Abstract
Purpose of Review
The incidence of onychomycosis by Aspergillus has shown an increase in recent years, representing 34–60% of onychomycosis due to non-dermatophyte molds. At least 26 species of Aspergillus causing onychomycosis have been reported, some of which may be morphologically indistinguishable but genetically distinct, even in their susceptibility profile to antifungals. So in the diagnosis of this pathology, it is necessary to use both conventional and molecular methods to get to the identification of the fungus at the species level and thus establish the appropriate treatment.
Recent Findings
The current taxonomy of the genus Aspergillus includes sections that are made up of species whose morphology is almost identical but have different patterns of susceptibility to antifungals. Advances in the taxonomy of these fungi reveal the need to combine phenotypic methods (analysis of microscopic and macroscopic characteristics) with molecular ones (amplification and sequencing of fragments of the β-tubulin and calmodulin genes) to achieve their correct identification at the level of species.
Summary
From the demonstration of Aspergillus as the primary agent of onychomycosis, an increase in the incidence of this pathology worldwide has been reported, whose treatment is usually complicated. Various species of Aspergillus can cause nail infection but may respond differently to antifungal treatment, so it is important to know their epidemiology, clinical characteristics, etiologic agents, diagnostic methods, and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The onychomycosis (from the Greek onychos—nail and mycosis—fungal infection) is produced by three types of microorganisms: dermatophytes, yeasts, and non-dermatophyte molds (NDM). According to the Society for Human and Animal Mycology, the term onychomycosis is exclusive for the infections caused by dermatophytes, while the ones caused by yeasts are known as onyxis; if it is Candida, they are called nail candidiasis, and those caused by an opportunistic mold are known as nail mycosis [1•, 2••].
Onychomycosis is one of the main nail infections at a global level; it represents up to 50% of all onychopathies, and of this, 33% is related to diabetic patients and 30% to HIV-positive patients [3••, 4]. In recent years, an increase in the incidence of this disease has been reported due to various factors, such as the trimmings of nails, use of artificial nails, advanced age, peripheral vascular disease, diabetes, autoimmune diseases, and swimming regularly [5••].
Currently, the frequency of onychomycosis by NDM has increased appearing in 1 to 45.8% of the population, depending on the geographical region, and exceeding 20% in population over 60 years [2••, 6, 7, 8•, 9, 10, 11•, 12, 13••, 14•]. Among the main NDM, there is Scopulariopsis brevicaulis, Aspergillus spp., Fusarium spp., Neoscytalidium spp., Acremonium spp., Paecilomyces spp., Penicillium spp., Rhizopus spp., Alternaria alternata, Tritirachium oryzae, Ulocladium spp., Trichoderma spp., and Nattrassia mangifereae. In recent years, there has been an increase in the diagnosis of onychomycosis by NDM in dermatology services, where Aspergillus species have been considered as emerging and represent between 34 and 60% of diagnoses [1•, 3••, 12, 15•, 16•, 17•].
The species of genus Aspergillus are saprobes and are found in the environment (soil, air, water, and vegetation). This group of fungi is normally considered as a contaminant as it does not produce keratinases and it depend on other conditions to cause nail onychomycosis, such as a previous nail trauma, anatomical alterations, bacterial infections, circulatory alterations, and immunosuppression; however, it has also been found causing damage in immunocompetent patients [1•, 3••, 12, 15•, 16•, 17•].
The first case of onychomycosis due to Aspergillus was reported by Émile-Weil and Gaudin in 1919. Subsequently, Sartory (1920) and Ota (1923) reported other cases. However, Thom and Church (1926) strongly questioned the relationship between onychomycosis and Aspergillus because it was considered a contaminant fungus; in addition, the clinical manifestations were similar to those caused by yeasts, so there was a lack of reports in the literature. Despite this controversy, cases such as those of Sartory et al. (1930) and Smith (1934) continued to be reported, and the reports remained controversial as the genus Aspergillus was still considered as a simple pollutant. From 1935 to 1941, no case was reported [18]. In this paper, we present a review on the behavior (epidemiology, clinical characteristics, diagnostic methods, treatment, and identified species) of the onychomycosis produced by Aspergillus spp. as primary agents.
Epidemiology
There are remarkable geographical differences in the epidemiology of onychomycosis, being the heat and humidity of the tropical and subtropical regions responsible for promoting the broad dissemination of the same [16•, 19•]. It is known to the present date that dermatophytes are the main cause of onychomycosis; however, it has been reported in some countries that the NDM have increased. Malaysia is one example, where in 1999, its main etiologic agents were dermatophytes, but in 2012, the NDM (45.4%) ranked first as responsible agents for onychomycosis being Aspergillus spp. the most common one (59.8%) [20•].
The onychomycosis caused by NDM is present throughout the world; in European countries, it has been reported between 5 and 17.2%, in North America between 4.3 and 33%, in South America 1 and 9.5%, in Central America 0.76%, East Asia 12 and 45.8%, Africa 2.78 and 9.0%, and in Mexico 1.49% (Fig. 1) [17•, 20•, 21•, 22•, 23••].
Among the NDM, genus Aspergillus presents a variable frequency according to the geographical region, being found in a 5% in North America, 0.6% in Brazil, 22 to 35.33% in India, 13.15% in Italy, 0.26% in Guatemala, 9.5–50% in Iran, 30.3% in Turkey, 2.0% in Pakistan, 63% in England, and 59.8% in Malaysia (Fig. 1) [1•, 16•, 17•, 20•, 24•, 25••, 26•, 27•, 28•, 29•, 30••, 31•].
The presence of Aspergillus as primary agent of onychomycosis was demonstrated in 1941 by Bereston and Keil, whom described a case of aspergillosis in a 30-year-old woman that referred a deformation of the first toenail of her right foot, which started with a dark spot in the proximal portion and a subsequent discoloration. Dr. Charles Tom isolated and identified A. flavus as the causative agent [32]. From this demonstration, numerous cases of onychomycosis caused by several species of Aspergillus have been reported in various parts of the world, among which the following have been identified: A. niger, A. sydowii, A. flavus, A. fumigatus, A. repens, A. sclerotiourum, A. versicolor, A. terreus, A. candidus, A. nidulans, A. clavatus, A. melleus, A. uvarum, A. nomius, A. ochraceopetaliformis, A. persii, A. tamarii, A. tubingensis, Emericella quadrilineata (Teleomorph of A. tetrazonus), A. hongkongenesis, A. unguis, A. welwitschiae (synonym A. awamori), A. austroafricanus, A. protuberus, A. alliaceus, and A. ochraceus (Table 1) [30••, 33••, 34••, 35•, 36•, 37•, 38•, 39•, 40•, 41, 42•, 43•].
The onychomycosis due to Aspergillus occurs in both sexes; however, there is no accurate data on which group is more vulnerable as men generally do not seek medical attention due to esthetic disinterest unlike women, which entails that in some reports females have higher incidence [21•, 29•].
As for age, onychomycosis due to Aspergillus can occur in any age group; there are reports in both pediatric and geriatric patients [43•]. However, it has been observed that the most affected group is the one between 30 and over 60 years [16•, 24•].
Clinical Manifestations
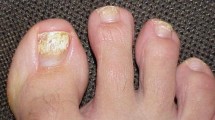
The clinical manifestations of onychomycosis caused by Aspergillus are quite varied, depending on the host and the species. In general, onycholysis, inflammation of the periungual fold, leukonychia, onychomadesis, onychodystrophy, hyperkeratosis, onychoclasis, brittle nails, changes in the coloration, and melanonychia in some cases, both in feet and hand nails (Table 2) [48], can be found. With regard to classification, it has been observed that the most frequent types of onychomycosis caused by Aspergillus spp. are distal lateral subungual onychomycosis (DLSO), white superficial onychomycosis (WSO), and proximal white subungual onychomycosis (PSO) [34••].
Diagnostic Methods
To diagnose onychomycosis due to Aspergillus spp., conventional techniques are used such as direct examination, considered the gold standard, with KOH 10 to 40%, alone or with dimethyl sulfoxide (DMSO). The most used mycological culture media are Sabouraud dextrose agar with and without antibiotics (cycloheximide, chloramphenicol, and gentamicin), modified Sabouraud agar, Czapek agar, and malt extract medium, being these two last specific for the isolation of Aspergillus. It can also be used potato dextrose agar which favors the sporulation of fungus. All cultures are incubated at temperatures ranging from 25 to 30 °C in a time range from 3 days to 3 weeks [14•, 15•, 38•, 51•, 52•, 63].
After it was demonstrated that genus Aspergillus is a primary pathogen in onychomycosis, certain criterion was established to discard it as a contaminant and determine the accurate diagnosis: presence of hyphae or spores in the direct microscopic examination of the clinical sample, isolation of the same fungal species from a second sample taken from nails with extreme hygiene measures after an interval of 5 to 9 days, and identification of Aspergillus in 5 of 20 inoculated nail fragments [14•, 36•].
Direct Examination
The observation of fungal structures with the combination of KOH 40% and DMSO enables a faster clearance of the sample, as it defragments keratin [15•]; this result is also attained by mixing KOH with chlorazol black [9]. Despite the high sensitivity of these techniques, it is possible to obtain in some cases false-negative results [64••]. In case of a negative result, the use of KOH/CFW (aqueous solution of calcofluor white at 0.1% mixed in equal volumes with KOH) allows early recognition of fungi in tissue under ultraviolet light [65•]. Systematic use of calcofluor white in the laboratories is not recommended given that it has not shown additional benefits when compared to KOH. This is of utmost importance when resources are limited [66••].
Molecular Tests
Classic diagnosis has limitations; direct examination may show false results up to 30%, while the mycological culture is not positive in all cases. In addition, its interpretation is often complicated, particularly when the NDM are isolated, as they are generally considered to be pollutants. These limitations lead to an empirical treatment which is not always effective.
Given the taxonomical complexity of genus Aspergillus, the morphological similarity, and different susceptibility to antifungal agents among the species of the same section, its identification is difficult. For example, within the section Nigri, it includes A. welwitschiae, A. carbonarius, A. brasiliensis, and A. tubingensis, which are morphologically similar species to A. niger, but A. tubingensis presents different susceptibility to antifungals [67]. Therefore, the precise identification of species in the diagnosis of onychomycosis due to Aspergillus is necessary. The use of morphological criteria to achieve the identification of Aspergillus spp. is insufficient because it lacks accuracy and requires highly trained staff; hence, more accurate methods are required such as molecular ones. Among these, the polymerase chain reaction (PCR) technique stands out, being the gene fragments (calmodulin and β-tubulin), the 28-s region of the rDNA and the internal transcribed spacer (ITS) regions, the most common amplification targets to identify, based on the sequence of these genes, any Aspergillus species [39•, 40•, 41, 42•, 52•].
It is important to point out that even though molecular biology techniques undoubtedly represent a step forward in the direct diagnosis of onychomycosis, surpassing in occasions the sensitivity limitations of direct examination and cultures, they do not substitute conventional tests, but are complementary; that is, the sensitivity of the diagnosis is greater when conventional and molecular procedures are combined.
Treatment
The treatment modalities for onychomycosis due to Aspergillus spp. include nail avulsion, surgical debridement [51•], topical therapy, oral therapy, or a combination of oral and topical antifungal agents. When it comes to topical treatment, the Whitfield ointment has been used, tioconazole 28%, amorolfine 5%, and calcipotriol, with failed results in infections caused by A. fumigatus, A. persii, and A. clavatus, while the iodochlorhydroxyquin (Vioform) ointment, urea cream 40%, terbinafine, bifonazole, and urea 40% have achieved clinical cure in onychomycosis caused by A. fumigatus, A. tamarii, and A. niger [42•, 43•, 50•, 60]. Currently, there is a new triazole antifungal topical solution, the eficonazole, which has shown an excellent activity against A. fumigatus, A. niger, A. flavus, and A. terreus, as well as against other NDM, which turns it into an alternative for the onychomycosis treatment [68•].
Oral therapy with 100 to 200 mg daily of itraconazole for 2 to 3 months has shown successful results against A. niger, A. clavatus, and A. candidus, except A. sydowii [39•, 52•, 55•, 56••, 59]. The terbinafine at a dose of 250 mg daily for 6 weeks has shown negative results against A. candidus [55•].
The therapy that has shown best results is the combined one. Reported combinations are ciclopiroxolamine and terbinafine 250 mg/day, amphotericin B 1% and terbinafine (200 mg/3 months), terbinafine 250 mg and amorolfine 5%, itraconazole 400 mg for 4 months with amorolfine nail lacquer, ketoconazole 2% and terbinafine 250 mg for 3 months, and amorolfine and fluconazole, for A. ochraceopetaliformis, A. persii, A. niger, A. nomius, A. uvarum, and A. sclerotiorum, respectively [37•, 38•, 40•, 41, 42•, 61].
It is important to note that the clinical cure rates with systemic or combined therapy, and even more with topical therapy, may be limited due to the lack of compliance by the patient [40,41,42]. Another factor that may interfere in the response to onychomycosis treatment by Aspergillus is the etiological agent, since within the current taxonomic classification of this genera, there are several species that have different susceptibility profiles and are morphologically indistinguishable, even though they pertain to the same section; therefore, an adequate treatment of onychomycosis due to Aspergillus must be based on the identification of the fungus at the species level.
Conclusion
Nail pathology caused by Aspergillus spp. is emerging and accounts for 34 to 60% of onychomycosis caused by NDM. It is developed mainly in patients presenting some types of immunosuppression, such as diabetic patients, HIV-positive patients, and those who engage in certain sports activities such as swimming. During diagnosis, it is important to determine the species of Aspergillus to avoid therapeutic failure, since resistance has been observed in some of them. The accurate identification of species must be done through the combination of conventional and molecular methods. For the treatment of this mycosis, it is recommended to use combined therapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Martínez-Herrera EO, Arroyo-Camarena S, Tejada-García DL, Porras-López CF, Arenas R. Onychomycosis due to opportunistic molds. An Bras Dermatol. 2015;90(3):334–7. This paper mentions general aspects of onychomycosis by Aspergillus.
•• López-Jodra O, Torres-Rodríguez JM. Unusual fungal species causing onychomycosis. Rev Iberoam Micol. 1999;16(S):S11–5. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
•• Wijesuriya T, Kottahachchi J, Gunasekara TDCP, Bulugahapitiya U, Ranasinghe KNP, Neluka Fernando S, et al. Aspergillus species: An emerging pathogen in onychomycosis among diabetics. Indian J Endocrinol Metab. 2015;19(6):811. This paper reports clinical aspects of onychomycosis by Aspergillus.
de Magalhães LK, Machado Barbosa de Castro CM, Nogueira Cambuim F II, de Oliveira JC, Delgado M, de Melo Rego RS. Hongos filamentosos no dermatofitos: onicomicosis en cuatro pacientes infectados con el virus de la inmunodeficiencia humana. Rev Iberoam Micol. 2008;25(1):45–9.
•• Nouripour-Sisakht S, Mirhendi H, Shidfar MR, Ahmadi B, Rezaei-Matehkolaei A, Geramishoar M, et al. Aspergillus species as emerging causative agents of onychomycosis. J Mycol Med. 2015;25(2):101–7. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
Ballesté R, Mousqués N, Gezuele E. Onicomicosis. Revisión del tema. Rev Medica Urug. 2003;19:93–106.
Baran R, Tosti A, Piraccini BM. Uncommon clinical patterns of Fusarium nail infection: report of three cases. Br J Dermatol. 1997;136(3):424–7.
• Escobar ML, Carmona-Fonseca J. Onicomicosis por hongos ambientales no dermatofíticos. Rev Iberoam Micol. 2003;20:6–10. This paper mentions general aspects of onychomycosis by Aspergillus.
Cavallera E, Asbati M. Onicomicosis por hongos filamentosos no dermatófitos en Cádiz. Dermatol Venezol. 2006;44(1):4–10.
Palacio A, Pazos C, Cuétara S. Onicomicosis por hongos filamentosos no dermatófitos en Cádiz. Enferm Infecc Microbiol Clin. 2001;19(7):345–6.
• Bonifaz A, Cruz-Aguilar P, Ponce RM. Onychomycosis by molds. Report of 78 cases. Eur J Dermatol. 2007;17(1):70–2. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
Ramirez Hobak L, Gomez-Stenz A, Vega Sanchez DC, Arenas R. Onicomicosis por mohos no dermatofitos. Una revisión. Dermatol CMQ. 2017;15(3):184–94.
•• Gupta AK, Drummond-Main C, Cooper EA, Brintnell W, Piraccini BM, Tosti A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J Am Acad Dermatol. 2012;66(3):494–502. This paper mentions general aspects of onychomycosis by Aspergillus .
• Negroni R, Arechavala A, Bohnvel P. Hongos miceliales no dermatofitos en onicodistrofias. Experiencia de un centro médico privado en Buenos Aires. Dermatol Argent. 2008;14(2):118–23. This paper mentions general aspects of onychomycosis by Aspergillus .
• Mahmoudabadi AZ, Zarrin M. Onychomycosis with Aspergillus flavus; a case report from Iran. Pak J Med Sci. 2005;21(4):497–8. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
• Godoy-Martinez P, Nunes FG, Tomimori-Yamashita J, Urrutia M, Zaror L, Silva V, et al. Onychomycosis in São Paulo, Brazil. Mycopathologia. 2009;168(3):111–6. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
• Hilmioǧlu-Polat S, Metin DY, Inci R, Dereli T, Kilinç I, Tümbay E. Non-dermatophytic molds as agents of onychomycosis in Izmir, Turkey—a prospective study. Mycopathologia. 2005;160(2):125–8. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
Bereston MES, Waring WS. Onychomycosis and dermatomycosis caused by Trichophyton Rubrum and Aspergillus nidulans. Arch Dermatol Syphilol. 1945;52(3):162–5.
• Nkondjo Minkoumou S, Fabrizi V, Papini M. Onychomycosis in Cameroon: A clinical and epidemiological study among dermatological patients. Int J Dermatol. 2012;51(12):1474–7. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
• Chadeganipour M, Nilipour S, Ahmadi G. Study of onychomycosis in Isfahan, Iran. Mycoses. 2010;53(2):153–7. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
• Ranawaka RR, de Silva N, Ragunathan RW. Non-dermatophyte mold onychomycosis in Sri Lanka. Dermatol Online J. 2012;18(1):7. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
• Hajoui ZM, Zeroual Z, Ghfir B, Moustachi A, Lyagoubi M, Aoufi S. The mold onychomycosis in Morocco: About 150 isolated cases in 20 years. J Mycol Med. 2012;22(3):221–4. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
•• Gupta AK, Nakrieko KA. Molecular determination of mixed infections of dermatophytes and nondermatophyte molds in individuals with onychomycosis. J Am Podiatr Med Assoc. 2014;104(4):330–6. This paper reports diagnostic methods for onychomycosis by Aspergillus .
• Raghavendra K, Yadav D, Kumar A, Sharma M, Bhuria J, Chand A. The nondermatophyte molds: Emerging as leading cause of onychomycosis in south-east Rajasthan. Indian Dermatol Online J. 2015;6(2):92. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
•• Ramani R, Srinivas CR, Ramani A, Kumari TGR, Shivananda PG. Molds in Onychomycosis. Int J Dermatol. 1993;32(12):877–8. This paper mentions general aspects of onychomycosis by Aspergillus.
• Romano C, Gianni C, Difonzo EM. Retrospective study of onychomycosis in Italy: 1985–2000. Mycoses. 2005;48(1):42–4. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
• Leelavathi M, Tzar MN, Adawiah J. Common microorganisms causing onychomycosis in tropical climate. Sains Malays. 2012;41(6):697–700. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
• Bokhari MA, Hussain I, Jahangir M, Haroon TS, Aman S, Khurshid K. Onychomycosis in Lahore, Pakistan. Int J Dermatol. 1999;38(8):591–5. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
• Hashemi SJ, Gerami M, Zibafar E, Daei M, Moazeni M, Nasrollahi A. Onychomycosis in Tehran: Mycological study of 504 patients. Mycoses. 2010;53(3):251–5. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
•• English MP, Atkinson R. Onychomycosis in elderly chiropody patients. Br J Dermatol. 1974;91(1):67–72. This paper reports clinical aspects of onychomycosis by Aspergillus.
• Soltani M, Khosravi AR, Shokri H, Sharifzadeh A, Balal A. A study of onychomycosis in patients attending a dermatology center in Tehran, Iran. J Mycol Med. 2015;25(2):e81–7. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
Bereston MES, Keil H. Onychomycosis due to Aspergillus flavus. Arch Dermatol Syphilol. 1941;44(3):420–5.
• Tsang CC, Hui TWS, Lee KC, Chen JHK, Ngan AHY, Tam EWT, et al. Genetic diversity of Aspergillus species isolated from onychomycosis and Aspergillus hongkongensis sp. nov., with implications to antifungal susceptibility testing. Diagn Microbiol Infect Dis. 2016;84(2):125–34. This paper reports a new species in onychomycosis.
•• Gianni C, Romano C. Clinical and histological aspects of toenail onychomycosis caused by Aspergillus spp.: 34 cases treated with weekly intermittent terbinafine. Dermatology. 2004;209(2):104–10. This paper mentions the treatment for onychomycosis by Aspergillus .
• Kim DM, Suh MK, Ha GY, Sohng SH. Fingernail onychomycosis due to Aspergillus niger. Ann Dermatol. 2012;24(4):459–63. This paper mentions general aspects of onychomycosis by Aspergillus.
• Gugnani HC, Vijayan VK, Tyagi P, Sharma S, Stchigel AM, Guarro J. Onychomycosis due to Emericella quadrilineata. J Clin Microbiol. 2004;42(2):914–6. This paper reports a new species in onychomycosis.
• Garcia-Martos P, Guarro J, Gene J, Mira J, Linares M, Ortoneda M. Onychomycosis caused by Aspergillus sclerotiorum. J Mycol Med. 2001;11(4):222–4. This paper reports a new species in onychomycosis.
• Zotti M, Machetti M, Persi A, Barabino G, Parodi A. Onychomycosis: First case due to Aspergillus nomius. Acta Derm Venereol. 2011;91(5):591–2. This paper reports a new species in onychomycosis.
• Falahati M, Ghojoghi A, Abastabar M, Ghasemi Z, Farahyar S, Roudbary M, et al. The first case of total dystrophic onychomycosis caused by Aspergillus clavatus resistant to antifungal drugs. Mycopathologia. 2016;181(3–4):273–7. This paper reports a new species in onychomycosis.
• Zarei F, Mirhendi H, Fakhim H, Geramishoar M. The first case of onychomycosis due to Aspergillus uvarum (section Nigri). Mycoses. 2015;58(4):239–42. This paper reports a new species in onychomycosis.
Brasch J, Varga J, Jensen JM, Egberts F, Tintelnot K. Nail infection by Aspergillus ochraceopetaliformis. Med Mycol. 2009;47(6):658–62. This paper reports a new species in onychomycosis.
• Zotti M, Machetti M, Perotti M, Barabino G, Persi A. A new species, Aspergillus persii, as an agent of onychomycosis. Med Mycol. 2010;48(4):656–60. This paper reports a new species in onychomycosis.
• Kristensen L, Stenderup J, Otkjær A. Onychomycosis due to Aspergillus tamarii in a 3-year-old boy. Acta Derm Venereol. 2005;85:261–2. This paper reports a new species in onychomycosis.
Nyongesa BW, Okoth S, Ayugi V. Identification key for Aspergillus species isolated from maize and soil of Nandi Country, Kenya. Adv Microbiol. 2015;5:205–29.
Chen AJ, Frisvad JC, Sun BD, Varga J, Kocsubé S, Dijksterhuis J, et al. Aspergillus section Nidulantes (formerly Emericella): Polyphasic taxonomy, chemistry and biology. Stud Mycol. 2016;84:1–118.
•• Balajee SA, Houbraken J, Verweij PE, Hong SB, Yaghuchi T, Varga J, et al. Aspergillus species identification in the clinical setting. Stud Mycol. 2007;59:39–46. This paper reports diagnostic methods for onychomycosis by Aspergillus .
• Lee BJ, Kim IJ, Suh SB. Two cases of onychomycosis due to Aspergillus repens. Korean J Dermatol. 1981;19:881–6. This paper reports a new species in onychomycosis.
Uribe B, Colin J, Arce M, Arenas R. Aspergillus niger. Dermatol CMQ. 2017;15(3):197–8.
• Moore M, Weiss RS. Onychomycosis caused by Aspergillus terreus. J Invest Dermatol. 1948;11(3):215–23. This paper mentions historical aspects of onychomycosis by Aspergillus.
• Rosenthal SA, Stritzler R, Villafane J. Onychomycosis ca 52(3): used by Aspergillus fumigatus. Report of a case. Arch Derm. 1968;97:685–7. This paper mentions historical aspects of onychomycosis by Aspergillus.
• Torres-Rodriguez JM, Madrenys-Brunet N, Siddat M, López-Jodra O, Jimenez T. Aspergillus versicolor as cause of onychomycosis: Report of 12 cases and susceptibility testing to antifungal drugs. J Eur Acad Dermatol Venereol. 1998;11(1):25–31. This paper mentions the treatment for onychomycosis by Aspergillus.
• Takahata Y, Hiruma M, Sugita T, Muto M. A case of onychomycosis due to Aspergillus sydowii diagnosed using DNA sequence analysis. Mycoses. 2008;51(2):170–3. This paper reports a new species in onychomycosis.
Choudhary SV, Koley S, Mallick S, Bose S, Basak S. Proximal subungual onychomycosis caused by Aspergillus flavus in a HIV- positive patient. Indian J Dermatol Venereol Leprol. 2009;75(4):410–2.
• Veraldi S, Chiaratti A, Harak H. Onychomycosis caused by Aspergillus versicolor. Mycoses. 2009;53(4):363–5. This paper reports a new species in onychomycosis.
• Ahmadi B, Hashemi SJ, Zaini F, Shidfar MR, Moazeni M, Mousavi B, et al. A case of onychomycosis caused by Aspergillus candidus. Med Mycol Case Rep. 2012;1(1):45–8. This paper reports a new species in onychomycosis.
•• Banu A, Anand M, Eswari L. A rare case of onychomycosis in all 10 fingers of an immunocompetent patient. Indian Dermatol Online J. 2013;4(4):302–4. This paper reports clinical aspects of onychomycosis by Aspergillus.
• Zotti M, Agnoletti AF, Vizzini A, Cozzani E, Parodi A. Onychomycosis from Aspergillus melleus, a novel pathogen for humans. Fungal identification and in vitro drug susceptibility. Exp Dermatol. 2015;24(12):966–8. This paper reports a new species in onychomycosis.
• Noguchi H, Hiruma M, Miyashita A, Makino K, Miyata K, Ihn H. A case of fingernail onychomycosis due to Aspergillus flavus. Jpn J Med Mycol. 2016;57(2):21–5. This paper reports clinical aspects of onychomycosis by Aspergillus.
Matsuyama Y, Nakamura T, Hagi T, Asanuma K, Sudo A. Subungual onychomycosis due to Aspergillus niger mimicking a glomus tumor: A case report. Biomed Rep. 2017;6:532–4.
Álvarez-Salafranca M, Hernández-Ostiz S, Salvo Gonzalo S, Ara MM. Onicomicosis subungueal proximal por Aspergillus niger: un simulador de melanoma maligno subungueal. Actas Dermosifiliogr. 2016;108(5):482–5.
Tamer F, Yuksel ME. Onychomycosis due to Aspergillus niger without black nail discoloration: A case report. Our Dermatol Online. 2017;8(2):233–4.
Akpinar Kara Y, Erdogan FG, Cologlu D. A case of onychomycosis due to Aspergillus flavus in all fingernails and toenails of an immunocompromised patient. J Clin Exp Dermatol Res. 2017;9(1):9–11.
Fernández MS, Rojas FD, Cattana ME, Sosa MA, Mangiaterra ML, Giusiano GE. Aspergillus terreus complex: An emergent opportunistic agent of Onychomycosis. Mycoses. 2013;56(4):477–8.
•• Grover C, Reddy BSN, Chaturvedi KU. Onychomycosis and the diagnostic significance of nail biopsy. J Dermatol. 2003;30(2):116–22. This paper reports diagnostic methods for onychomycosis by Aspergillus .
• Afshar P, Khodavaisy S, Kalhori S, Ghasemi M, Razavyoon T. Onychomycosis in North-East of Iran. Iran J Microbiol. 2014;6(2):98–103. This paper mentions aspects of the epidemiology of onychomycosis by Aspergillus.
•• Bonifaz A, Rios-Yuil JM, Arenas R, Araiza J, Fernández R, Mercadillo-Pérez P, et al. Comparison of direct microscopy, culture and calcofluor white for the diagnosis of onychomycosis. Rev Iberoam Micol. 2013;30(2):109–11. This paper reports diagnostic methods for onychomycosis by Aspergillus .
Frías-De-León MG, Rosas-de Paz E, Arenas R, Atoche C, Duarte-Escalante E, Molina de Soschin D, et al. Identification of Aspergillus tubingensis in a primary skin infection. J Mycol Med. 2018; https://doi.org/10.1016/j.mycmed.2018.02.013.
• Tupaki-Sreepurna A, Jishnu B, Thanneru V, Sharma S, Gopi A, Sundaram M, et al. An assessment of in vitro antifungal activities of efinaconazole and itraconazole against common non-dermatophyte fungi causing onychomycosis. J Fungi. 2017;3(2):20. This paper mentions the treatment for onychomycosis by Aspergillus .
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This paper is part of the Topical Collection on Fungal Infections of Skin and Subcutaneous Tissue
Rights and permissions
About this article
Cite this article
Frías-De-León, M.G., Espinosa-Hernández, V.M., Bonifaz, A. et al. Onychomycosis Due to Aspergillus spp.: a Current Review. Curr Fungal Infect Rep 12, 112–119 (2018). https://doi.org/10.1007/s12281-018-0319-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-018-0319-8