Abstract
Purpose of Review
This review describes the clinical presentation, predisposing factors, pathophysiology, mycology, diagnosis, and therapy of sino-orbital invasive fungal infections in immunocompetent individuals. Recent advances published in the literature were reviewed with special consideration of current diagnostic methods and treatment regimens.
Recent Findings
Owing to the rarity of this condition literature is scarce. Majority of the studies are from tropical countries such as India where the disease is relatively more common. Treatment strategies are variable and response to medical treatment is favorable in significant number of patients.
Summary
Invasive sino-orbital fungal infection in immunocompetent hosts is a rare clinical condition. Aspergillus and Mucorales group of fungi cause infection in the presence of certain risk factors. Other species of fungi may occasionally be involved. Diagnosis requires imaging and laboratory back up. CT scan remains the primary imaging modality of choice although MRI is more sensitive in picking up orbital soft tissue inflammation. With early diagnosis and appropriate medical and surgical therapy, globe salvage and visual recovery may be achieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With their severe local and systemic morbidity, infections of the orbit and periorbital tissues form important subsets of orbital inflammatory diseases. Infectious and non-infectious causes of orbital inflammatory disease may have similar clinical and radiologic features. Infective orbital cellulitis patients often have a history of sinus or dental disease or recent facial trauma and may be febrile at presentation. Significant number of patients may also have visual loss [1]. Several factors predispose the orbit to infection such as inferior, middle, and superior relationship of orbit to the paranasal sinuses; separation of the orbit from the ethmoid sinuses by a paper-thin bone of the lamina papyracea; and lack of valves in the inferior and superior ophthalmic veins in the midface permitting direct spread of infectious organisms. Patients with systemic immunosuppression due to any cause are more susceptible to orbital infection; however, immunocompetent patients can also be affected in presence of a risk factor. Orbital infection is relatively less common in immunocompetent individuals. Until 2003, there were 17 cases of sino-orbital aspergillosis over 33 years’ period in healthy individuals [2]. A study from South India reported 20 cases seen over a period of 8 years suggesting higher prevalence in tropical parts of the world [3••]. Some of the risk factors associated with orbital infection include pre-existing orbital disease, direct injury, contiguous spread from neighboring structures, and hematogenous spread [4]. Driven by the predisposing factor, the infection could be bacterial, fungal, or parasitic. Of these, bacterial infections of the orbit are more common. Clinical features may often be suggestive of the organism involved and may guide the initiation of treatment pending culture results. This report focuses on fungal infections of the sinuses and the orbit, especially in immunocompetent hosts.
Clinical Presentation and Predisposing Factors
Invasive sino-orbital fungal infection in immunocompetent patients is a rare condition. Both the diagnosis and management of this condition are challenging. Of all fungal species, members of the genus Aspergillus are the commonest that colonize the paranasal sinuses and lungs. In tropical areas of the world, Aspergillus is a common saprophytic fungus and the spores are abundantly present in the atmosphere. Aspergillus flavus is the predominant species isolated from sino-orbital aspergillosis in Asia, Africa, and Middle East. The high frequency of this species may be related to high environmental prevalence of the fungus [5]. Once inhaled, the spores may develop into fungal masses, especially if the host immunity is low. Due to close proximity of the paranasal sinuses to the orbit, a contiguous spread may occur to the orbit. While the infection spreads rapidly in immunocompromised hosts, it is slow and chronic in immunocompetent hosts. It can be potentially fatal due to spread to the middle cranial fossa via superior orbital fissure and optic canal [6]. Barring some case reports from tropical countries like Sudan and India, invasive fungal infection of the orbit is under-reported from healthy individuals [7,8,9, 10••, 11•]. Out of 35 patients with orbital aspergillosis in immunocompetent individuals, Mody et al. found A. flavus in 30 (86%) and A. fumigatus in the remaining [11•]. In this series, the mean age at presentation was 37.63 (8–73) years and mean duration of symptoms was 12.03 (0.5–84) months. The commonest presenting features were proptosis (22.63%) and mass lesion (13.37%). Presenting visual acuity was better than 6/9 in 21 (60%) and no perception of light in 3 (8%). In 10.29%, the commonest clinical differential diagnosis was non-specific orbital inflammatory disease (NSOID) followed by malignancy (7.20%). Infiltrative lesions with bone destruction were seen in 22 (63%) patients along with contiguous paranasal sinus involvement. Ten patients (29%) had intracranial extension [11•]. A large case series of 20 patients has been reported recently by Adulkar et al. [3••]. Most common presentation (15/20) was proptosis followed by diplopia (5/20). Vision was affected in 4 out of 20 patients. Two patients in this series had the unique feature of periocular discharging sinuses. The most common early signs are sinusitis or facial pain, pharyngitis, and a foul-smelling seropurulent nasal discharge. Facial and retrobulbar pain can be severe enough to require narcotic analgesics [2]. In severe cases, there may be loss of smell sensation, numbness over the face, and black necrotic mass in the nose or back of the mouth. Mauriello et al. [12] have reported three immunocompetent patients with Aspergillus sino-orbital infection who presented with acute proptosis and loss of vision. Majority of the patients reported in the literature are young [3••, 8, 10••, 11•]. However, some studies have reported advanced age as a predisposing risk factor [12, 13]. Predisposing factors may include orbital inflammatory disease, rhinosporidiosis, tuberculosis, Tolosa-Hunt syndrome, and orbital foreign body [3••].
Several conditions may mimic sino-orbital fungal infection such as malignancy [12, 14], temporal arteritis [12, 15], optic neuritis [16], idiopathic orbital inflammatory syndrome [15], orbital apex syndrome [13, 17], bacterial cellulitis, and orbital abscess [18]. Prior treatment with steroid and absence of sinus-related symptoms might lead to difficulty in diagnosis. A high index of suspicion is crucial to overcome the diagnostic dilemma in such cases.
Diagnostic Investigations
Computed tomography (CT) is the most common imaging method that is recommended in patients suspected to have sino-orbital disease. Ancillary investigations such as white blood cell count, culture of pus or blood, and orbital ultrasonography may not be useful in initiation of treatment in suspected postseptal disease, which should not be delayed on account of such investigations [19]. Erythrocyte sedimentation rate and white blood cell counts may be elevated. Blood cultures are usually negative. Biopsy may show wide sparsely septate or aseptate branching filaments with characteristic ribbon-like folds if a Mucorales (Mucor, Rhizopus, Apophysomyces, etc.) is involved. Broad aseptate hyphae with right-angled branching are easily seen on hematoxylin and eosin–stained sections that spread along nerves across tissue planes, and into blood vessels. Filaments of Aspergillus are usually thin, septate, and branch at acute angles. Inflammation ranges from negligible to large numbers of neutrophils, eosinophils, and histiocytes within granulation tissue. Non-caseating multinucleate giant cell granuloma, fibrosis and chronic inflammatory response consisting of eosinophils, lymphocytes, and plasma cells were seen in 18 patients with sino-orbital infection [3••]. In immunocompetent individuals, a minimal inflammatory reaction of granulation tissue with micro-abscesses or multinucleated giant cells can be seen [20]. Histopathology stains for the examination of the biopsy material should include Gomori’s methenamine silver (GMS) in addition to hematoxylin and eosin and periodic acid Schiff. Failure to identify fungus on initial biopsy has been reported by many authors [3••, 7, 18]. The role of fine needle aspiration biopsy (FNAB) in the diagnosis of orbital fungal infection is not clear although it can be tried in patients with sino-orbital mass involving the posterior orbit, especially when biopsy from involved paranasal sinuses is negative and incisional biopsy from the orbital mass is difficult [10••]. Kuruba et al. [21] reportedly established definitive diagnosis using FNAB in two cases of sino-orbital fungal disease. Specific antibodies to Aspergillus can be used for immunohistochemistry to identify Aspergillus sp. in histopathology sections [22]. Serum galactomannan and β-d glucan tests as fungal biomarkers have been used in the diagnosis of invasive fungal infections but seem to have limited value [23].
Histopathology helps to differentiate allergic aspergillosis from infection. Aspergillus hyphae with no tissue invasion and presence of mucin along with eosinophils and Charcot-Leyden crystals are the characteristic features [24]. Tissue invasion by fungal filaments, especially angio-invasion and perineural invasion, is usually present in cases of sino-orbital invasive fungal infections.
Direct examination of the sample after Gram stain or potassium hydroxide with or without calcofluor white may demonstrate the presence of the fungal filaments. The latter stain would require a fluorescence microscope for observation. Culture on blood agar and Sabouraud dextrose agar would usually suffice for the growth of the fungus. The fungal isolates are identified by their colony characteristic and spores seen under lactophenol cotton blue mount. In the absence of sporulation, the isolate may be identified by DNA sequencing of the ITS region of the 18S rRNA gene of fungus [25]. If the fungus fails to grow in culture but is present in histopathology sections, the paraffin sections can be used for DNA extraction followed by polymerase chain reaction and DNA sequencing [26, 27, 28•]. The same can be applied directly to the biopsy sample.
CT scan is essential for identifying sinus disease and subperiosteal or orbital abscess. An abscess can be differentiated from inflammatory phlegmon by intravenous contrast. Investigation of possible cavernous sinus, intracranial extension, or radiolucent foreign bodies may require magnetic resonance imaging (MRI). Lumbar puncture is rarely needed and is reserved for children with meningism [29]. CT scan in rhino-cerebral mucormycosis may show mucosal thickening, bony destruction, and findings suggestive of thrombosis. CT scan features of sino-orbital aspergillosis may be non-specific and confused with idiopathic orbital inflammatory disease. Observation of paranasal involvement usually points to the diagnosis. Adulkar et al. [3••] observed pansinusitis in 70% of their patients, while bony erosion was seen in 50%.
Pathophysiology and Mycology
The etiological agents of sino-orbital fungal infections include Aspergillus spp., Mucorales, dematiaceous fungi, and, less commonly, hyaline septate fungi like Fusarium spp. Based on the phylogenetic data using molecular techniques, the older term Zygomycetes is abandoned [30, 31•, 32].
Aspergillus is a genus that includes large number of species such as A. fumigatus, A. flavus, A. terreus, and A. glaucus; all of which are identified based on colony characteristics and microscopic features of asexual sporulation. Aspergillus has been reported from orbital infections in healthy host, although it has greater predilection for an immunocompromised host [33, 34]. Sino-orbital aspergillosis, although said to be rare in immunocompetent hosts [2, 8], in a series of 20 patients from a tertiary eye care center in India, 18 cases were due to Aspergillus [3••]. Aspergillus hyphae were identified in histopathology based on uniform diameter, dichotomous branching at approximately 45 degrees, and presence of septation.
Fungal genera Mucor, Rhizopus, Absidia, Apophysomyces etc., belonging to the order Mucorales under the phylum Mucoromycota, are the most species-rich clinical order and are commonly reported from sino-orbital mucormycosis in immunocompromised hosts. This group of fungi seems to be the most common cause of rhino-orbital cerebral fungal infection in the debilitated host [35]. Six clinical types of mucormycosis include cerebral, pulmonary, gastrointestinal, and central nervous system, and subcutaneous and disseminated. Beginning in the nose, rhino-orbital cerebral infection spreads to the maxillary sinus, ethmoids, and then to the orbit. Infection spreads to the central nervous system through roof of the orbit and cribriform plate. These fungal species are angio-invasive and particularly destructive in ketotic diabetic patients. The infection may however at times occur in immunocompetent individuals [36]. Two out of a series of 20 patients with sino-orbital fungal infection in immunocompetent patients showed presence of Mucor colonies (identified by broad, ribbon-like collapsed aseptate fungal filaments with obtuse angle branching) in orbital tissue with angio-invasion [3••]. Apophysomyces elegans belongs to the family Mucoraceae and is generally transmitted through contaminated soil following trauma. A. elegans has been earlier reported as an emerging pathogen causing rhino-orbital mucormycosis in immunocompetent patients [36, 37]. On microscopy, tissues show chronic inflammation with local invasion into adjacent structures. Acute inflammation with necrosis is attributed to vessel thrombosis by A. elegans. Optic nerve invasion can lead to vision loss. The fungus generally originates in the sino-nasal region and extends into the orbit. Histopathology can, to a certain extent, differentiate the fungal hyphae based on their morphological characteristics, but microbiological culture confirmation is essential for the species identification. Cultures on staining with lactophenol cotton blue can help identify the species based on the different shapes of sporangia as follows:
-
Globose sporangium—Rhizopus, Mucor, Rhizomucor, and Mortierella spp.
-
Pyriform or teardrop-shaped sporangia—Absidia and Apophysomyces elegans. The apophysis differentiates between the two; Absidia spp. produce a flask-shaped apophysis with a large columella. A. elegans produces either a “funnel-shaped” or “bell-shaped” apophysis.
-
Vasiform sporangium—Saksenaea vasiformis.
One case is illustrated below.
Case Presentation
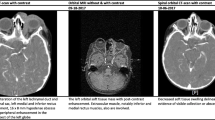
A well-built and nourished 50-year-old male presented with painful swelling and loss of vision since a week. There was no prior history of ocular injury or optical prescription. Past history included diagnosis of Hansen’s disease and malaria. He was positive for hepatitis C virus. On radiological imaging, CT scan of the right eye showed pre-septal homogenous shadows with proptosis and tenting of the globe. Ethmoid sinus fullness was seen involving mainly the posterior sinuses. Contrast-enhanced CT of the right side showed smaller eyeball with tenting and enlarged superior orbital vein. Patient was suspected to have cavernous venous thrombosis and was referred to neurosurgical service; however, he returned after 5 days and required orbital exenteration. Histopathology revealed extensive areas of necrosis surrounded by intense foreign body giant cell reaction. Within the necrotic area elongated, ribbon-shaped with variable thickness, amphophilic, right-angle branched refractile structures were seen with angio-invasion and perineural invasion. These refractile structures were highlighted on GMS stain and microbiological culture of the tissue showed off-white cottony growth on chocolate agar which under lactophenol cotton blue mount showed broad aseptate hyphae, long sporangiophore with the apex of the sporangiophore widening to form a funnel-shaped apophysis along with half circle columella (Fig. 1).
a Clinical image showing lid edema, proptosis, conjunctival congestion, and crusting over the lower cornea and conjunctiva. b Computed tomography image showing proptosis, tenting of globe (arrow), and enlarged superior orbital vein (arrowhead). c Hematoxylin and eosin stain of the exenterated tissue sections revealed extensive areas of necrosis with broad, ribbon-shaped, amphophilic, right-angle branched fungal hyphae (× 100) highlighted on the Gomori methenamine silver stain, × 400 (inset). d Culture of the tissue showed off-white cottony growth on chocolate agar which under lactophenol cotton blue mount showed broad aseptate hyphae and long sporangiophore with the apex of the sporangiophore widening to form a funnel-shaped apophysis along with half circle columella, × 400 (inset)
Among the dematiaceous fungi, Exserohilum rostratum has been reported in an immunocompetent child [38]. Aged 11 years, a female child presented with bilateral nasal stuffiness and nasal discharge since 1 year and left-sided proptosis since 8 months. The patient had a history of bronchial asthma and recurrent allergic fungal sinusitis since she was 2 years old. On the basis of CT findings that showed involvement of left maxillary, frontal, sphenoid, and ethmoidal sinuses along with breach in the lamina papyracea and bony erosion of the medial wall of maxillary sinus, a diagnosis of pansinusitis with intraorbital extension was made. Left-sided functional endoscopic surgery was done and culture of excised tissue grew E. rostratum. The child was treated with oral itraconazole [38]. Alternaria species is another dematiaceous fungus that was seen in an immunocompetent female patient with a 10-year history of nasal congestion. On investigation, she was found to have tumor-like lesion in right maxillary sinus with extension into the right nasal cavity, right ethmoidal cells, and right orbit. Surgically removed lesion showed growth of Alternaria alternaria in culture and medical therapy with itraconazole and surgical debridement led to eradication of the infection [39]. A common cause of fungal keratitis in tropical parts of the world including India, Fusarium sp. has been reported to cause periorbital necrotizing infection in a female patient following cataract surgery. The clinical features were complicated with concomitant endophthalmitis in the operated eye and herpes zoster ophthalmicus. However, the patient tested negative for antibodies to human immunodeficiency virus by ELISA [40].
Therapy
Treatment of sino-orbital fungal infection is challenging in the absence of standard guidelines. Being angio-invasive, aspergillosis causes necrosis by infarction, which reduces drug penetration. Surgical debridement is often needed. Surgical debridement is difficult in the orbit in the presence of vital structures and the inability to determine the extent of the disease. Patients with retro-bulbar and apical involvement may need exenteration [8, 12]; however, exenteration does not guarantee complete eradication of apical disease and may require long-term antifungal therapy to clear the infection. Some surgeons prefer globe-conserving debulking surgery, coupled with antifungal therapy. A good response to antifungal therapy (amphotericin B and itraconazole) in immunocompetent patients has been reported [41]. Adulkar et al. [3••] treated all patients in their series of immunocompetent patients with oral itraconazole with variable results. They found good response in 14 out of 20 patients and relief from pain in 18 out of 20. Intravenous amphotericin B followed by oral itraconazole achieved good results in 10 out of 15 patients in another report [10••]. Liver function tests need to be monitored in patients on antifungal drugs. With a lower toxicity, liposomal amphotericin B is considered a good antifungal formulation that is effective against a wide range of fungal species including Aspergillus and Mucorales. The recommended dosage is 3–10 mg/kg body weight/day. Another drug that has been used with favorable response is a newer triazole, posaconazole [42, 43]. In mucormycosis, radical surgical debridement of necrotic tissue along with antifungal therapy is the mainstay of effective management.
Visual prognosis is usually poor in patients with sino-orbital invasive fungal disease despite treatment. Orbital debulking preserving the vital structures along with oral itraconazole may result in relief of pain, resolution of proptosis, and salvage of the globe; however, vision may be preserved in not more than 40% of patients [3••]. Favorable visual outcome of 62.5% was reported in one study [11•]. Causes of visual compromise include central retinal occlusion and optic neuropathy due to ischemia. Fungal invasion of the vessels can often lead to localized formation of thrombi with a tendency to dislodge. Raised intraocular pressure along with thrombus formation can cause vascular occlusions. Voriconazole has been used successfully in some of the studies as the antifungal drug of choice [23].
In conclusion, sino-orbital invasive fungal infection is a rare condition that can occur in otherwise healthy individuals. Characteristic clinical features, history, and appropriate imaging often guide the initial therapy. Surgical biopsy or debridement may be required. Histopathological study of the tissue helps confirm the fungal etiology and smear; culture or molecular methods determine the type of offending fungus. Aspergillus and Mucorales group of fungi are most commonly associated although other species may be involved. Early institution of antifungal therapy may lead to resolution with variable levels of visual recovery.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tomac S, Turgut S. Orbital cellulitis and irreversible visual loss owing to acute sinusitis. Ann Ophthalmol. 2006;38:131–3.
Sivak-Callcott JA, Livesley N, Nugent RA, Rasmussen SL, Saeed P, Rootman J. Localised invasive sino-orbital aspergillosis: characteristic features. Br J Ophthalmol. 2004;88:681–7.
•• Adulkar NG, Radhakrishnan S, Vidhya N, Kim U. Invasive sino-orbital fungal infections in immunocompetent patients: a clinic-pathological study. Eye. 2019;33:988–94 This paper from South India is the latest in the literature with account of 20 patients with sino-orbital fungal infections in immunocompetent patients along with review.
Verity DH, Rose GE. Infectious processes of the orbit. Chapter 232. In: Albert DM, Miller JW, editors. Albert Jakobiec’s Principals and practice of ophthalmology. Canada: Saunders Elseviers; 2008. p. 2962.
Chakrabarti A, Chatterjee SS, Das A, Shivaprakash MR. Invasive aspergillosis in developing countries. Med Mycol. 2011;49:S35–47.
Shamim MS, Siddiqui AA, Enam SA, Shah AA, Jooma R, Anwar S. Craniocerebral aspergillosis in immunocompetent hosts: surgical perspective. Neurol India. 2007;55:274–81.
Kameswaran M, al-Wadei A, Khurana P, Okafor BC. Rhinocerebral aspergillosis. J Laryngol Otol. 1992;106:981–5.
Dhiwakar M, Thakar A, Bahadur S. Invasive sino-orbital aspergillosis: surgical decisions and dilemmas. J Laryngol Otol. 2003;117:280–5.
Green WR, Font RL, Zimmerman LE. Aspergillosis of the orbit: report of ten cases and review of the literature. Arch Ophthalmol. 1969;82:302–13.
•• Pushker N, Meel R, Kashyap S, Bajaj M, Sen S. Invasive aspergillosis of orbit in immunocompetent patients: treatment and outcome. Ophthalmology. 2011;118:1886–91 The study reports efficacy of newer antifungal drug voriconazole along with other antifungals, in the treatment of invasive orbital aspergillosis.
• Mody KH, Ali MJ, Vemuganti GK, Nalamada S, Naik MN, Honavar SG. Orbital aspergillosis in immunocompetent patients. Br J Ophthalmol. 2014;98:1379–84 In a large series of 35 cases of orbital aspergillosis in immunocompetent patients, nearly 50% responded to medical therapy alone. The study highlights the importance of histopathology and microbiology in the diagnosis. Avoiding steroid therapy is emphasized.
Mauriello JA Jr, Yepez N, Mostafavi R, Barofsky J, Kapila R, Baredes S, et al. Invasive rhino-sino- orbital aspergillosis with precipitous visual loss. Can J Ophthalmol. 1995;30:124–30.
Marcet MM, Yang W, Albert DM, Salamat MS, Appen RE. Aspergillus infection of the orbital apex masquerading as Tolosa-Hunt syndrome. Arch Ophthalmol. 2007;125:563–6.
Yumoto E, Kitani S, Okamura H, Yanagihara N. Sino-orbital aspergillosis associated with total ophthalmoplegia. Laryngoscope. 1985;95:190–2.
Austin P, Dekker A, Kennerdell JS. Orbital aspergillosis: report of a case diagnosed by fine needle aspiration biopsy. Acta Cytol. 1983;27:166–9.
Spoor TC, Hartel WC, Harding S, Kocher G. Aspergillosis presenting as a corticosteroid-responsive optic neuropathy. J Clin Neuroophthalmol. 1982;2:103–7.
Slavin ML. Primary aspergillosis of the orbital apex. Arch Ophthalmol. 1991;109:1502–3.
Whitehurst FO, Liston TE. Orbital aspergillosis: report of a case in a child. J Pediatr Ophthalmol Strabismus. 1981;18:50–4.
Rose GE. Suspicion, speed, sufficiency and surgery: keys to management of orbital infection. Orbit. 1998;17:223–6.
Garrish MT, Podnos SD, Meyerhoff WL. Chronic progressive aspergillosis in an immunocompetent host. Otolaryngol Head Neck Surg. 1987;96:565–8.
Kuruba SL, Prabhakaran VC, Nagarajappa AH, Biligi DS. Orbital Aspergillus infection diagnosed by FNAC. Diagn Cytopathol. 2011;39:523–6.
Challa S, Uppin SG, Uppin MS, Umabala P, Vemu L. Diagnosis of filamentous fungi on tissue sections by immunohistochemistry using anti-Aspergillus antibody. Med Mycol. 2015;53:470–6.
Sugai A, Oyake M, Umeda M, Umeda Y, Fujita N. Case of orbital apex syndrome caused by invasive aspergillosis successfully treated during the diagnostic procedure by the use of voriconazole. Rinsho Shinkeigaku. 2008;48:746–9.
Das A, Bal A, Chakrabarti A, Panda N, Joshi K. Spectrum of fungal rhinosinusitis; histopathologist’s perspective. Histopathology. 2009;54:854–9.
Embong Z, Wan Hitam WH, Yean CY, Rashid NHA, Kamarudin B, Abidin SKZ, et al. Specific detection of fungal pathogens by 18S rRNA gene PCR in microbial keratitis. BMC Ophthalmol. 2008;8:7.
Salehi E, Hedayati MT, Zoll J, Rafati H, Ghasemi M, Doroudinia A, et al. Discrimination of aspergillosis, mucormycosis, fusariosis, and scedosporiosis in formalin-fixed paraffin-embedded tissue specimens by use of multiple real-time quantitative PCR assays. J Clin Microbiol. 2016;54:2798–803.
Rickerts V, Khot PD, Myerson D, Ko DL, Lambrecht E, Fredricks DN. Comparison of quantitative real time PCR with sequencing and ribosomal RNA-FISH for the identification of fungi in formalin fixed, paraffin-embedded tissue specimens. BMC Infect Dis. 2011;11:202.
• Rickerts V. Identification of fungal pathogens in formalin-fixed, paraffin embedded tissue samples by molecular methods. Fungal Biol. 2016;120:279–87 Limitations of culture and histopathology in confirming the diagnosis of invasive fungal infections can be overcome to some extent by molecular approach using paraffin-embedded tissue. The study describes this alternative approach in great details along with its limitations.
Antoine GA, Grundfast KM. Periorbital cellulitis. Int J Pediatr Otorhinolaryngol. 1987;13:273–8.
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher-level phylogenetic classification of the fungi. Mycol Res. 2007;111:509–47.
• Walther G, Wagner L, Kurzai O. Updates on the taxonomy of Mucorales with an emphasis on clinically important taxa. J Fungi. 2019;5:106. https://doi.org/10.3390/jof5040106This extensive review provides the latest account of the constantly evolving taxonomy of Mucorales.
Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301.
Siddiqui A, Shah A, Bashir S. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: clinical spectrum and outcome in 25 cases. Neurosurgery. 2004;55:602–13.
Dortzbach R, Segresr DR. Orbital aspergillosis. Ophthalmic Surg. 1983;14:240–4.
Gutierrez Diaz A, del Palacio HA, Larregla S, Sanz LA. Orbital phycomycosis. Ophthalmologica. 1981;182:165–70.
Fairley C, Sullivan TJ, Bartley P, Allworth T, Lewandowski R. Survival after rhino-orbital-cerebral mucormycosis in an immunocompetent patient. Ophthalmology. 2000;107:555–8.
Radner AB, Witt MD, Edwards JE Jr. Acute invasive rhinocerebral zygomycosis in an otherwise healthy patient: case report and review. Clin Infect Dis. 1995;20:163–6.
Gupta A, Immaculata X, Sharma SC, Mallick S. Invasive rhinosinusitis by Exserohilum rostratum in an immunocompetent child. BMJ Case Rep. 2014.
Pesic Z, Otasevic S, Mihailovic D, Petrovic S, Arsic-Arsenijevic V, Stojanov D, et al. Alternaria –associated fungus ball of orbit, nose and paranasal sinuses: case report of a rare clinical entity. Case Rep Mycopathol. 2015;180:99–103.
Pushker N, Chra M, Bajaj MS, Ghose S, Naik N, Kashyap S, et al. Necrotizing periorbital Fusarium infection-an emerging pathogen in immunocompetent individuals. J Inf Secur. 2002;44:236–9.
Greenberg RN, Mullane K, van Burik JA, Raad I, Abzug MJ, Anstead G, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006;50:126–33.
Sun QN, Fothergill AW, MscCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, amphotericin B and fluconazole against 37 clinical isolates of Zygomycetes. Antimicrob Agents Chemother. 2002;46:1581–2.
van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;43:1376.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Savitri Sharma and Saumya Jakati declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Clinical Pathology
Rights and permissions
About this article
Cite this article
Sharma, S., Jakati, S. Sino-Orbital Invasive Fungal Infections in Immunocompetent Hosts. Curr Fungal Infect Rep 14, 246–251 (2020). https://doi.org/10.1007/s12281-020-00400-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-020-00400-8