Abstract
Background
We prospectively compared the diagnostic accuracies of PET/CT and BS in patients with suspected bone metastases from breast cancer.
Methods
This single-institution prospective study included consecutive patients with suspected bone metastases from biopsy-proven breast cancer seen at Tokai University Hospital between September 2011 and March 2014. Inclusion criteria included suspicions for bone metastases (bone pain, elevated alkaline phosphatase, elevated tumor markers, or suspected bone metastases by BS). Two nuclear medicine physicians evaluated PET/CT and BS images.
Results
Thirty patients were initially enrolled in this study. Two were excluded from the analyses because they declined to undergo imaging during follow-up. PET/CT successfully detected bone metastases in all 10 patients finally diagnosed with the condition, whereas BS identified 2. The two methods were not highly concordant in detecting osseous metastases. In 19 of 28 paired studies (68 %), 2 (10 %) were positive for metastasis, and 17 (90 %) were negative. Nine occurrences (32 %) were discordant; of these, 2 were PET/CT positive and BS negative; 5 were PET/CT positive and BS equivocal; one was PET/CT negative and BS equivocal, and one was PET/CT equivocal and BS negative.
Conclusions
Our results indicated that PET/CT was superior to BS for the diagnosis of bone metastases. On the basis of the results of previous studies as well as ours, PET/CT could replace BS as the initial modality to detect bone metastases in patients suspected for the condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early diagnosis of bone metastases is needed for precise distant staging, optimal management, and prevention of complications. The combination of physical exam and appropriate imaging procedure can prevent emergencies and lasting morbidity through early detection, allowing timely delivery of necessary therapy. The bone is one of the most frequent sites for distant metastasis in breast cancer patients [1]. The median survival duration after diagnosis of bone metastasis is 25.2–72 months [2–5]. Conventional bone scintigraphy (BS) is the most frequently used imaging modality for the detection of bone metastases in breast cancer patients owing to its availability and ability to evaluate the entire skeleton with low cost [6]. Costelloe et al. noted recommendations regarding the use of imaging modalities for detecting osseous metastases. The first choice for screening should be BS; however, because this method shows only bone mineral turnover, another imaging study might be needed for an accurate diagnosis [radiography, computed tomography (CT), or magnetic resonance imaging (MRI)] [7]. They also recommended the use of 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET)/CT if MRI and CT cannot detect the disease, and clinical suspicion of bone metastasis remains [7].
BS shows the osteoblastic response to bone destruction by cancer cells. One of the most important disadvantages of BS is that benign processes (such as osteoarthritis, fractures, and degenerative changes) may lead to a high false-positive rate and decrease the specificity of BS [8, 9].
In contrast, 18FDG-PET shows increased metabolic activity of tumor cells and can detect both osteoblastic and osteolytic lesions at an earlier stage [10]. However, PET imaging alone is limited for anatomical localization. Therefore, PET is expected to detect lytic lesions not seen on BS, whereas CT is expected to visualize sclerotic lesions not seen on PET alone [11] [12]. Thus, dual-modality PET/CT seems to have an advantage over BS in detecting osseous metastases [7]. Sclerotic lesions are easily recognized in the CT image of a PET/CT examination because of their radiographic density. Furthermore, several studies have demonstrated that the evaluation of metabolic response by using the standardized uptake value (SUV) in PET/CT may predict tumor response and survival [13–15]. PET/CT might therefore be the best use of imaging modality for evaluating bone metastases and their response to treatment.
Several retrospective studies have reported the diagnostic accuracy of 18FDG-PET/CT and BS in detecting bone metastases from breast cancer [16–21]. These studies demonstrate that, in detecting bone metastases from breast cancer, PET/CT is superior to BS in terms of sensitivity and specificity. However, the differences in the efficacy between 18FDG-PET/CT and BS were not confirmed.
We hypothesized that bone metastases could be detected more accurately by PET/CT than BS. In this prospective study, we compared the diagnostic performance of PET/CT and BS in patients with suspected bone metastases from breast cancer.
Patients and methods
Patients
Study design
This single-institution prospective study included consecutive patients with suspected bone metastases from biopsy-proven breast cancer seen at the Breast Diseases Unit at Tokai University Hospital, Kanagawa, Japan between September 2011 and March 2014. Inclusion criteria were signs of suspected bone metastases such as bone pain, elevated alkaline phosphatase, elevated tumor markers, suspected bone metastases by other imaging results, and locally advanced breast cancer. Other inclusion criteria were histologically proven breast cancer, age over 20 years or less than 75 years, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Exclusion criteria were prior history of breast or other cancer, ECOG performance status of 2 or 3, symptomatic brain metastases, uncontrolled diabetes mellitus, and pregnancy. The main objective was to compare the diagnostic accuracy of bone metastases, as detected by 18FDG-PET/CT and BS. We have planned accrual of 50 patients to prove this hypothesis.
This study was approved by the Institutional Review Board of Tokai University School of Medicine. All patients provided written informed consent. The study is registered with the University Hospital Medical Information Network (UMIN number 000006003).
Imaging protocols
BS was performed first, followed by PET/CT within a two-week interval.
For BS, images were obtained 3 h after injecting 555–740 MBq (15–20 mCi) of 99mTc-hydroxymethylene diphosphonate (HMDP) with an e.cam (Siemens Medical Solutions, Knoxville, TN). Whole-body anterior and posterior images were obtained in a 256 × 1024 matrix using a low-energy, high-resolution collimator at a scan speed of 15 cm/min.
For PET/CT, patients fasted 5 h before 18F-FDG administration. The tracer (185–370 MBq or 5–10 mCi) was intravenously injected in the arm of the opposite side to the tumor. Imaging was performed 60 min later, from the midthigh level to the top of the skull with the arms down using a lutetium oxyorthosilicate-based PET + 16-slice CT system TruePoint Biograph 16 (Siemens Medical Solutions, Knoxville, TN). CT data were acquired without contrast enhancement and using the following parameters: 130 kV, 60 mA, pitch 1.4, and slice thickness 5 mm. PET data was collected in a three-dimensional mode at 2 min per table position and reconstructed. Images were viewed on a Siemens syngo MultiModality Workplace workstation (Siemens Medical Solutions, Knoxville, TN). Maximum standardized uptake value (SUVmax), a semiquantitative measure of FDG uptake, was calculated.
Image interpretation
Bone scintigraphy
BS images were read by two nuclear medicine physicians (J.H. and T.K.) who were blinded to each patient’s clinical and other imaging findings. First, they independently interpreted the BS images, and then consensus was achieved upon discussion. Bone metastases and benign conditions were differentiated based on site, size, shape and distribution of tracer uptake, and patients’ clinical conditions including history of trauma and surgery. For example, round-shaped uptake(s) in a rib (or the adjacent ribs) of a patient with a history of trauma was diagnosed as a traumatic change, whereas extended uptake was considered malignant. Uptakes on the edges of the vertebrae were diagnosed as benign degeneration, whereas uptakes in the center of the vertebrae without compression fracture were judged as metastases. The readers scored for the presence or absence of bone metastasis and classified into three categories: 1, negative for bone metastasis; 2, equivocal for bone metastasis, and 3, positive for bone metastasis. In cases of equivocal or positive findings, the site of abnormal findings was recorded.
18FDG-PET/CT
PET, CT, and PET/CT fusion images were considered altogether. Two physicians who were blinded to patients’ clinical and other imaging findings independently interpreted PET/CT results. For bone metastases, the form and intensity of 18FDG uptake as well as CT findings were considered simultaneously. 18FDG uptake corresponding to degenerative findings on the underlying CT (e.g., on a facet articulation) and uptake in a rib fracture with a history of trauma were considered non-suspicious. However, high uptake on a typical area of metastasis (e.g., the body of a vertebra, pedicle, sternum, and pelvis) was considered malignant even if the CT part showed subtle or no changes, in agreement with the well-known high sensitivity of 18FDG-PET compared with CT for the detection of early bone marrow involvement [22].
Criteria for the final diagnosis of bone metastasis
Bone involvement was confirmed by biopsy, especially in cases of oligometastases. If biopsy proved difficult to perform, conventional imaging, additional directed radiological studies, and follow-up were helpful.
Bone metastases were confirmed by at least one other method as follows: bone biopsy (1 patient), CT (2 patients), and MRI (4 patients). However, such a confirmation was not performed in patients with evidence of other metastases at diagnosis because they were treated as having metastatic breast cancer.
In all of the above image interpretations, when the two physicians provided different diagnoses, the final diagnosis was decided by discussion.
Statistical analysis
Sensitivity and specificity were determined on the basis of number of patients instead of number of lesions. The McNemar’s Chi squared test was used to compare the diagnostic performance of PET/CT and BS. A P value of less than 0.05 was considered significant. All analyses were performed by using R software v 3.1.1 (http://www.r-project.org).
Results
Patients
Thirty patients were initially enrolled, but two were excluded from the analyses because they declined further follow-up imaging. The median patient age at diagnosis was 59 years (range 31–74 years). Patient characteristics are shown in Table 1. The reasons for suspected bone metastases are presented in Table 2.
PET/CT successfully detected bone metastases in all 10 patients finally diagnosed with the condition, whereas BS identified 2. The two modalities were not highly concordant in detecting osseous metastases; of the 19/28 paired studies (68 %), 2 (10 %) were positive for metastasis, and 17 (90 %) were negative. Of the patients with concordant positive results, one underwent MRI to confirm osseous metastases, and the other did not undergo other imaging procedures owing to multiple bone metastases. Both patients were clinically treated as having bone metastases.
There were nine discordant cases (32 %); of these, one was PET/CT positive and BS negative; seven were PET/CT positive and BS equivocal, and one was PET/CT equivocal and BS negative (Table 3). The Chi square test showed statistically significant difference between the results of PET/CT and those of BS (P = 0.029).
PET/CT positive, BS negative case
One of the 9 discordant cases manifested positive PET/CT and negative BS (Table 3). This patient did not undergo bone biopsy because the tumor in the sternum was very small. Since multiple lung metastases were also detected by PET/CT, the patient was treated with tamoxifen as having metastatic breast cancer.
PET/CT positive, BS equivocal cases
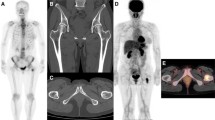
Of the seven patients with positive PET/CT and equivocal BS, one underwent bone biopsy after PET/CT examination, and osseous metastasis was confirmed (Fig. 1). Of the six patients who did not undergo bone biopsy or fine needle aspiration (FNA), three had clinical or radiographic evidence of bone metastasis, whereas the other three did not. Other metastatic lesions were detected in the lymph nodes (two patients) and lungs (one patient) of the three patients without bone metastasis. Progression of bone metastases was confirmed in one patient 3 months after diagnosis. Therefore, all seven patients received treatment as having metastatic breast cancer. Two cases with positive PET/CT and equivocal BS findings are presented in Figs. 1 and 2.
A case of lumbar metastasis in which bone scintigraphy (BS) findings (a) were inconsistent with positron emission tomography/computed tomography (PET/CT) findings (b). Biopsy-proven L4 spinal bone metastasis was present in this case and was detected on PET/CT (b), whilst the BS findings were equivocal (a)
A case of multiple bone metastases in which bone scintigraphy (BS) findings (a) were inconsistent with positron emission tomography/computed tomography (PET/CT) findings (b). PET/CT clearly demonstrated multiple bone metastases (b), but the BS findings were equivocal, with only a slightly increased uptake in the metastatic lesions (a)
PET/CT equivocal, BS-negative cases
In the one patient with equivocal PET/CT and negative BS, an osteoblastic change in the spine was detected on CT. Since increased FDG uptake was not observed for the lesion, the patient did not undergo bone biopsy or FNA. The patient was followed up without any signs of bone metastasis 24 months after the PET/CT examination.
Discussion
To our knowledge, this is the first prospective study comparing PET/CT and BS for the detection of bone metastases. The number of patients did not reach the planned number of patients. However, PET/CT successfully detected bone metastases in 10 of 10 patients, whereas BS findings were positive in 2, equivocal in 7, and negative in one. The two modalities were not highly concordant in detecting osseous metastases. Our data demonstrated that PET/CT was superior to BS in detecting bone metastases.
No consensus criteria are available for the selection of imaging modalities in diagnosing bone metastasis. Since BS images reflect bone mineral turnover and provide only indirect information about tumor activity, they are less sensitive in detecting lytic and/or indolent metastatic lesions compared to PET/CT [23]. On the other hand, PET/CT could detect bone metastases even in patients with a small number of metastatic lesions in the current study. Of the seven equivocal patients, four had a single bone metastasis. Bone biopsy is required to confirm the diagnosis of single bone metastasis owing to the high false-positive rate of BS and conventional imaging [24]. 18FDG-PET/CT has a high potential for more accurate assessment of bone metastases than does either PET or CT alone, because osteolytic lesions can be detected by PET and sclerotic lesions by CT [12] [11]. Nonetheless, in our study, a patient who had a sclerotic lesion detected by CT without increased 18FDG uptake did not exhibit any evidence of disease for 2 years. Furthermore, PET/CT is advantageous for whole-body imaging for the detection of metastases in soft tissues and visceral organs. In point of disadvantage, PET/CT is high cost and high radiation exposure than BS. However, equivocal patients diagnosed BS need additional imaging, that is additional cost and radiation exposure.
The current study has some limitations. First, the number of enrolled patients was small. Our data were collected from a single institution and might reflect selection bias. Second, not all patients underwent a completed follow-up imaging evaluation. Third, histopathologic findings were not available for all areas of suspected bone metastases because clinicians tended to avoid biopsy in patients with suspected multiple metastases, particularly when findings on additional imaging studies were highly suggestive of metastatic disease, and treatment needed to be expedited in cases of rapidly progressive disease. For patients with presumed false-negative findings on PET/CT who did not undergo biopsy, we did not have a definite answer regarding whether the patient actually had bone metastases.
Conclusions
The results of our prospective study strongly supported findings from previous retrospective studies showing that PET/CT might be superior to BS in detecting bone metastases. On the basis of the results of previous studies, as well as ours, PET/CT could replace BS as the first choice for detecting suspected bone metastases.
References
Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–80.
Niikura N, Liu J, Hayashi N, Palla SL, Tokuda Y, Hortobagyi GN, et al. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: a single-institution retrospective analysis. Oncologist. 2011;16:155–64.
Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336–40.
Domchek SM, Younger J, Finkelstein DM, Seiden MV. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer. 2000;89:363–8.
Briasoulis E, Karavasilis V, Kostadima L, Ignatiadis M, Fountzilas G, Pavlidis N. Metastatic breast carcinoma confined to bone: portrait of a clinical entity. Cancer. 2004;101:1524–8.
Roberts CC, Daffner RH, Weissman BN, Bancroft L, Bennett DL, Blebea JS, et al. ACR appropriateness criteria on metastatic bone disease. J Am Coll Radiol. 2010;7:400–9.
Costelloe CM, Rohren EM, Madewell JE, Hamaoka T, Theriault RL, Yu TK, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–14.
Tryciecky EW, Gottschalk A, Ludema K. Oncologic imaging: interactions of nuclear medicine with CT and MRI using the bone scan as a model. Semin Nucl Med. 1997;27:142–51.
Arano Y. Recent advances in 99mTc radiopharmaceuticals. Ann Nucl Med. 2002;16:79–93.
Abe K, Sasaki M, Kuwabara Y, Koga H, Baba S, Hayashi K, et al. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005;19:573–9.
Shie P, Cardarelli R, Brandon D, Erdman W, Abdulrahim N. Meta-analysis: comparison of F-18 Fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med. 2008;33:97–101.
Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–9.
Du Y, Cullum I, Illidge TM, Ell PJ. Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25:3440–7.
Gebhart G, Gamez C, Holmes E, Robles J, Garcia C, Cortes M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med. 2013;54:1862–8.
Groheux D, Giacchetti S, Hatt M, Marty M, Vercellino L, de Roquancourt A, et al. HER2-overexpressing breast cancer: FDG uptake after two cycles of chemotherapy predicts the outcome of neoadjuvant treatment. Br J Cancer. 2013;109:1157–64.
Fuster D, Duch J, Paredes P, Velasco M, Munoz M, Santamaria G, et al. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol. 2008;26:4746–51.
Rong J, Wang S, Ding Q, Yun M, Zheng Z, Ye S. Comparison of 18 FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients. A meta-analysis. Surg Oncol. 2013;22:86–91.
Morris PG, Lynch C, Feeney JN, Patil S, Howard J, Larson SM, et al. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol. 2010;28:3154–9.
Niikura N, Costelloe CM, Madewell JE, Hayashi N, Yu TK, Liu J, et al. FDG-PET/CT compared with conventional imaging in the detection of distant metastases of primary breast cancer. Oncologist. 2011;16:1111–9.
Houssami N, Costelloe CM. Imaging bone metastases in breast cancer: evidence on comparative test accuracy. Ann Oncol. 2012;23:834–43.
Ohta M, Tokuda Y, Suzuki Y, Kubota M, Makuuchi H, Tajima T, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Commun. 2001;22:875–9.
Nakamoto Y, Cohade C, Tatsumi M, Hammoud D, Wahl RL. CT appearance of bone metastases detected with FDG PET as part of the same PET/CT examination. Radiology. 2005;237:627–34.
Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–53.
Niikura N, Odisio BC, Tokuda Y, Symmans FW, Hortobagyi GN, Ueno NT. Latest biopsy approach for suspected metastases in patients with breast cancer. Nat Rev Clin Oncol. 2013;10:711–9.
Acknowledgments
We would like to thank Editage for providing editorial assistance. This study was supported by MEXT KAKENHI Grant Number 26870597, and Tokai University School of Medicine Research Aid, 2013–2014.
Conflict of interest
Yutaka Tokuda received a research grant from Chugai Pharmaceutical Co., Ltd, Eisai Co. Ltd and Novartis Pharma L.K. The other authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Niikura, N., Hashimoto, J., Kazama, T. et al. Diagnostic performance of 18F-fluorodeoxyglucose PET/CT and bone scintigraphy in breast cancer patients with suspected bone metastasis. Breast Cancer 23, 662–667 (2016). https://doi.org/10.1007/s12282-015-0621-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-015-0621-z