid synthase (FAS) expression in human breast cancer cell culture supernatants and in breast cancer patients. Cancer Lett. 2001;167(1):99–104.
Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26(35):5713–20.
Bauerschlag DO, Maass N, Leonhardt P, Verburg FA, Pecks U, Zeppernick F, et al. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. J Transl Med. 2015;13(1):146.
Hou W, Fei M, Qin X, Zhu X, Greshock J, Liu P, et al. High overexpression of fatty acid synthase is associated with poor survival in Chinese patients with gastric carcinoma. Exp Ther Med. 2012;4(6):999–1004.
Liu H, Liu Y, Zhang JT. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther. 2008;7(2):263–70.
Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–72.
Chen EY, Fujinaga M, Giaccia AJ. Hypoxic microenvironment within an embryo induces apoptosis and is essential for proper morphological development. Teratology. 1999;60(4):215–25.
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12(2):149–62.
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394(6692):485–90.
Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26(14):5336–47.
Shi YH, Fang WG. Hypoxia-inducible factor-1 in tumour angiogenesis. World J Gastroenterol. 2004;10(8):1082–7.
Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(Supplement 5):10–7.
Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80(2):51.
Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21(10):3436–44.
Minchenko A, Leshchinsky I, Opentanova I, Sang N, Srinivas V, Armstead V, et al. Hypoxia-inducible Factor-1-mediated Expression of the 6-Phosphofructo-2-kinase/fructose-2, 6-bisphosphatase-3 (PFKFB3) Gene ITS POSSIBLE ROLE IN THE WARBURG EFFECT. J Biol Chem. 2002;277(8):6183–7.
Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16(10):1151–62.
Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J. 2003;22(16):4082–90.
Nepal M, Gong YD, Park YR, Soh Y. An activator of PHD2, KRH102140, decreases angiogenesis via inhibition of HIF-1α. Cell Biochem Funct. 2011;29(2):126–34.
Choi H, Song BJ, Gong YD, Gwak W, Soh Y. Rapid degradation of hypoxia-inducible factor-1α by KRH102053, a new activator of prolyl hydroxylase 2. Br J Pharmacol. 2008;154(1):114–25.
Temes E, Martín-Puig S, Acosta-Iborra B, Castellanos MC, Feijoo-Cuaresma M, Olmos G, et al. Activation of HIF-prolyl hydroxylases by R59949, an inhibitor of the diacylglycerol kinase. J Biol Chem. 2005;280(25):24238–44.
Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6(4):551–62.
Acknowledgments
MS, UD and SR carried out the literature review for this paper. PRG, GK and SAS guided them for literature survey. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest.
Additional information
Manjari Singh and Uma Devi have contributed equally.
SHIATS-Deemed to be University is formerly Allahabad Agricultural Institute.
About this article
Cite this article
Singh, M., Devi, U., Roy, S. et al. Prolyl hydroxylase mediated inhibition of fatty acid synthase to combat tumor growth in mammary gland carcinoma. Breast Cancer 23, 820–829 (2016). https://doi.org/10.1007/s12282-016-0683-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-016-0683-6