Abstract
The Astragalus polysaccharide is an important bioactive component derived from the dry root of Astragalus membranaceus. This review aims to provide a comprehensive overview of the research progress on the immunomodulatory effect of Astragalus polysaccharide and provide valuable reference information. We review the immunomodulatory effect of Astragalus polysaccharide on central and peripheral immune organs, including bone marrow, thymus, lymph nodes, spleen, and mucosal tissues. Furthermore, the immunomodulatory effect of Astragalus polysaccharide on a variety of immune cells is summarized. Studies have shown that Astragalus polysaccharide can promote the activities of macrophages, natural killer cells, dendritic cells, T lymphocytes, B lymphocytes and microglia and induce the expression of a variety of cytokines and chemokines. The immunomodulatory effect of Astragalus polysaccharide makes it promising for the treatment of many diseases, including cancer, infection, type 1 diabetes, asthma, and autoimmune disease. Among them, the anticancer effect is the most prominent. In short, Astragalus polysaccharide is a valuable immunomodulatory medicine, but further high-quality studies are warranted to corroborate its clinical efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astragalus is a plant from the legume family, known as “Huangqi” in China. According to the Chinese Pharmacopoeia, the medicinal source of Astragalus is the dried root of Astragalus membranaceus (Fisch.) Bge.var.mongholicus (Bge.) Hsiao or Astragalus membranaceus (Fisch.) Bge. (Zhang et al. 2021). In this regard, Fu et al. reported that Astragalus membranceus (Fisch.) Bge. and Astragalus membranceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao are two varieties of the same species (Fu et al. 2014). Prior in vivo and in vitro studies have demonstrated that Astragalus possesses multiple biological functions, such as immunomodulation, antioxidant, anti-inflammation and antitumor properties and thus widely used for the treatment of cardiovascular diseases, diabetes mellitus, cancer, respiratory diseases, nervous system diseases and other diseases (Song et al. 2011, 2013; Qin et al. 2012; Jung et al. 2016). Astragalus polysaccharide is an important natural active component derived from Astragalus (Jin et al. 2014). An increasing body of evidence from pharmacological studies has shown that Astragalus polysaccharide has a variety of biological activities such as regulating blood glucose and blood lipids, anticancer, anti-aging, and immune regulation, amongst which the immunomodulatory effect is the most important (Chen et al. 2020; Zheng et al. 2020).
Therefore, this review systematically reviewed the evidence on the immunomodulatory effect of Astragalus polysaccharides. It has been established that Astragalus polysaccharide can regulate the activities of immune organs (bone marrow, thymus, lymph node, spleen and mucosa-associated lymphoid tissue) and immune cells (macrophage, natural killer cell, dendritic cell, T lymphocyte, B lymphocyte and microglia) and the release of immune active substances (such as IL-2 (interleukin-2), IL-6, TNF-α (tumor necrosis factor-α), IFN-γ (interferon-γ), IgA (immunoglobulin A), IgG, and IgM) (Xie et al. 2016; Farag and Alagawany 2018; Wang et al. 2019; Wan et al. 2022). Then, we systematically reviewed the therapeutic effect of Astragalus polysaccharides on immune-related diseases. Astragalus polysaccharide can be used for anticancer, anti-infection and as vaccine adjuvants by enhancing the activity of immune cells and the release of immune active substances. Moreover, Astragalus polysaccharide is indicated for autoimmune diseases, including type 1 diabetes, asthma and inflammatory demyelinating disease, given its ability to inhibit an overactivated immune system (Zhang et al. 2019b; Kong et al. 2021). In addition, the immunomodulatory effect of ASPC (Astragalus polysaccharide component) and pure polysaccharide (by further purification and separation) are distinguished to broaden current understanding and trigger further interest in exploring the relationship between the structure and immunomodulatory activity of Astragalus polysaccharide.
Pharmacological activities on immune organs
The immune organs represent an important part of the immune system and can be divided into central and peripheral immune organs according to their functions. The central immune organs of humans or other mammals include the bone marrow and thymus (Ganea et al. 2015). Peripheral immune organs include lymph nodes, spleen and mucosa-associated lymphoid tissues. They are the sites where mature lymphocytes colonize and the main site for lymphocytes to produce immune responses (Scheffer and Latini 2020).
Pharmacological activities on the bone marrow
The bone marrow can produce pluripotent hematopoietic stem cells and is the birthplace of various blood cells and immune cells (Zhao et al. 2012). Current evidence suggests that Astragalus polysaccharide has a protective effect on bone marrow stem cells. ASPC (50 µg/ml) could protect bone marrow mesenchymal stem cells from radiation-induced apoptosis by inhibiting the production of reactive oxygen species, upregulating the proteins B-cell lymphoma-2 (Bcl-2) and B-cell lymphoma extra-large (Bcl-xl), and downregulating the proteins Bcl-2-associated X (Bax) and Bcl-2 homologous killer (Bak) (Zhang et al. 2020b). Moreover, ASPC (40–400 µg/ml) could protect bone marrow mesenchymal stem cells from formaldehyde-induced cytotoxicity and genotoxicity by upregulating the expression of xeroderma pigmentosum group A, xeroderma pigmentosum group C, excision repair cross-complementation group 1, replication protein A1, and replication protein A2 (She et al. 2021). Exploring the immunomodulatory effect of Astragalus polysaccharides on immune organs is of great significance for an in-depth understanding of the regulation of immune cells and immune factors by Astragalus polysaccharides (Jia et al. 2019). In cyclophosphamide-induced immunosuppressive rats, ASPC (100 mg/kg) improved the chemotactic capacity of polymorphonuclear leukocytes among mature bone marrow granulocytes and peripheral blood neutrophils by regulating the L-selectin signaling pathway, and upregulating the expression of P-selectin glycoprotein ligand-1, CD11b (cluster of differentiation 11b)/CD18, TNF-α and IL-8 (Zhang et al. 2015). In radiation-induced bone marrow immunosuppressive mice, ASPC (50–500 mg/kg) promoted the colony formation of megakaryocytes, granulocyte monocytes, and erythroid cells and inhibited apoptosis of megakaryocytic cells by inhibiting the activation of the mitochondrial apoptotic pathway (Li et al. 2021a). In addition, some pure polysaccharides obtained by further purification and separation can stimulate immune activity in the immunosuppressive bone marrow. It has been established that the glucose, galactose, arabinose, rhamnose, and the galacturonic acid ratio is 1.5:1:5.4:0.08:0.1 in Astragalus polysaccharide-I (APS-I) (molecular weight above 500 kDa) and 9:1:1.4:0.04:0.001 in APS-II (Astragalus polysaccharide-II) (molecular weight 10 kDa) (Li et al. 2020d). APS-I and APS-II (5–20 mg/kg) could increase the levels of white blood cells, lymphocytes, monocytes and neutrophils, and the expression of IL-2, IL-6, IgG, and granulocyte-macrophage colony-stimulating factor (GM-CSF), and decrease CD4+/CD8+ level in cyclophosphamide-induced immunosuppressive mice (Li et al. 2020d). The pure polysaccharide Radix Astragali polysaccharide (RAP) consists of rhamnose, arabinose, glucose, galactose and galacturonic acid with a molar ratio of 0.03:1.00:0.27:0.36:0.30, and a molecular weight of 1334 kDa. The backbone consists of 1,2,4-linked Rhap, α-1,4-linked Glcp, α-1,4-linked GalAp6Me, and β-1,3,6-linked Galp (Yin et al. 2012). RAP (150 mg/kg) has been shown to protect mice hematopoietic stem cells from cyclophosphamide-induced myelosuppression by inhibiting bone marrow cell apoptosis, promoting bone marrow cell proliferation, and increasing the number of hematopoietic stem cells, CD34+ cells, lineage-negative cells, Lin−c-Kit+ cells, and Lin−Sca1+c-Kit+ cells (Bao et al. 2021).
Pharmacological activities on the thymus
The thymus is another important central immune organ, which is the site of T lymphocyte development, differentiation and maturation (Sakaguchi et al. 2011). Current research shows that Astragalus polysaccharides can promote proliferation and improve drug-induced apoptosis in thymocytes. In cyclophosphamide-induced immunosuppressive mice, ASPC (50–80 mg/kg) enhanced the thymus index and serum levels of immunoglobulins (IgA, IgG, and IgM) and inhibited the overproduction of IL-2 (Meng et al. 2017). In doxorubicin hydrochloride-induced immunosuppressive lung cancer mice, the pure polysaccharide APS-III (Astragalus polysaccharide-III), with a backbone consisting of Glc(1–4), Xyl(1–2), Gal(1–4), and GalA(1–3), (15%) also significantly increased the thymus index. Further research showed that it might be related to the inhibition of drug-induced thymocytes apoptosis by suppressing the activation of the mitochondrial apoptotic pathway, upregulating the proteins Bcl-2 and Bcl-xl, and downregulating the proteins Bax and Bak (Zhou et al. 2018). The pure polysaccharide AX-I-3b [Astragalus radix (radix) polysaccharide-I-3b] consists of arabinose, xylose, and glucose with a molar ratio of 10.4:79.3:1.1, and a molecular weight of 7.87 kDa (Li et al. 2019a). AX-I-3b (50–200 mg/kg) also improved cisplatin-induced decrease in thymus organ index and increased the ratio of CD4+ T cells to CD8+ T cells and the expression of IL-2, IL-6 and TNF-α in lung cancer mice (Li et al. 2019a).
Pharmacological activities on the spleen
The spleen is the largest peripheral immune organ, which can synthesize and secrete complement, interferon and other substances and trigger immune responses to blood-derived antigens (Bronte and Pittet 2013). Growing evidence shows that Astragalus polysaccharide can promote the proliferation of spleen cells and the release of biologically active substances. In this respect, ASPC (1–500 µg/ml) could enhance in vitro mice splenic lymphocytes proliferation and increase the T-cell CD4+/CD8+ ratio (Xu et al. 2019). Polysaccharide-rich Astragalus extract (1 g/kg) promoted splenocytes proliferation and increased the levels of peripheral white blood cells, splenic lymphocyte subset, IgG and IgM in cyclophosphamide-induced immunosuppressive mice (Li et al. 2021d). In ovalbumin subcutaneously immunized mice, ASPC (1–4 mg/ml) promoted the proliferation of splenocytes, the secretion of IFN-γ and IL-6 and the specific immunoglobulins antibody response (IgG, IgG1 and IgG2a) (Fan et al. 2013). In carboplatin-induced immunosuppression in B16 tumor-bearing mice, ASPC (10 g/kg) alleviated immune suppression by inducing the proliferation of spleen cells and secretion of immune regulatory factors, such as IL-1β, IL-6 and TNF-α (Wu et al. 2016). Paternal dietary ASPC (10 g/kg) transgenerationally could induce an endotoxin tolerance-like immune response in the spleen of broiler chickens by increasing serum IFN-α and IFN-β levels by activating the TLR4 (toll-like receptor 4) signaling pathway in jejunal mucosa (Li et al. 2018b).
Pharmacological activities on mucosal tissue
Mucosal-associated lymphoid tissue, also called the mucosal immune system, mainly responds to microorganisms that enter through the mucosal surface (Tesher et al. 2019). Yupingfeng polysaccharide fraction (ASPC as the main ingredient, 10 ml/kg), could improve rabbits intestinal barrier integrity and functionality and increase the levels of IL-1, IL-2, IL-4, IL-6, IL-10, IL-12, TNF-α, and IFN-γ by activating TLR2 and TLR4 signaling pathways (Sun et al. 2016). The Bu-Zhong-Yi-Qi-Tang polysaccharide fraction (ASPC as the main ingredient, 45 mg/kg) also increased mucosal immune activity in mice, enhanced the activity of T lymphocytes in Peyer’s patches and induced the production of IL-2, IL-4, IL-5 and IFN-γ (Liu et al. 2019b). In ovo injection of ASPC (1–4 mg/kg) was found to enhance the intestinal mucosal immunity of broiler chickens by increasing the levels of IL-2, IL-4, IFN-γ, and TLR-4 and the number of IgA+ cells in the early stage after hatching (Yang et al. 2021). ASPC (0.6 mg/ml) could regulate the intestinal mucosal immune function of reovirus-infected muscovy ducklings by increasing the ileal secretory IgA and duodenal cytokine levels of IL-4, IL-6, IL-15, TNF-α, and INF-γ (Liao et al. 2021). For rats vaccinated with recombination urease subunit B, using ASPC (1.25–10 mg/ml) as an adjuvant could increase the levels of ovalbumin-specific IgG in serum and secretory IgA in saliva, vaginal wash and intestinal lavage fluid by activating the TLR2 signaling pathway and resulting in mixed specific T helper 1 (Th1) cells and Th17 cells immune response (Liu et al. 2019a). The pure polysaccharide Astragalus polysaccharide (APS) consists of glucose, arabinose, xylose and mannose with a molar ratio 95.0:2.9:0.7:0.7:0.6, and a molecular weight of 17.39 kDa. APS (50 mg/kg) could activate the dendritic cells, cytotoxic T lymphocytes, and natural killer cells of the mesenteric lymph nodes, thereby promoting the inhibitory effect of programmed death ligand-1 (PD-L1) antibody on the growth of metastatic lung melanoma in mice (Hwang et al. 2021). The pure polysaccharide AMA-1-b-PS2 (Arabino-3,6-galactan) consists of arabinose, fucose, galactose, glucose, mannose, rhamnose, xylose, galacturonic acid, glucuronic acid and glucose acid with a molar ratio of 12.8:4.5:25.6:23.6:24.8:5.1:0.7:1.5:1.5:1.4. The backbone is composed of β-D-(1→3) linked galactans. In mice small intestines, AMA-1-b-PS2 (10–100 µg/ml) could contribute to immunomodulation of Peyer’s patch cells by activating the T cell response and promoting the production of IL-2, IL-6 and TNF-α (Lim et al. 2016).
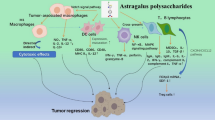
In short, current evidence suggests that Astragalus polysaccharide can stimulate the immune function of the bone marrow, thymus, spleen and mucosal immune system and further activate a variety of immune cells to produce immune factors, including macrophages, glial cells, natural killer cells, dendritic cells, and T lymphocytes.
Pharmacological activities on immune cells
Pharmacological activities on macrophages
It is widely acknowledged that monocytes leave the blood vessels and distribute to various tissues, becoming macrophages. Macrophages are not only the main effector cells of innate immunity but also play an important role in the process of the adaptive immune response (Poupot et al. 2018). Cell research has shown that ASPC (6.25–100 µg/ml) could increase the expression of IL-1β, IL-6, IL-8, TNF-α, IFN-γ and iNOS (inducible nitric oxide synthase) in primary head kidney macrophages (Zhang et al. 2020a). Moreover, ASPC (12.5–100 µg/ml) could stimulate the activity of RAW264.7 macrophages and increase the production of NO, IL-1β, IL-6 and TNF-α (Zhao et al. 2011). Studies have shown that this stimulation effect was related to the activation of.
TLR4/MyD88 (myeloid differentiation factor 88) signaling pathway (Zhou et al. 2017; Li et al. 2019d). In addition, studies have also shown that this promotion effect was related to the activation of NF-κB (nuclear factor κ-B) and MAPK (mitogen-activated protein kinase) signaling pathways (Lee and Jeon 2005; Zhu et al. 2018; Feng et al. 2020,). Wang et al. found that secondary messengers were involved in the immune regulation of RAW 264.7 macrophages by ASPC (25 µg/ml), including increasing the levels of NO, diglycerides, inositol 1,4,5-triphosphate, and cyclic adenosine monophosphate (Wang et al. 2017). In addition, ASPC could improve immune stress in macrophages. In this respect, ASPC (10–30 µg/ml) inhibited the expression of IL-1β, IL-6 and TNF-α in ochratoxin A-induced porcine alveolar macrophages (Liu et al. 2018b). In LPS-induced RAW264.7 macrophages, ASPC (10–300 µg/ml) also decreased the expression of NO, IFN-γ, IL-1β, IL-22 and TNF-α (Liao et al. 2018). In vivo research further confirmed the immunomodulatory effect of ASPC on macrophages. In Brucella-infected mice, ASPC (50–200 µg/ml) promoted the activation of macrophages and the production of proinflammatory cytokines, including TNF-α, IL-12 and IFN-γ (Shi et al. 2019). ASPC (200 mg/kg) promoted defective alveolar macrophage phagocytosis and decreased IL-6, IL-8, and TNF-α levels in bronchoalveolar lavage fluid and serum in chronic obstructive pulmonary disease mice (Chu et al. 2016).
In addition, scholars have explored the immunomodulatory effect of several pure polysaccharides on macrophages. The pure polysaccharide AP (Astragalus polysaccharide), an α-d-glucan (300 mg/kg), promoted macrophage phagocytosis and the secretion of immune-related cytokines such as GM-CSF, TNF-α, IL-4 and IL-10 by activating the TLR4 signaling pathway in normal mice (Moreno-Mendieta et al. 2017; Peng et al. 2019). The pure polysaccharide APSII (Astragalus polysaccharide II) consists of xylose, glucuronic, arabinose, rhamnose, mannose, and galactose with a molar ratio of 9.22:77.89:1:5.18:4.54:2.17, and a molecular weight of 11.4 kDa (Lv et al. 2016). APSII (10–80 µg/ml) promoted the maturation of RAW264.7 macrophages by increasing the expression of MHC II (major histocompatibility complex II), CD40, CD80 and CD86 (Lv et al. 2016). The pure polysaccharide AMP (Astragalus membranaceus polysaccharide) consists of glucose, arabinose, and galactose with a molar ratio of 91.0:6.2:2.8 and a molecular weight of 804 kDa (Li et al. 2020a). AMP (10–100 µg/ml) has been reported to stimulate RAW264.7 macrophages to produce NO by activating the NF-κB and MAPK signaling pathways (Li et al. 2020a). The pure polysaccharide RAP (30–300 µg/ml) could also promote the production of NO, TNF-α and IL-6 in RAW264.7 macrophages by activating the NF-κB and MAPK signaling pathways (Wei et al. 2016; Li et al. 2017, 2018c). In addition, RAP (30–300 µmol/L) could induce mice macrophages polarization to the M1 phenotype by inducing higher expression of M1 marker genes, including iNOS, IL-6, TNF-α and CXCL10 (chemokine (C-X-C motif) ligand 10) (Wei et al. 2019). In addition, RAP (10–100 µg/ml) could protect RAW264.7 macrophages from cell cycle arrest and apoptosis induced by paclitaxel by downregulating the protein levels of phospho-histone H2A. X, poly ADP-ribose polymerase, checkpoint kinase 1, p53 and p21, and upregulating the levels of Bax and myeloid cell leukemia-1 (Bao et al. 2018). In short, Astragalus polysaccharide can promote the activity of macrophages by activating the NF-κB and MAPK signaling pathways and induce the expression of a variety of cytokines.
Pharmacological activities on T and B lymphocytes
T and B lymphocytes are two important lymphocytes in the body. T lymphocytes can promote the proliferation and activation of other immune cells through the production of cytokines and directly kill target cells. The basic function of B lymphocytes is to produce antibodies, present antigens and secrete cytokines (Locatelli et al. 2013). In vivo studies showed that ASPC (8 mg/kg) improved the immunity of cyclophosphamide-induced immunosuppressive mice by promoting proliferation of T lymphocytes and B lymphocytes, and inducing the production of a variety of cytokines, including IgA, IgG, IgM, IL-6, IL-2, IFN-γ, complement 3, complement 4 and TNF-α (Yu et al. 2016; Meng et al. 2017). For mice vaccinated with HBV (hepatitis B virus)-DNA vaccine, ASPC (500 µg/mouse) as an adjuvant promoted the proliferation and the activity of T lymphocytes, induced CD4+ T cells to produce IL-4, IL-2 and IFN-γ, enhanced the expression of IFN-γ in CD8+ T cells, and reduced the frequency of regulatory T cells (Du et al. 2012). In spleen deficiency syndrome mice (induced by a high-fat, low-protein diet and exhausting swimming), ASPC (300–1200 mg/kg) increased the percentages of CD3+ and CD3+CD4+ T cells and the ratio of CD3+CD4+/CD3+CD8+, and reduced the expression of IL-6, IL-10 and TNF-α (Kang and Yu 2018; Zhao et al. 2019). CD4+CD25+ Treg cell is a kind of T lymphocyte with immunosuppressive effect and can inhibit the activation and proliferation of T lymphocytes (Haufe et al. 2011). In the microenvironment of human hepatocellular carcinoma, ASPC (10–200 µg/ml) could inhibit the growth, proliferation and migration of CD4+CD25+ Treg cells by inhibiting the activation of chemokine (C-X-C motif) receptor 4 (CXCR4)/CXCL12 signaling pathway, blocking chemokine stromal-derived factor-1 and its receptor and restoring the imbalance of cytokines (increasing IFN-γ expression and decreasing IL-4 and IL-10 expression) (Li et al. 2012). The pure polysaccharide Astragalus polysaccharide-IV (APS-IV) is an α-1,4-d-glucan with a molecular weight of 20.7 kDa. The pure polysaccharide Astragalus polysaccharide-V ( APS-V) consists only of arabinose, and the molecular weight was 40.1 kDa. The pure polysaccharide Astragalus polysaccharide-VI (APS-VI) consists of rhamnose, glucose, galactose and arabinose with a molar ratio of 1:10.76:6.55:12, and a molecular weight of 15.3 kDa (Niu et al. 2011; Jiang et al. 2016). In vitro research has shown that APS-IV (100–250 µg/ml), APS-V (6.25–800 µg/ml) and APS-VI (6.25–800 µg/ml) could effectively stimulate the proliferation of mouse T lymphocytes and B lymphocytes (Niu et al. 2011; Jiang et al. 2016). In short, Astragalus polysaccharides can improve immune suppression and enhance tumor immunity by promoting the proliferation of T lymphocytes and B lymphocytes, increasing the secretion of related cytokines and inhibiting the activity of regulatory T cells.
Pharmacological activities on natural killer cells
The natural killer cell is a kind of lymphocyte from bone marrow lymphoid cells distributed in peripheral blood and the spleen (Huntington et al. 2020). Given that the cytotoxic effect of natural killer cells does not require antigen pre-sensitization and there is no MHC restriction, natural killer cells play an important role in antitumor immunity (Battella et al. 2016). In vitro research has shown that the pure polysaccharide AMP (10–100 µg/ml) could activate and promote the proliferation of mouse natural killer cells by up-regulating the expression of IFN-γ, TNF-α, granzyme-B, and NKp44 (a natural cytotoxicity receptor) (Li et al. 2020a). Mu et al. further explored the mechanism of Astragalus polysaccharide in activating natural killer cells. In H22 tumor-bearing mice, ASPC (10–100 mg/kg) upregulated the expression of major histocompatibility complex class I chain-related molecule A (MICA) and MICB on the tumor cell surface which represent the major ligands of activating receptor NKG2D on natural killer cells. The binding of MICA/B and NKG2D led to natural killer cell activation by increasing the phosphorylated extracellular signal-regulated kinase level. Activated natural killer cells released IFN-γ, granzyme-B and perforin and promoted the clearance of cancer cells (Mu et al. 2019). In addition, ASPC (10 g/kg) ameliorated natural killer cell-mediated cytotoxicity in breeder cock testes by reducing levels of IFN-γ, perforin, DNA-binding protein (high mobility group-2 (HMG-2), and lysin (Wu et al. 2017). In conclusion, experimental studies have confirmed that Astragalus polysaccharide can regulate the activity of natural killer cells and the expression of immune factors such as IFN-γ, TNF-α, granzyme-B and perforin.
Pharmacological activities on dendritic cells
Dendritic cells differentiate from myeloid stem cells and lymphoid stem cells and are widely distributed in various organs except for brain tissue (Shang et al. 2017). Research has shown that Astragalus polysaccharide can promote the maturation of dendritic cells. ASPC (500 µg/mouse) as an adjuvant could stimulate the maturation of dendritic cells and upregulate the expression of MHC I/II, CD40, CD80 and CD86 in mice vaccinated with the HBV-DNA vaccine (Du et al. 2012). The pure polysaccharide APSII (1.67–45 µg/ml) could also promote the maturation of dendritic cells by inducing the production of NO and increasing the expression of CD40, CD80, CD86 and MHC-II (Zhu et al. 2016). In the normal mice, the pure polysaccharide AMP (100–500 mg/kg) increased the levels of CD40, CD80, CD86, MHC I and MHC II in splenic dendritic cells and their subsets and promoted the production of proinflammatory cytokines such as IL-6, IL-12p70 and TNF-α (Lim et al. 2021). Since dendritic cells are the most powerful antigen-presenting cells in the body, their activity is essential for developing, differentiating, and activating T and B lymphocytes (Kambayashi and Laufer 2014). It has been reported that ASPC (50–200 μm/ml) induced the differentiation of splenic dendritic cells to IL-12-producing CD11chigh CD45RBlow dendritic cells and further induced activation of T lymphocytes with the transformation of T helper 2 cells to T helper 1 cells (Liu et al. 2011). ASPC (10 mg/kg) induced phenotypic maturation of dendritic cells by upregulating the expression of MHC-II, CD80 and CD86, which in turn induced cytotoxic T lymphocyte activation and immune factor TNF-α, IFN-γ, IL-4, IL-10, and IgG1 production in 4T1 tumor-bearing mice (Xiong et al. 2020). In non-small cell lung cancer-bearing mice, ASPC (3 mg/kg) could promote the functional maturation of dendritic cells and increase the population of CD80+, CD103+, and CD86+ functionally matured dendritic cells, thereby enhancing the anticancer immune response mediated by T lymphocytes (Bamodu et al. 2019). In conclusion, upregulation of the expression of IL-6, IL-12p70, TNF-α, CD40, CD80, CD86 and MHC I/II by Astragalus polysaccharide indicates that it can stimulate the activity of dendritic cells and thus promote the activation of T and B lymphocytes.
Pharmacological activities on microglia
Microglia function as a sentinel for innate immunity in the central nervous system. Under different cell microenvironment conditions, microglia can differentiate into two phenotypes: the M1 type with a proinflammatory effect and the M2 type with an anti-inflammatory effect (Saijo et al. 2013). ASPC (200 µg/ml) could inhibit LPS-induced microglial activation and the expression of various proinflammatory factors, including prostaglandin E2, iNOS, cyclooxygenase-2, IL-1β and TNF-α by inhibiting the NF-κB and AKT (protein kinase B) signaling pathways (Luo et al. 2015). Jia et al. found that ASPC (22.5–45 mg/kg) reversed the polarization of rat microglia M1/M2, decreased the matrix metalloproteinaseprotein-9 expression, and maintained the integrity of blood brain barrier in the middle cerebral artery occlusion rats by inhibiting the expression of P2 × 7R (purinergic receptor) and promoting the degradation of ATP in microglia (2022). In addition, Liu et al. found that miR-155 mediated the polarization of microglia M1/M2 phenotype. ASPC (0.4–0.8 mg/ml) regulated the polarization of microglia from the M1 to M2 phenotype by inhibiting miR-155 expression, reducing the secretion of inflammatory factors IL-1α and TNF-α, and inhibiting the activation of neurotoxic astrocytes in experimental autoimmune encephalomyelitis mice (Liu et al. 2021). In mice treated with a high-fat diet and low-dose streptozotocin, ASPC (500 mg/kg) reduced metabolic stress-induced astrogliosis and microglial activation in brain plaques by diminishing the immunoreactivity of the ionized calcium-binding adaptor molecule-1 (Huang et al. 2017). In conclusion, Astragalus polysaccharide can inhibit the activation of microglia and inhibit the inflammatory response of the nervous system.
Pharmacological activities on immunological diseases
Pharmacological activities on cancer
The body’s immune response to tumors involves innate immunity and adaptive immunity. For cancer cells with weak immunogenicity, innate immunity plays a major role, and the main participating cells include macrophages, natural killer cells and γδ T cells (Sun et al. 2018). It has been shown that Astragalus polysaccharides can boost the body’s immune response to cancer by activating macrophages, dendritic cells, and natural killer cells by promoting their proliferation and differentiation, increasing the secretion of cytokines such as IL-2, TNF-α and IFN-γ, and alleviating the state of immune suppression (Balakrishnan et al. 2021; Li et al. 2021c). Macrophages are not only used as antigen-presenting cells during tumor immunity but also as effector cells that kill cancer cells (Van den Bossche et al. 2017). Research has shown that ASPC (0.1–100 mg/ml) could induce MCF-7 human breast cancer cell apoptosis by activating RAW 246.7 macrophages and promoting the expression of NO and TNF-α (Li et al. 2018a, 2019d). In 4T1 tumor-bearing mice, ASPC (50–1000 µg/ml) enhanced the proliferation of spleen lymphocytes and phagocytosis of peritoneal macrophages and upregulated the expression of IL-2, TNF-α and IFN-γ in the peripheral blood (Li et al. 2020e). In Ehrlich ascites carcinoma mice models, ASPC (500 mg/kg) also promoted the production of TNF-α, IL-1β, and IL-6 by activating RAW 264.7 macrophages, thereby inducing apoptosis of cancer cells. The qRT-PCR and Western blotting indicated that it was related to the activation of the MyD88-dependent signaling pathway mediated by TLR4 (Zhou et al. 2017; Li et al. 2020f). Natural killer cells and γδ T cells are effector cells that play an important role in early antitumor immunity and represent the body’s first line of defense against tumors (Johnsrud et al. 2020). In S180 sarcoma-bearing mice, ASPC (150–300 mg/kg) induced apoptosis of cancer cells by promoting the proliferation of γδ T cells and inducing the expression of IFN-γ, Fas ligand and granzyme B (Sun et al. 2014). In addition to enhancing the body’s immune response to cancer, the immunomodulatory effect of ASPC is reflected in its ability to improve cancer-related inflammation. Clinical research has found that ASPC (250–500 mg/day) could reduce the levels of IL-1β, IL-6, IL-12, GM-CSF, transforming growth factor β1 (TGF-β1) and IFN-γ and improve complications of cancer including pain, nausea, vomiting, and fatigue in cancer patients (Huang et al. 2019). The pure polysaccharide cAMPs-1 A (cold-water-soluble Astragalus polysaccharide-1 A) consists of fucose, arabinose, galactose, glucose, xylose with a molar ratio of 0.01:0.06:0.20:1.00:0.06, and a molecular weight of 12.3 kDa (Liu et al. 2018a). The pure polysaccharide ASP (alcohol-soluble Astragalus polysaccharide) consists of arabinose, galactose, glucose and mannose with a molar ratio of 1.00:0.98:3.01:1.52, and a molecular weight of 2.1 kDa (Yu et al. 2018). Studies have shown that cAMPs-1 A (300 mg/kg) and ASP (100–300 mg/kg) could induce tumor cell apoptosis by promoting the activities of natural killer cells, macrophages and T lymphocytes and the expression of TNF-α, IL-2 and IFN-γ in B16 or H22 tumor-bearing mice (Liu et al. 2018a; Yu et al. 2018). This finding confirms that the tumor immunity induced by Astragalus polysaccharide involves a variety of immune cells.
T lymphocyte is another important cell of tumor immunity. Tumor antigen mainly induces two types of T lymphocyte subpopulations in the body to respond, including CD4+ T cell restricted by MHC II and CD8+ T cell restricted by MHC I (Rivoltini et al. 2002). In H22 tumor-bearing mice, ASPC (50–400 mg/kg) induced apoptosis and inhibited the growth of tumor cells by increasing the spleen and thymus indexes and promoting T lymphocytes to produce IL-2, IL-6 and TNF-α (Lai et al. 2017). In S180 tumor-bearing mice, ASPC (150–300 mg/kg) induced the apoptosis of tumor cells by upregulating the proportion of CD3+, CD4+, CD8+ T cells and CD19+ B cells in the thymus, peripheral blood and spleen (Yu et al. 2021). In mouse mammary carcinoma 4T1 cells, ASPC (100–200 µg/ml) enhanced the expression of CD40, CD80 and CD86 markers in dendritic cells, immunomodulatory cytokines and chemokines including TNF-α, IL-6, CCL1 (chemokine (C-C motif) ligand 1), CCL3, CXCL1, CXCL2, CXCL10 and the proliferation of CD4+ T cells and CD8+ T cells (Chang et al. 2015; Pang et al. 2019a, b). In Lewis tumor-bearing mice, the pure polysaccharide AX-I-3b (50–200 mg/kg) improved the immunosuppression caused by cisplatin by increasing the ratio of CD4+ T cells to CD8+ T cells and the expression of IL-2, IL-6 and TNF-α (Li et al. 2019a). The pure polysaccharide AP-I (extract of Astragalus-I), an α-(1→4)-d-glucan with α-(1→6)-linked branches, could promote the proliferation of spleen lymphocytes and increase the levels of IL-2, IgA, IgG and IgM and natural killer cells activities in gastric cancer rats at 300 mg/kg (Li et al. 2009). In addition, ASPC (10–200 µg/ml) could inhibit the activation of CD4+CD25+ Treg cells in the microenvironment of human hepatocellular carcinoma by restoring cytokine imbalance (increasing IFN-γ expression and decreasing IL-4 and IL-10 expression) and reducing FOXp3 (forkhead box protein p3) expression, thereby eliminating the immunosuppression and promoting the apoptosis of cancer cells (Li et al. 2012).
Programmed death 1 (PD-1) is an immune checkpoint that can inhibit the function of immune cells, prevent the body from producing an effective antitumor immune response, and induce immune escape of tumor cells (Xu-Monette et al. 2018). It has been reported that ASPC (50 mg/kg) could downregulate the expression of PD-L1 on the surface of breast cancer 4T1 cells and colorectal cancer CT26 cells and block the effect of PD-L1-induced T cell exhaustion by inhibiting the AKT/mammalian target of rapamycin/ribosomal protein S6 kinase β-1 signaling pathway in tumor-bearing mice (Chang et al. 2020a, b). A study involving 53 lung cancer patients showed that ASPC (500 mg/person) could alleviate poor response and improve survival after treatment with immune checkpoint inhibitors by normalizing the neutrophil-to-lymphocyte ratio (Tsao et al. 2021). Myeloid-derived suppressor cells also play a role in immune suppression in cancer. Myeloid-derived suppressor cells are pathologically activated myeloid cells that are potent suppressors of T cells and natural killer cells (Gabrilovich 2017). In the co-culture of peripheral blood mononuclear cells with HeLa cervical cancer cells, ASPC (15–1000 µg/ml) inhibited myeloid-derived suppressor cells proliferation and the induction of Treg cells and promoted the production of TGF-β, IL-6, IFN-γ and IL-10 by peripheral blood mononuclear cells (Shokati et al. 2021). In the co-culture of bone marrow-derived mesenchymal stem cells with A549 lung cancer cells, ASPC (50 µg/ml) inhibited myeloid-derived suppressor cells proliferation and morphological changes by inhibiting the activation of the MAPK/NF-κB signaling pathway and reducing the protein expression levels of acetylated H4K5 (histone H4 lysine 5), acetylated H4K8, and acetylated H3K9 (Zhang et al. 2019a). In melanoma-bearing mice, ASPC (50–200 mg/kg) reduced the number of myeloid-derived suppressor cells and the expression of Arg-1, IL-10 and TGF-β, which increased the cytotoxic effect of CD8+ T cells against tumor cells. Spearman correlation analysis showed that it was related to ASPC remodeling the gut microbiota (Ding et al. 2021; Tian et al. 2012). In addition, ASPC (10 mg/kg) could inhibit vascular endothelial growth factor (VEGF) production in 4T1 tumor-bearing mice and inhibit the proliferation and migration of cancer cells by inducing the production of single-chain fragment variable antibody 4E (scFv 4E). Molecular docking simulations revealed that the main driving force for the interaction of scFv 4E and VEGF involved the hydrophobic interactions and hydrogen bonds of Tyr108 and Tyr 109 of the complementarity-determining region H3 loop with VEGF (Lee et al. 2020).
In summary, these studies substantiate that Astragalus polysaccharide can promote tumor immune response by stimulating the activity of a variety of immune cells. In addition, Astragalus polysaccharide can inhibit tumor immune escape by inhibiting the activity of regulatory T cells and immune checkpoints.
Pharmacological activities against viral infection
ASPC (120 µg/ml) could protect mouse astrocytes from herpes simplex virus (HSV)-1 infection by increasing the expression of TNF-α and IL-6 by activating the TLR3/NF-κB signaling pathway (Shi et al. 2014). ASPC (10 µg/ml) could modulate the immune activity of porcine endothelial cells exposed to CSFV (classical swine fever virus) by increasing the levels of IFN-α, IFN-β, IL-1, IL-6, and IL-8 (Zhuge et al. 2017). In vivo studies have further shown that Astragalus polysaccharide could exert an antiviral effect by improving the body’s immune function. Current evidence suggests that ASPC (2 g/kg) could improve the resistance of crucian carp against SVCV (spring viremia of carp virus) infection by upregulating the expression of IgM, IL-8, IL-10, IL-1β, IFN-α, IFN-γ, MyD88, TGF-β and TNF-α in the spleen, kidney, liver and intestine (Liu et al. 2022). In zebrafish infected by the SVCV, ASPC (0.01%) upregulated the levels of IL-10, tight junction protein 1b, and occludin1 and increased the expression of antiviral genes in the spleen, including IFNφ1, IFNφ2, and IFNφ3 (Li et al. 2021b). In intestinal inflammatory damage of goslings infected with parvovirus, ASPC (0.3 ml) decreased the expression of secreted IgA, IL-1β, IL-6 and TNF-α in the jejunal tissue and increased the levels of serum IgG, IgM, complement 3, complement 4, IFN-γ (Luo et al. 2021). ASPC (0.2 g/kg) enhanced immune response in shrimp attacked by white spot syndrome virus by promoting hemocyte proliferation and phagocytic activity and increasing the activities of phenoloxidase, total superoxide dismutase and lysozyme (Chang et al. 2018).
In addition, Astragalus polysaccharide has been reported as an adjuvant for viral vaccine by activating macrophages, natural killer cells, T and B lymphocytes, and the complement system, promoting the secretion of immune factors, and so on (Wang et al. 2021). ASPC used as a vaccine carrier enhanced the immune response in Swiss albino mice vaccinated with seasonal influenza A (H3N2) vaccine by inducing Th1/Th2 balance and the production of IL-17 and IgG1 antibody (Yakuboğulları et al. 2019). ASPC as a vaccine delivery system increased the immune response to the newcastle disease vaccine in zebrafish by enhancing the production of neutralizing antibodies and the minor augmentation of IFN-α and IL-2 levels (Yakubogullari et al. 2021). For mice vaccinated with HBV-DNA vaccine, ASPC (500 µg/mouse) as an adjuvant stimulated the maturation of dendritic cells, upregulated the expression of MHC I/II, CD40, CD80 and CD86, induced CD4+ T cells to produce IL-4, IL-2 and IFN-γ, enhanced the expression of IFN-γ in CD8+ T cells, and reduced the frequency of regulatory T cells (Du et al. 2012). In ovo administration of live newcastle disease vaccine with ASPC (0.5 ml) as an adjuvant could stimulate stronger humoral and cellular responses in newly hatched chicks by increasing the concentrations of IFN-γ, IL-2, IL-4 and IL-6, promoting lymphocyte proliferative capability as well as improving the frequencies of CD4+ and CD8+ T cells (Shan et al. 2019; Xue et al. 2020). ASPC (10–100 mg/kg ) could be used as an adjuvant for the avian infectious bronchitis virus vaccine and promote lymphocyte proliferation and the expression of IL-1β, IL-2, IL-8, and TNF-α in chicken (Zhang et al. 2017). These findings indicate that Astragalus polysaccharide may be an effective adjuvant to optimize the response to viral vaccines.
Pharmacological activities against bacterial infection
Studies have shown that Astragalus polysaccharide could exert an antibacterial effect by improving the body’s immune function. The use of ASPC (1500 mg/kg) enhanced the immune function of Nile tilapia and improved several immune parameters, including the serum bactericidal activity, phagocytic activity of blood phagocytes, respiratory burst activity of the whole blood, and serum lysozyme activity (Zahran et al. 2014). Moreover, ASPC (1 g/kg) could modulate the intestinal microbiota and activate the immune response to protect grass carp from Aeromonas hydrophila infection by increasing the expression of TNF-α, IL-1β, GM-CSF, interleukin enhancer-binding factor 2 homolog, and serine/threonine-protein kinase doublecortin like kinase protein 1 (Shi et al. 2021). ASPC (20–50% of the diet) modulated immunity in Aeromonas veronii TH0426 infected crucian carp by increasing the activities of serum acid phosphatase, alkaline phosphatase, and lysozyme and levels of the IL-10, IL-1β, IFN-γ and TNF-α in the spleen (Song et al. 2022). In Aeromonas hydrophila-infected mice, ASPC (250 mg/kg) improved the phagocytic activity of neutrophils in intestinal tissues, increased the number of CD4+ T cells in intestinal tissues and thymus, and reduced the number of CD8+ T cells in spleen and thymus (Abuelsaad 2014). ASPC (100–400 mg/kg) modulated immunity in Vibrio parahaemolyticus-infected shrimp by increasing the expression of the immune-related factors, including anti-lipopolysaccharide factor, cathepsin B, crustin, lectin, lysozyme, and toll-like receptor (Zhai et al. 2019). In Brucella-infected mice, ASPC (50–200 µg/ml) promoted the secretion of proinflammatory cytokines such as TNF-α, IL-12 and IFN-γ and the activation of macrophages, thereby enhancing the host’s immune response (Shi et al. 2019). It is widely acknowledged that sepsis is a systemic inflammatory response caused by bacterial infection. Balancing pro- and anti-inflammatory responses is a potential therapeutic approach for sepsis (Johansen et al. 2021). In polymicrobial sepsis mice, ASPC (100–200 mg/kg) increased the percentage of Th1 cells in the spleen and Peyer’s patches, decreased the percentage of Treg cells and Th2 cells in the blood circulation, and inhibited the polarization of blood CD4+ T cells towards T helper cell 2 response (Hou et al. 2015).
Mounting evidence suggests that Astragalus polysaccharide can be used as an adjuvant for bacterial vaccines. Inactivated Edwardsiella ictaluri vaccine, formulated with ASPC (100 µg) as an adjuvant, could improve the survival rate of yellow catfish and protect intestine tissue from the injury of Edwardsiella ictaluri by increasing the expression of IL-1β, IL-2, IFN-γ2 and IgM in the spleen (Zhu et al. 2019). Inactivated Vibrio harveyi formalin-killed cells vaccine, formulated with ASPC (4 mg/ml) as an adjuvant, could increase IL-1β, IL-16, TNF-α, MHC-Iα and IgM levels in the spleen in groupers (Gwab et al. 2020). For rats vaccinated with recombination urease subunit B, using ASPC (1.25–10 mg/ml) as an adjuvant could increase the levels of ovalbumin-specific IgG in serum and secretory IgA in saliva, vaginal wash and intestinal lavage fluid by activating the TLR2 signaling pathway and resulting in mixed specific Th1 cells and Th17 cells immune response (Liu et al. 2019a). Overall, these findings indicate that Astragalus polysaccharide may be an effective adjuvant for bacterial vaccines.
Pharmacological activities against autoimmune diseases
Multiple sclerosis is an inflammatory demyelinating disease of the central nervous system. ASPC (500 mg/kg) could effectively suppress autoimmune encephalomyelitis in mice by inhibiting MOG35−55-specific T cell proliferation, downregulating the levels of IFN-γ, TNF-α, IL-2, and IL-17, upregulating the costimulatory molecules PD-1/PD-Ls signaling pathway, and leading to inhibition of T cell-mediated immune response (Sun et al. 2019). In addition, promoting remyelination is an important strategy for treating multiple sclerosis. In cuprizone-induced demyelination mice, ASPC (500 mg/kg) relieved the neurobehavioral dysfunction and efficaciously facilitated remyelination by activating the Sonic hedgehog signaling pathway and inducing neural stem cells to differentiate into oligodendrocytes (Ye et al. 2021). Systemic scleroderma is an autoimmune disease characterized by fibrotic changes involving excessive collagen deposition in the skin and other organs. ASPC (200 mg/kg) could reduce collagen production and the expression of Smad2 and Smad3 in bleomycin-induced scleroderma mice by suppressing the TGF-β signaling pathway (Hao et al. 2015).
It is widely acknowledged that type 1 diabetes is characterized by the autoimmune destruction of pancreatic β cells. Type 1 diabetes is an autoimmune disease mediated by T cells that destroy insulin-producing β cells in the pancreatic islets (Marfil-Garza et al. 2021). In streptozocin-induced type 1 diabetes mice, ASPC (100–400 mg/kg) could protect pancreatic β cells from apoptosis induced by CD8+ T cells by upregulating the expression of galectin-1 and causing CD8+ T cell apoptosis (Zhou et al. 2011). Li et al. also found that ASPC (100–400 mg/kg) protected β cells from apoptosis in type 1 diabetic rats by downregulating the Th1/Th2 cytokine ratio, decreasing the level of IFN-g and increasing the level of IL-4 by upregulating the peroxisome proliferator-activated receptor γ expression in the spleen (Li et al. 2007).
In type II collagen-induced rheumatoid arthritis rats, ASPC (4 g/kg) reduced paw swelling, serum concentrations of IL-1β and TNF-α, and the levels of NF-κB-p65 and IκBα (inhibitor of NF-κB α) in synovial membranes (Cao et al. 2019a). Moreover, ASPC (10 mg/kg) could mitigate the proinflammatory response, reduce TNF-α, IL-17, and IL-6 levels in myocardial tissues and inhibit the apoptosis in complete Freund’s adjuvant-induced rheumatoid arthritis rats by suppressing the activation of TLR4/MAPK/NF-κB signaling pathway (Cao et al. 2019b).
Other pharmacological activities
Astragalus polysaccharides can improve inflammatory response by regulating immune function. In 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease rats, ASPC (0.5–1.0 g/kg) could enhance the therapeutic effect of prednisone by promoting the expression of T helper cell 1 and T helper cell 2 specific transcription factors T-bet and GATA-3 (GATA binding protein 3) and ultimately contributing to the transition to the T helper cell 2 phenotype (Gao et al. 2016). In dextran sulfate sodium-induced acute colitis mice, ASPC (100–300 mg/kg) reestablished the immune balance by decreasing the levels of IL-1β, TNF-α, and IL-6 and upregulating IL-22 expression by activating aryl hydrocarbon receptor (present in Th17 cells, Th22 cells or innate lymphoid cells) (Tang et al. 2021). In ovalbumin-induced asthmatic mice, ASPC (100 mg/kg) could promote the therapeutic effect of budesonide by reducing the number of dendritic cells and the levels of IL-4 and IL-10 and increasing the number of Treg cells (Zhang and Ma 2020). Wang et al. found that ASPC (5–15 mg/kg) could reduce eosinophil infiltration and IL-4 expression in the nasal mucosa tissue of ovalbumin-induced asthmatic rats (Wang et al. 2020). Furthermore, Wu et al. documented that ASPC (10–50 mg/kg) attenuated eosinophil and neutrophil-dominant infiltration by reducing the levels of CXCL5, IL-8, CCL20, IL-13RA (IL-13 receptor α) and IL-17RA in ovalbumin-induced asthmatic mice (Lu et al. 2016). Moreover, ASPC (50 µg) has been reported as an adjuvant for the ovalbumin vaccine, which induced Th1 and Th2 immune responses, increased IL-2, IL-4, IL-10, IL-12 and IFN-γ levels and enhanced ovalbumin-specific IgG and IgG1 antibody responses in ovalbumin-induced asthmatic mice (Zhou et al. 2021). Allergic rhinitis is an IgE-mediated chronic inflammatory disease of the allergic nasal mucosa. ASPC (10–50 mg/kg) could improve the inflammatory symptoms of the nasal mucosa and reduce Th2-related cytokines of IL-4, IL-5, and IL-13 levels in serum and nasal mucosa tissue of allergic rhinitis rats by inhibiting the NLRP3 (NLR family pyrin domain-containing protein 3) inflammasome and NF-κB signaling pathway (Xu et al. 2021). In addition, in experimental periodontitis rats, ASPC (50–100 ng/ml) protected the alveolar bone from inflammatory erosion by decreasing the proportion of CD4+Foxp3+ cells and the levels of receptor activator of NF-κB ligand, osteoprotegerin, TGF-β and IL-10 and upregulating the level of CD4+IL-10+ cells in the gingiva (Han et al. 2021). In addition, the pure polysaccharide AMP (20–75 mg/kg) could ameliorate the immunity and spermatogenesis of mice with impaired reproductive function induced by cyclophosphamide by increasing the levels of IL-11, TNF-α and IFN-γ (Qiu and Cheng 2019).
Relationship between the Astragalus polysaccharide structure and immunomodulatory effect
Xia et al. compared the immunomodulatory effects of Astragalus polysaccharides with different molecular weights (157.7 kDa, 69.9 kDa, 22.4 kDa, 13.2 kDa, and 1.4 kDa) on cyclophosphamide-induced immunosuppression mice. The results showed that the higher the molecular weight, the stronger the phagocytic ability of peritoneal macrophages and the higher the level of secretory IgA in the small intestine (Xia et al. 2011). Li et al. compared the immunomodulatory effects of three Astragalus polysaccharides with different molecular weights (> 2000 kDa, 10 kDa and 300 Da) on cyclophosphamide-induced immunosuppression mice. The results showed that the Astragalus polysaccharide with a molecular weight of 10 kDa had the best immunomodulatory effect, including promoting the proliferation of T and B lymphocytes and the secretion of IL-2, IL-4, INF-γ and increasing the phagocytic activities of peritoneal macrophages and the activities of spleen natural killer cells (Li et al. 2020b, c). In conclusion, these findings suggest that Astragalus polysaccharides with extremely high or low molecular weight exhibit poor immunomodulatory activity. In addition, Ren et al. found that treatment of the Astragalus polysaccharide with γ-irradiation (24 kGy) could enhance its immunomodulatory activity on Caco2 cells including promoting NO, TNF-α, IL-1β and IL-8 production via TLR4 signaling pathway activation. Unlike general structural modification, γ-irradiation does not affect the functional groups of polysaccharides but affects physicochemical properties, such as apparent viscosity (Ren et al. 2018). Li et al. subsequently found that γ-irradiation-enhanced Astragalus polysaccharide immune activity was associated with decreased molecular weight and viscosity and increased water solubility. γ-irradiated Astragalus polysaccharide (300–900 mg/kg) elevated the level of IgA produced by duodenal cells, the jejunal expression of IL-2, IL-10, and IFN-γ, serum IgG concentration, and thymus index, and promoted T and B lymphocytes proliferation in cyclophosphamide-induced immunosuppressed broilers (Li et al. 2019b, c). In addition, Astragalus polysaccharides can be chemically modified or functionalized to increase their immunomodulatory activities. In this regard, Se-enriched Astragalus polysaccharide nanoparticles showed a stronger ability to enhance T lymphocyte proliferation than Astragalus polysaccharides in vitro (Meng et al. 2018). In short, the relationship of Astragalus polysaccharide structure on immunomodulatory effect warrants further research in the future.
Conclusion and perspectives
In short, Astragalus polysaccharide is a valuable immunomodulatory medicine. Table 1 summarizes the immunomodulatory effect, and Fig. 1 summarizes the molecular mechanism. Astragalus polysaccharide can improve immunosuppression caused by drugs in central immune organs and peripheral immune organs, including bone marrow, thymus, lymph nodes, spleen and mucosal tissues. Since these immune organs are the sites where various immune cells differentiate, mature and produce an immune response, Astragalus polysaccharide can further regulate the activity of a variety of immune cells. These immune cells include macrophages, natural killer cells, dendritic cells, T lymphocytes, B lymphocytes and microglia. Astragalus polysaccharides can induce these immune cells to produce a variety of cytokines and chemokines, thereby enhancing the immune response. The immunomodulatory effect of Astragalus polysaccharide makes it useful for the treatment of various diseases, including cancer, infection, type 1 diabetes, asthma, and autoimmune disease. Among these, the anticancer effect of Astragalus polysaccharide is the most prominent, which is closely related to its activation of tumor immune response and inhibition of tumor immune escape.
Although the current research has deeply explored the immunomodulatory effect of Astragalus polysaccharide, there are few studies on the relationship between polysaccharide structure and immunomodulatory activity. Table 2 summarizes the structural information of these pure polysaccharides with immunomodulatory effects and hopes to provide useful information for further research. In addition, there are few reports on structure modification of Astragalus polysaccharides. Therefore, the immunomodulatory function of different modified Astragalus polysaccharides can be investigated in the future. At present, although there are many experimental studies on the immunomodulation of Astragalus polysaccharides, there is still a lack of sufficient clinical research. Insufficient studies on the structural characteristics of Astragalus polysaccharides may be the key to restricting clinical research. Therefore, conducting more clinical research based on sufficient structural characterization studies in the future may be an important research direction.
References
Abuelsaad A (2014) Supplementation with Astragalus polysaccharides alters Aeromonas-induced tissue-specific cellular immune response. Microb Pathog 66:48–56. https://doi.org/10.1016/j.micpath.2013.12.005
Balakrishnan B, Liang Q, Fenix K, Tamang B, Hauben E, Ma L, Zhang W (2021) Combining the anticancer and immunomodulatory effects of Astragalus and Shiitake as an integrated therapeutic approach. Nutrients. https://doi.org/10.3390/nu13082564
Bamodu OA, Kuo KT, Wang CH, Huang WC, Wu ATH, Tsai JT, Lee KY, Yeh CT, Wang LS (2019) Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients 11:2264. https://doi.org/10.3390/nu11102264
Bao WR, Li Z, Zhang Q, Li L, Liu H, Ma D, Leung C, Lu A, Bian Z, Han Q (2018) Astragalus polysaccharide RAP selectively attenuates paclitaxel-induced cytotoxicity toward RAW 264.7 cells by reversing cell cycle arrest and apoptosis. Front Pharmacol 9:1580. https://doi.org/10.3389/fphar.2018.01580
Bao WR, Zhang QW, Zheng HM, Li LF, Liu M, Cheng HY, Wong TL, Zhang G, Lu AP, Bian ZX, Ma DL, Leung CH, Han QB (2021) Radix Astragali polysaccharide RAP directly protects hematopoietic stem cells from chemotherapy-induced myelosuppression by increasing FOS expression. Int J Biol Macromol 183:1715–1722. https://doi.org/10.1016/j.ijbiomac.2021.05.120
Battella S, Cox MC, Santoni A, Palmieri G (2016) Natural killer (NK) cells and anti-tumor therapeutic mAb: unexplored interactions. J Leukoc Biol 99:87–96. https://doi.org/10.1189/jlb.5VMR0415-141R
Bronte V, Pittet MJ (2013) The spleen in local and systemic regulation of immunity. Immunity 39:806–818. https://doi.org/10.1016/j.immuni.2013.10.010
Cao L, Yu M, Wang CH, Bao YH, Zhang MH, He P, Zhang Y, Yang TT, Li LL, Li G, Gong Y (2019a) Cellulase-assisted extraction, characterization, and bioactivity against rheumatoid arthritis of Astragalus polysaccharides. Int J Polym Sci. https://doi.org/10.1155/2019/8514247
Cao YX, Huang D, Liu J, Zong RK, Wan L, Huang CB, Zhang WD, Wang Y (2019b) A Novel Chinese Medicine, Xinfeng Capsule, Modulates Proinflammatory Cytokines via Regulating the Toll-Like Receptor 4 (TLR4)/Mitogen-Activated Protein Kinase (MAPK)/Nuclear Kappa B (NF-κB) Signaling Pathway in an Adjuvant Arthritis Rat Model. Med Sci Monit 25:6767–6774. https://doi.org/10.12659/msm.916317
Chang WT, Lai TH, Chyan YJ, Yin SY, Chen YH, Wei WC, Yang NS (2015) Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PLoS ONE. https://doi.org/10.1371/journal.pone.0122374
Chang ZQ, Ge QQ, Sun M, Wang Q, Lv HY, Li J (2018) Immune responses by dietary supplement with Astragalus polysaccharides in the Pacific white shrimp, Litopenaeus vannamei. Aquacult Nutr 24:702–711. https://doi.org/10.1111/anu.12599
Chang FL, Tsai KC, Lin TY, Yang TW, Lo YN, Chen WC, Chang JH, Lu MK, Chiou CT, Chen PH, Yen Y, Pan SL, Lee YC (2020a) Astragalus membranaceus-derived anti-programmed death-1 monoclonal antibodies with immunomodulatory therapeutic effects against tumors. BioMed Res Int. https://doi.org/10.1155/2020/3415471
Chang HL, Kuo YH, Wu LH, Chang CM, Cheng KJ, Tyan YC, Lee CH (2020b) The extracts of Astragalus membranaceus overcome tumor immune tolerance by inhibition of tumor programmed cell death protein ligand-1 expression. Int J Med Sci 17:939–945. https://doi.org/10.7150/ijms.42978
Chen ZJ, Liu LJ, Gao CF, Chen WJ, Vong CT, Yao PF, Yang YH, Li XZ, Tang XD, Wang SP, Wang YT (2020) Astragali Radix (Huangqi): a promising edible immunomodulatory herbal medicine. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2020.112895
Chu X, Liu XJ, Qiu JM, Zeng XL, Bao HR, Shu J (2016) Effects of Astragalus and Codonopsis pilosula polysaccharides on alveolar macrophage phagocytosis and inflammation in chronic obstructive pulmonary disease mice exposed to PM2.5. Environ Toxicol Pharmacol 48:76–84. https://doi.org/10.1016/j.etap.2016.10.006
Ding GQ, Gong QY, Ma JY, Liu XJ, Wang YH, Cheng XD (2021) Immunosuppressive activity is attenuated by Astragalus polysaccharides through remodeling the gut microenvironment in melanoma mice. Cancer Sci 112:4050–4063. https://doi.org/10.1111/cas.15078
Du X, Zhao B, Li J, Cao X, Diao M, Feng H, Chen X, Chen Z, Zeng X (2012) Astragalus polysaccharides enhance immune responses of HBV DNA vaccination via promoting the dendritic cell maturation and suppressing Treg frequency in mice. Int Immunopharmacol 14:463–470. https://doi.org/10.1016/j.intimp.2012.09.006
Fan YP, Ma L, Zhang WM, Cui XQ, Zhen Y, Suolangzhaxi, Song X (2013) Liposome can improve the adjuvanticity of Astragalus polysaccharide on the immune response against ovalbumin. Int J Biol Macromol 60:206–212. https://doi.org/10.1016/j.ijbiomac.2013.05.030
Farag MR, Alagawany M (2018) The role of Astragalus membranaceus as immunomodulator in poultry. World’s Poult Sci J 75:43–54. https://doi.org/10.1017/S0043933918000739
Feng S, Ding H, Liu L, Peng C, Wu J (2020) Astragalus polysaccharide enhances the immune function of RAW264.7 macrophages via the NFκB p65/MAPK signaling pathway. Exp Ther Med 21:20. https://doi.org/10.3892/etm.2020.9452
Fu J, Wang ZH, Huang LF, Zheng SH, Wang DM, Chen SL, Zhang HT, Yang SH (2014) Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother Res 28:1275–1283. https://doi.org/10.1002/ptr.5188
Gabrilovich DI (2017) Myeloid-derived suppressor cells. Cancer Immunol Res 5:3–8. https://doi.org/10.1158/2326-6066.cir-16-0297
Ganea D, Hooper KM, Kong W (2015) The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol 213:442–452. https://doi.org/10.1111/apha.12427
Gao YJ, Zhu F, Qian JM, Dai JY (2016) Therapeutic and immunoregulatory effect of GATA-binding protein-3/T-box expressed in T-cells ratio of Astragalus polysaccharides on 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Chin J Integr Med 22:918–924. https://doi.org/10.1007/s11655-015-2151-0
Gwab C, Scab C, Ywab C, Smab C, Yhab C (2020) Immune effect of Vibrio harveyi formalin-killed cells vaccine combined with chitosan oligosaccharide and Astragalus polysaccharides in ♀Epinephelus fuscoguttatus×♂Epinephelus lanceolatus. Fish Shellfish Immunol 98:186–192. https://doi.org/10.1016/j.fsi.2020.01.015
Han YK, Yu CC, Yu Y (2021) Astragalus polysaccharide alleviates alveolar bone destruction by regulating local osteoclastogenesis during periodontitis. J Appl Biomed 19:97–104. https://doi.org/10.32725/jab.2021.010
Hao ZF, Su YM, Liu JY, Wang CM, Yang RY (2015) Astragalus polysaccharide suppresses excessive collagen accumulation in a murine model of bleomycin-induced scleroderma. Int J Clin Exp Med 8:3848–3854
Haufe S, Haug M, Schepp C, Kuemmerle-Deschner J, Hansmann S, Rieber N, Tzaribachev N, Hospach T, Maier J, Dannecker GE, Holzer U (2011) Impaired suppression of synovial fluid CD4+CD25-T cells from patients with juvenile idiopathic arthritis by CD4+CD25+Treg cells. Arthritis Rheum 63:3153–3162. https://doi.org/10.1002/art.30503
Hou YC, Wu JM, Wang MY, Wu MH, Chen KY, Yeh SL, Lin MT (2015) Modulatory effects of Astragalus polysaccharides on T-cell polarization in mice with polymicrobial sepsis. Mediat Inflamm. https://doi.org/10.1155/2015/826319
Huang YC, Tsay HJ, Lu MK, Lin CH, Yeh CW, Liu HK, Shiao YJ (2017) Astragalus membranaceus-polysaccharides ameliorates obesity, hepatic steatosis, neuroinflammation and cognition impairment without affecting amyloid deposition in metabolically stressed APPswe/PS1dE9 mice. Int J Mol Sci. https://doi.org/10.3390/ijms18122746
Huang WC, Kuo KT, Bamodu OA, Lin YK, Wang CH, Lee KY, Wang LS, Yeh CT, Tsai JT (2019) Astragalus polysaccharide (PG2) ameliorates cancer symptom clusters, as well as improves quality of life in patients with metastatic disease, through modulation of the inflammatory cascade. Cancers. https://doi.org/10.3390/cancers11081054
Huntington ND, Cursons J, Rautela J (2020) The cancer-natural killer cell immunity cycle. Nat Rev Cancer 20:437–454. https://doi.org/10.1038/s41568-020-0272-z
Hwang J, Zhang W, Dhananjay Y, An E, Kwak M, You S, Lee P, Jin J (2021) Astragalus membranaceus polysaccharides potentiate the growth-inhibitory activity of immune checkpoint inhibitors against pulmonary metastatic melanoma in mice. Int J Biol Macromol 182:1292–1300. https://doi.org/10.1016/j.ijbiomac.2021.05.073
Jia N, Qiao HR, Zhu W, Zhu MH, Meng QH, Lu Q, Zu YG (2019) Antioxidant, immunomodulatory, oxidative stress inhibitory and iron supplementation effect of Astragalus membranaceus polysaccharide-iron (III) complex on iron-deficiency anemia mouse model. Int J Biol Macromol 132:213–221. https://doi.org/10.1016/j.ijbiomac.2019.03.196
Jia X, Xie LY, Liu Y, Liu TF, Yang PQ, Hu JF, Peng ZC, Luo KR, Du M, Chen CJ (2022) Astragalus polysaccharide (APS) exerts protective effect against acute ischemic stroke (AIS) through enhancing M2 micoglia polarization by regulating adenosine triphosphate (ATP)/purinergic receptor (P2 × 7R) axis. Bioengineered 13:4468–4480. https://doi.org/10.1080/21655979.2021.1980176
Jiang YP, Qi XH, Gao K, Liu WJ, Li N, Cheng NB, Ding G, Huang WZ, Wang ZZ, Xiao W (2016) Relationship between molecular weight, monosaccharide composition and immunobiologic activity of Astragalus polysaccharides. Glycoconj J 33:755–761. https://doi.org/10.1007/s10719-016-9669-z
Jin ML, Zhao K, Huang QS, Shang P (2014) Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol 64:257–266. https://doi.org/10.1016/j.ijbiomac.2013.12.002
Johansen JR, Perner A, Brodtkorb JH, Moller MH (2021) Use of hydroxyethyl starch in sepsis research: a systematic review with meta-analysis. Acta Anaesthesiol Scand 65:1355–1364. https://doi.org/10.1111/aas.13954
Johnsrud AJ, Jenkins SV, Jamshidi-Parsian A, Quick CM, Galhardo EP, Dings RP, Vang KB, Narayanasamy G, Makhoul I, Griffin RJ (2020) Evidence for early stage anti-tumor immunity elicited by spatially fractionated radiotherapy-immunotherapy combinations. Radiat Res 194:688–697. https://doi.org/10.1667/rade-20-00065.1
Jung Y, Jerng U, Lee S (2016) A systematic review of anticancer effects of Radix Astragali. Chin J Integr Med 22:225–236. https://doi.org/10.1007/s11655-015-2324-x
Kambayashi T, Laufer TM (2014) Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol 14:719–730. https://doi.org/10.1038/nri3754
Kang CS, Yu CY (2018) Effect of Astragalus membranaceus polysaccharides on improves immune response after exhaustive exercise rats. Korean J Med Crop Sci 26:72–81. https://doi.org/10.7783/kjmcs.2018.26.1.72
Kong FM, Chen TQ, Li XJ, Jia YJ (2021) The current application and future prospects of Astragalus polysaccharide combined with cancer immunotherapy: a review. Front Pharmacol. https://doi.org/10.3389/fphar.2021.737674
Lai XY, Xia WB, Wei J, Ding XH (2017) Therapeutic effect of Astragalus polysaccharides on hepatocellular carcinoma H22-bearing mice. Dose-Response. https://doi.org/10.1177/1559325816685182
Lee KY, Jeon YJ (2005) Macrophage activation by polysaccharide isolated from Astragalus membranaceus. Int Immunopharmacol 5:1225–1233. https://doi.org/10.1016/jintimp200502020
Lee YC, Huang HT, Chang CD, Chen CT, Lin TY, Yang TW, Chang FL, Lu MK, Chiou CT, Chen WC, Pan SL, Tsai KC (2020) Isolation of anti-VEGF monoclonal antibodies with neutralizing effects from an Astragalus-induced immune antibody library. Int Immunopharmacol. https://doi.org/10.1016/j.intimp.2020.107007
Li RJ, Qiu SD, Chen HX, Tian H, Wang HX (2007) The immunotherapeutic effects of Astragalus polysaccharide in type 1 diabetic mice. Biol Pharm Bull 30:470–476. https://doi.org/10.1248/bpb.30.470
Li R, Chen WC, Wang WP, Tian WY, Zhang XG (2009) Extraction, characterization of Astragalus polysaccharides and its immune modulating activities in rats with gastric cancer. Carbohydr Polym 78:738–742. https://doi.org/10.1016/j.carbpol.2009.06.005
Li Q, Bao JM, Li XL, Zhang T, Shen XH (2012) Inhibiting effect of Astragalus polysaccharides on the functions of CD4+CD25 highTreg cells in the tumor microenvironment of human hepatocellular carcinoma. Chin Med J 125:786–793. https://doi.org/10.3760/cma.j.issn.0366-6999.2012.05.012
Li ZP, Zhang QW, Wei W, Li LF, Ma DL, Leung CH, Lu AP, Bian ZX, Han QB (2017) Luteolin exerted less inhibitory effect on macrophage activation induced by Astragalus polysaccharide than by lipopolysaccharide. J Funct Foods 37:618–623. https://doi.org/10.1016/j.jff.2017.08.023
Li WF, Hu XY, Wang SP, Wang H, Parungao R, Wang YW, Liu TQ, Song KD (2018a) Detection and evaluation of anti-cancer efficiency of Astragalus polysaccharide via a tissue engineered tumor model. Macromol Biosci. https://doi.org/10.1002/mabi.201800223
Li YL, Lei XY, Yin ZC, Guo W, Yang XJ (2018b) Transgenerational effects of paternal dietary Astragalus polysaccharides on spleen immunity of broilers. Int J Biol Macromol 115:90–97. https://doi.org/10.1016/j.ijbiomac.2018.04.009
Li ZP, Liu HB, Zhang QW, Li LF, Bao WR, Ma DL, Leung CH, Bian ZX, Lu AP, Han QB (2018) Interference of quercetin on Astragalus polysaccharide-induced macrophage activation. Molecules. https://doi.org/10.3390/molecules23071563
Li K, Li S, Wang D, Li X, Wu X, Liu X, Du G, Li X, Qin X, Du Y (2019a) Extraction, characterization, antitumor and immunological activities of hemicellulose polysaccharide from Astragalus radix herb residue. Molecules. https://doi.org/10.3390/molecules24203644
Li S, Ren LN, Zhu XD, Li JL, Zhang L, Wang XF, Gao F, Zhou GH (2019b) Immunomodulatory effect of gamma-irradiated Astragalus polysaccharides on immunosuppressed broilers. Anim Sci J 90:117–127. https://doi.org/10.1111/asj.13133
Li S, Wang XF, Ren LN, Li JL, Zhu XD, Xing T, Zhang L, Gao F, Zhou GH (2019c) Protective effects gamma-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult Sci 98:6400–6410. https://doi.org/10.3382/ps/pez478
Li W, Song K, Wang S, Zhang C, Zhuang M, Wang Y, Liu T (2019d) Anti-tumor potential of Astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater Sci Eng C Mater Biol Appl 98:685–695. https://doi.org/10.1016/j.msec.2019.01.025
Li CS, Talapphet N, Palanisamy S, Ma N, Cho ML, You S (2020a) The relationship between structural properties and activation of RAW264.7 and natural killer (NK) cells by sulfated polysaccharides extracted from Astragalus membranaceus roots. Process Biochem 97:140–148. https://doi.org/10.1016/j.procbio.2020.06.021
Li K, Cao YX, Jiao SM, Du GH, Du YG, Qin XM (2020b) Structural Characterization and Immune Activity Screening of Polysaccharides With Different Molecular Weights From Astragali Radix. Front Pharmacol. 11https://doi.org/10.3389/fphar.2020.582091
Li K, Cui LJ, Cao YX, Li SY, Shi LX, Qin XM, Du YG (2020c) UHPLC Q-exactive MS-based serum metabolomics to explore the effect mechanisms of immunological activity of Astragalus polysaccharides with different molecular weights. Front Pharmacol. https://doi.org/10.3389/fphar.2020.595692
Li K, Li S, Du Y, Qin X (2020d) Screening and structure study of active components of Astragalus polysaccharide for injection based on different molecular weights. J Chromatogr B 1152:122255. Analytical technologies in the biomedical and life scienceshttps://doi.org/10.1016/j.jchromb.2020.122255
Li WF, Hu XY, Wang SP, Jiao ZR, Sun TY, Liu TQ, Song KD (2020e) Characterization and anti-tumor bioactivity of Astragalus polysaccharides by immunomodulation. Int J Biol Macromol 145:985–997. https://doi.org/10.1016/j.ijbiomac.2019.09.189
Li YL, Xu YJ, Pan C, Ren ZZ, Yang XJ (2020f) TRIF is essential for the anti-inflammatory effects of Astragalus polysaccharides on LPS-infected Caco2 cells. Int J Biol Macromol 159:832–838. https://doi.org/10.1016/j.jbiomac.2020.05.005
Li L, Xu WH, Yi CJ, Cheng YC, Xin HW, Xue HM, Li CK, Fang XY, Yang LM, Chen C, Yang M (2021a) Astragalus polysaccharide has a protective effect on hematopoiesis in an irradiated mouse model and decreases apoptosis in megakaryocytes. Mol Med Rep. https://doi.org/10.3892/mmr.2020.11653
Li Y, Ran C, Wei KJ, Xie YD, Xie MX, Zhou W, Yang YL, Zhang Z, Lv HY, Ma XF, Zhou ZG (2021b) The effect of Astragalus polysaccharide on growth, gut and liver health, and anti-viral immunity of zebrafish. Aquaculture. https://doi.org/10.1016/j.aquaculture.2021.736677
Li Y, Wang X, Ma X, Liu C, Wu J, Sun C (2021c) Natural polysaccharides and their derivates: a promising natural adjuvant for tumor immunotherapy. Front Pharmacol 12:621813. https://doi.org/10.3389/fphar.2021.621813
Li ZX, Zhao GD, Xiong W, Linghu KG, Ma QS, Cheang WS, Yu H, Wang Y (2021d) Immunomodulatory effects of a new whole ingredients extract from Astragalus: a combined evaluation on chemistry and pharmacology. Chin Med 16:12. https://doi.org/10.1186/s13020-021-00440-3
Liao JZ, Li CY, Huang J, Liu WP, Chen HC, Liao SY, Chen HY, Rui W (2018) Structure characterization of honey-processed Astragalus polysaccharides and its anti-inflammatory activity in vitro. Molecules. https://doi.org/10.3390/molecules23010168
Liao LY, Li J, Li J, Huang YF, Wu YJ (2021) Effects of Astragalus polysaccharides on intestinal morphology and intestinal immune cells of Muscovy ducklings infected with Muscovy duck reovirus. Poult Sci 100:64–72. https://doi.org/10.1016/j.psj.2020.10.021
Lim JD, Yu CY, Kim SH, Chung IM (2016) Structural characterization of an intestinal immune system-modulating arabino-3,6-galactan-like polysaccharide from the above-ground part of Astragalus membranaceus (Bunge). Carbohydr Polym 136:1265–1272. https://doi.org/10.1016/j.carbpol.2015.10.029
Lim SM, Park HB, Jin JO (2021) Polysaccharide from Astragalus membranaceus promotes the activation of human peripheral blood and mouse spleen dendritic cells. Chin J Nat Med 19:56–62. https://doi.org/10.1016/s1875-5364(21)60006-7
Liu QY, Yao YM, Zhang SW, Sheng ZY (2011) Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB(low) DCs in vitro. J Ethnopharmacol 136:457–464. https://doi.org/10.1016/j.jep.2010.06.041
Liu AJ, Yu J, Ji HY, Zhang HC, Zhang Y, Liu HP (2018a) Extraction of a novel cold-water-soluble polysaccharide from Astragalus membranaceus and its antitumor and immunological activities. Molecules. https://doi.org/10.3390/molecules23010062
Liu DD, Su JR, Lin JS, Qian G, Chen XX, Song SQ, Huang KH (2018b) Activation of AMPK-dependent SIRT-1 by Astragalus polysaccharide protects against ochratoxin A-induced immune stress in vitro and in vivo. Int J Biol Macromol 120:683–692. https://doi.org/10.1016/j.ijbiomac.2018.08.156
Liu C, Luo J, Xue RY, Guo L, Nie L, Li S, Ji L, Ma CJ, Chen DQ, Miao K, Zou QM, Li HB (2019a) The mucosal adjuvant effect of plant polysaccharides for induction of protective immunity against Helicobacter pylori infection. Vaccine 37:1053–1061. https://doi.org/10.1016/j.vaccine.2018.12.066
Liu L, Hu L, Yao Z, Qin Z, Idehara M, Dai Y, Kiyohara H, Yamada H, Yao X (2019b) Mucosal immunomodulatory evaluation and chemical profile elucidation of a classical traditional Chinese formula, Bu-Zhong-Yi-Qi-Tang. J Ethnopharmacol 228:188–199. https://doi.org/10.1016/j.jep.2018.08.003
Liu X, Ma J, Ding G, Gong Q, Wang Y, Yu H, Cheng X (2021) Microglia polarization from M1 toward M2 phenotype is promoted by Astragalus polysaccharides mediated through inhibition of miR-155 in experimental autoimmune encephalomyelitis. Oxid Med Cell Longev 2021:5753452. https://doi.org/10.1155/2021/5753452
Liu J, Zhang PJ, Wang B, Lu YT, Li L, Li YH, Liu SJ (2022) Evaluation of the effects of Astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp Biochem Physiol C. https://doi.org/10.1016/j.cbpc.2021.109249
Locatelli F, Bauquet A, Palumbo G, Moretta F, Bertaina A (2013) Negative depletion of alpha/beta(+) T cells and of CD19 + B lymphocytes: a novel frontier to optimize the effect of innate immunity in HLA-mismatched hematopoietic stem cell transplantation. Immunol Lett 155:21–23. https://doi.org/10.1016/j.imlet.2013.09.027
Lu Y, Xing QQ, Xu JY, Ding D, Zhao X (2016) Astragalus polysaccharide modulates ER stress response in an OVA-LPS induced murine model of severe asthma. Int J Biol Macromol 93:995–1006. https://doi.org/10.1016/j.ijbiomac.2016.09.058
Luo T, Qin J, Liu M, Luo J, Ding F, Wang ML, Zheng LM (2015) Astragalus polysaccharide attenuates lipopolysaccharide-induced inflammatory responses in microglial cells: regulation of protein kinase B and nuclear factor-kappa B signaling. Inflamm Res 64:205–212. https://doi.org/10.1007/s00011-015-0798-9
Luo D, Yang N, Liu Z, Li T, Wang H, Ge M, Zhang R (2021) Effects of Astragalus polysaccharide on intestinal inflammatory damage in goslings infected with gosling plague. Br Poult Sci 62:353–360. https://doi.org/10.1080/00071668.2020.1859094
Lv X, Chen D, Yang L, Zhu N, Li J, Zhao J, Hu Z, Wang FJ, Zhang LW (2016) Comparative studies on the immunoregulatory effects of three polysaccharides using high content imaging system. Int J Biol Macromol 86:28–42. https://doi.org/10.1016/j.ijbiomac.2016.01.048
Marfil-Garza BA, Hefler J, De Leon MB, Pawlick R, Dadheech N, Shapiro AMJ (2021) Progress in translational regulatory T cell therapies for type 1 diabetes and islet transplantation. Endocr Rev 42:198–218. https://doi.org/10.1210/endrev/bnaa028
Meng FX, Xu PJ, Wang X, Huang Y, Wu LY, Chen YL, Teng LR, Wang D (2017) Investigation on the immunomodulatory activities of Sarcodon imbricatus extracts in a cyclophosphamide (CTX)-induced immunosuppressanted mouse model. Saudi Pharm J 25:460–463. https://doi.org/10.1016/j.jsps.2017.04.006
Meng YB, Zhang YY, Jia N, Qiao HR, Zhu MH, Meng QH, Lu Q, Zu YG (2018) Synthesis and evaluation of a novel water-soluble high Se-enriched Astragalus polysaccharide nanoparticles. Int J Biol Macromol 118:1438–1448. https://doi.org/10.1016/j.ijbiomac.2018.06.153
Moreno-Mendieta S, Guillen D, Hernandez-Pando R, Sanchez S, Rodriguez-Sanoja R (2017) Potential of glucans as vaccine adjuvants: a review of the alpha-glucans case. Carbohydr Polym 165:103–114. https://doi.org/10.1016/j.carbpol.2017.02.030
Mu JY, Li YD, Zhao XK, Li J, Yang AJ (2019) Astragalus polysaccharide restores activation of NK cells in radiation therapy of tumors. Int J Clin Exp Med 12:8609–8621
Niu Y, Wang H, Xie Z, Whent M, Gao X, Xian Z, Zou S, Yao W, Yu L (2011) Structural analysis and bioactivity of a polysaccharide from the roots of Astragalus membranaceus (Fisch) Bge. var. mongolicus (Bge.) Hsiao. Food Chem 128:620–626. https://doi.org/10.1016/j.foodchem.2011.03.055
Pang GB, Chen C, Liu Y, Jiang TY, Yu H, Wu YX, Wang YY, Wang FJ, Liu ZY, Zhang LW (2019a) Bioactive polysaccharide nanoparticles improve radiation-induced abscopal effect through manipulation of dendritic cells. ACS Appl Mater Interfaces 11:42661–42670. https://doi.org/10.1021/acsami.9b16814
Pang GB, Zhang SL, Zhou XP, Yu H, Wu YX, Jiang TY, Zhang XH, Wang FJ, Wang YY, Zhang LW (2019b) Immunoactive polysaccharide functionalized gold nanocomposites promote dendritic cell stimulation and antitumor effects. Nanomedicine 14:1291–1306. https://doi.org/10.2217/nnm-2018-0390
Peng Y, Song Y, Wang Q, Hu Y, He Y, Ren D, Wu L, Liu S, Cong H, Zhou H (2019) In vitro and in vivo immunomodulatory effects of fucoidan compound agents. Int J Biol Macromol 127:48–56. https://doi.org/10.1016/j.ijbiomac.2018.12.197
Poupot R, Goursat C, Fruchon S (2018) Multivalent nanosystems: targeting monocytes/macrophages. Int J Nanomed 13:5511–5521. https://doi.org/10.2147/ijn.s146192
Qin QJ, Niu JY, Wang ZX, Xu WJ, Qiao ZD, Gu Y (2012) Astragalus membranaceus extract activates immune response in macrophages via heparanase. Molecules 17:7232–7240. https://doi.org/10.3390/molecules17067232
Qiu CJ, Cheng YX (2019) Effect of Astragalus membranaceus polysaccharide on the serum cytokine levels and spermatogenesis of mice. Int J Biol Macromol 140:771–774. https://doi.org/10.1016/j.ijbiomac.2019.08.191
Ren L, Wang X, Li S, Li J, Zhu X, Zhang L, Gao F, Zhou G (2018) Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. Int J Biol Macromol 120:641–649. https://doi.org/10.1016/j.ijbiomac.2018.08.138
Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais S, Parmiani G (2002) Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev 188:97–113. https://doi.org/10.1034/j.1600-065X.2002.18809.x
Saijo K, Crotti A, Glass CK (2013) Regulation of microglia activation and deactivation by nuclear receptors. Glia 61:104–111. https://doi.org/10.1002/glia.22423
Sakaguchi S, Tanaka S, Tanaka A, Ito Y, Maeda S, Sakaguchi N, Hashimoto M (2011) Thymus, innate immunity and autoimmune arthritis: interplay of gene and environment. FEBS Lett 585:3633–3639. https://doi.org/10.1016/j.febslet.2011.10.026
Scheffer DD, Latini A (2020) Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta. https://doi.org/10.1016/j.bbadis.2020.165823
Shan CL, Sun BD, Dalloul RA, Zhai ZC, Sun P, Li MH, Yang SB, Luan WM (2019) Effect of the oral administration of Astragalus polysaccharides on jejunum mucosal immunity in chickens vaccinated against Newcastle disease. Microb Pathog. https://doi.org/10.1016/j.micpath.2019.103621
Shang N, Figini M, Shangguan J, Wang B, Sun C, Pan L, Ma QH, Zhang ZL (2017) Dendritic cells based immunotherapy. Am J Cancer Res 7:2091–2102. https://doi.org/10.1080/08830180600992456
She YL, Zhao XW, Wu PF, Xue L, Liu Z, Zhu M, Yang J, Li YL (2021) Astragalus polysaccharide protects formaldehyde-induced toxicity by promoting NER pathway in bone marrow mesenchymal stem cells. Folia Histochem Cytobiol 59:124–133. https://doi.org/10.5603/FHC.a2021.0013
Shi L, Yin F, Xin X, Mao S, Sun X (2014) Astragalus polysaccharide protects astrocytes from being infected by HSV-1 through TLR3/NF-κB signaling pathway. Evid Based Complement Altern Med. https://doi.org/10.1155/2014/285356
Shi Q, Zhao L, Zhang L (2019) Astragalus polysaccharide strengthens the inflammatory and immune responses of Brucella suis S2-infected mice and macrophages. Exp Ther Med 18:4295–4302. https://doi.org/10.3892/etm.2019.8084
Shi F, Lu ZJ, Yang MX, Li F, Zhan FB, Zhao LJ, Li YN, Li QQ, Li JT, Li J, Lin L, Qin ZD (2021) Astragalus polysaccharides mediate the immune response and intestinal microbiota in grass carp (Ctenopharyngodon idellus). Aquaculture. https://doi.org/10.1016/j.aquaculture.2020.736205
Shokati E, Shokri MR, Entezami K, Khorrami S, Amani M, Motallebnezhad M, Safari E (2021) Immunomodulatory effects of Astragalus polysaccharide on Human peripheral blood mononuclear cells co-cultured with cervical cancer cell line. J Tradit Chin Med 41:684–694. https://doi.org/10.19852/j.cnki.jtcm.2021.05.004
Song Y, Yang J, Bai WL, Ji WY (2011) Antitumor and immunoregulatory effects of Astragalus on nasopharyngeal carcinoma in vivo and in vitro. Phytother Res 25:909–915. https://doi.org/10.1002/ptr.3354
Song J, Li J, Zheng SR, Jin Y, Huang Y (2013) Anti-inflammatory and immunoregulatory effects of Yupingfeng (cZ parts per thousand a +/- e Z) pound powder on chronic bronchitis rats. Chin J Integr Med 19:353–359. https://doi.org/10.1007/s11655-013-1442-6
Song HC, Zhang SQ, Yang BT, Liu YH, Kang YH, Li Y, Qian AD, Yuan ZH, Cong B, Shan XF (2022) Effects of four different adjuvants separately combined with Aeromonas veronii inactivated vaccine on haematoimmunological state, enzymatic activity, inflammatory response and disease resistance in crucian carp. Fish Shellfish Immunol 120:658–673. https://doi.org/10.1016/j.fsi.2021.09.003
Sun S, Zheng K, Zhao H, Lu C, Liu B, Yu C, Zhang G, Bian Z, Lu A, He X (2014) Regulatory effect of Astragalus polysaccharides on intestinal intraepithelial γδT cells of tumor bearing mice. Molecules 19:15224–15236. https://doi.org/10.3390/molecules190915224
Sun H, Ni XQ, Song X, Wen B, Zhou Y, Zou FQ, Yang MY, Peng ZR, Zhu H, Zeng Y, Wang HS, Fu XC, Shi YD, Yin ZQ, Pan KC, Jing B, Zeng D, Wang P (2016) Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl Microbiol Biotechnol 100:8105–8120. https://doi.org/10.1007/s00253-016-7619-0
Sun ZC, Fu YX, Peng H (2018) Targeting tumor cells with antibodies enhances anti-tumor immunity. Biophys Rep 4:243–253. https://doi.org/10.1007/s41048-018-0070-2
Sun Y, Jing YY, Huang MW, Ma JY, Peng XY, Wang JY, Li GL, Cheng XD (2019) The PD-1/PD-Ls pathway is up-regulated during the suppression of experimental autoimmune encephalomyelitis treated by Astragalus polysaccharides. J Neuroimmunol 332:78–90. https://doi.org/10.1016/j.jneuroim.2019.03.019
Tang S, Liu W, Zhao QQ, Li KD, Zhu JY, Yao WB, Gao XD (2021) Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2020.113280
Tesher MS, Esteban Y, Henderson TO, Villanueva G, Onel KB (2019) Mucosal-associated lymphoid tissue (MALT) lymphoma in association with pediatric primary sjogren syndrome: 2 cases and review. J Pediatr Hematol Oncol 41:413–416. https://doi.org/10.1097/mph.0000000000001321
Tian QE, Li HD, Yan M, Cai HL, Tan QY, Zhang WY (2012) Astragalus polysaccharides can regulate cytokine and P-glycoprotein expression in H22 tumor-bearing mice. World J Gastroenterol 18:7079–7086. https://doi.org/10.3748/wjg.v18.i47.7079
Tsao SM, Wu TC, Chen J, Chang F, Tsao T (2021) Astragalus polysaccharide injection (PG2) normalizes the neutrophil-to-lymphocyte ratio in patients with advanced lung cancer receiving immunotherapy. Integr Cancer Ther 20:1534735421995256. https://doi.org/10.1177/1534735421995256
Van Den Bossche J, O’neill LA, Menon D (2017) Macrophage immunometabolism: where are we (going)? Trends Immunol 38:395–406. https://doi.org/10.1016/j.it.2017.03.001
Wan XH, Yin YM, Zhou CZ, Hou L, Cui QH, Zhang XP, Cai XQ, Wang YL, Wang LZ, Tian JZ (2022) Polysaccharides derived from Chinese medicinal herbs: a promising choice of vaccine adjuvants. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2021.118739
Wang ZX, Liu ZJ, Zhou LJ, Long TT, Zhou X, Bao YX (2017) Immunomodulatory effect of APS and PSP is mediated by Ca2+-cAMP and TLR4/NF-κB signaling pathway in macrophage. Int J Biol Macromol 94:283–289. https://doi.org/10.1016/j.ijbiomac.2016.10.018
Wang J, Jia JY, Song L, Gong X, Xu JP, Yang M, Li MH (2019) Extraction, structure, and pharmacological activities of Astragalus polysaccharides. Appl Sci. https://doi.org/10.3390/app9010122
Wang SS, Sun YQ, Zhang JJ, Cui XM, Xu ZL, Ding DJ, Zhao LM, Li WT, Zhang WF (2020) Astragalus polysaccharides/chitosan microspheres for nasal delivery: preparation, optimization, characterization, and pharmacodynamics. Front Pharmacol. https://doi.org/10.3389/fphar.2020.00230
Wang D, Liu Y, Zhao W (2021) The adjuvant effects on vaccine and the immunomodulatory mechanisms of polysaccharides from traditional Chinese medicine. Front Mol Biosci. https://doi.org/10.3389/fmolb.2021.655570
Wei W, Xiao HT, Bao WR, Ma DL, Leung CH, Han XQ, Ko CH, Lau CB, Wong CK, Fung KP, Leung PC, Bian ZX, Han QB (2016) TLR-4 may mediate signaling pathways of Astragalus polysaccharide RAP induced cytokine expression of RAW264.7 cells. J Ethnopharmacol 179:243–252. https://doi.org/10.1016/j.jep.2015.12.060
Wei W, Li ZP, Bian ZX, Han QB (2019) Astragalus polysaccharide RAP induces macrophage phenotype polarization to M1 via the notch signaling pathway. Molecules. https://doi.org/10.3390/molecules24102016
Wu J, Xu H, Zhang L, Zhang X (2016) Radix Astragali and Tanshinone help carboplatin inhibit B16 tumor cell growth. Technol Cancer Res Treat 15:583–588. https://doi.org/10.1177/1533034615588682
Wu SR, Ren XC, Li YL, Guo W, Lei XY, Yao JH, Yang XJ (2017) Effect of dietary Astragalus polysaccharide supplements on testicular miRNA expression profiles and enzymatic changes of breeder cocks. Sci Rep 7:38864. https://doi.org/10.1038/srep38864
Xia WU, Yang W, Zhang L, Li DX (2011) Effect of Astragalus polysaccharide segments with different molecular weight on systematic/mucosal immunization in immunodepressive mice. Chin J Exp Tradit Med Formulae. https://doi.org/10.13422/j.cnki.syfjx.2011.18.015
Xie JH, Jin ML, Morris GA, Zha XQ, Chen HQ, Yi Y, Li JE, Wang ZJ, Gao J, Nie SP, Shang P, Xie MY (2016) Advances on bioactive polysaccharides from medicinal plants. Crit Rev Food Sci Nutr 56:S60–S84. https://doi.org/10.1080/10408398.2015.1069255
Xiong J, Jiang BL, Luo Y, Zou JZ, Gao X, Xu D, Du Y, Hao L (2020) Multifunctional nanoparticles encapsulating Astragalus polysaccharide and gold nanorods in combination with focused ultrasound for the treatment of breast cancer. Int J Nanomed 15:4151–4169. https://doi.org/10.2147/ijn.s246447
Xu SW, Wusiman A, Liu ZG, Gu PF, Ni HY, Zhang Y, Hu YL, Liu JG, Wu Y, Wang DY (2019) pH-responsive Astragalus polysaccharides-loaded poly(lactic-co-glycolic acid) nanoparticles and their in vitro immunogenicity. Int J Biol Macromol 125:865–875. https://doi.org/10.1016/j.ijbiomac.2018.12.156
Xu J, Zhang Q, Li Z, Gao Y, Pang Z, Wu Y, Li G, Lu D, Zhang L, Li D (2021) Astragalus polysaccharides attenuate ovalbumin-induced allergic rhinitis in rats by inhibiting NLRP3 inflammasome activation and NOD2-mediated NF-κB activation. J Med Food 24:1–9. https://doi.org/10.1089/jmf.2020.4750
Xue LG, Wang D, Zhang DX, Ju AQ, Duan AY, Xing JH, Qin YJ, Yang SB, Luan WM (2020) The immune adjuvant effect of Astragalus polysaccharide onin ovoinjection of Newcastle disease vaccine. J Anim Physiol Anim Nutr 104:1719–1726. https://doi.org/10.1111/jpn.13388
Xu-Monette ZY, Zhou JF, Young KH (2018) PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 131:68–83. https://doi.org/10.1182/blood-2017-07-740993
Yakubogullari N, Coven FO, Cebi N, Coven F, Coven N, Genc R, Bedir E, Nalbantsoy A (2021) Evaluation of adjuvant activity of Astragaloside VII and its combination with different immunostimulating agents in Newcastle Disease vaccine. Biologicals 70:28–37. https://doi.org/10.1016/j.biologicals.2021.01.005
Yakuboğulları N, Genç R, öven F, Nalbantsoy A, Bedir E (2019) Development of adjuvant nanocarrier systems for seasonal influenza A (H3N2) vaccine based on Astragaloside VII and gum tragacanth (APS). Vaccine 37:3638–3645. https://doi.org/10.1016/j.vaccine.2019.05.038
Yang SB, Qin YJ, Ma X, Luan WM, Sun P, Ju AQ, Duan AY, Zhang YN, Zhao DH (2021) Effects of in ovo Injection of Astragalus polysaccharide on the intestinal development and mucosal immunity in broiler chickens. Front Veterinary Sci. https://doi.org/10.3389/fvets2021738816
Ye N, Cruz J, Peng XY, Ma JY, Zhang AM, Cheng XD (2021) Remyelination is enhanced by Astragalus polysaccharides through inducing the differentiation of oligodendrocytes from neural stem cells in cuprizone model of demyelination. Brain Res. https://doi.org/10.1016/j.brainres.2021.147459
Yin JY, Chan BCL, Yu H, Lau IYK, Han XQ, Cheng SW, Wong CK, Lau CBS, Xie MY, Fung KP, Leung PC, Han QB (2012) Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali. Carbohydr Polym 87:667–675. https://doi.org/10.1016/j.carbpol.2011.08.045
Yu ZM, Huang XH, Yan CQ, Gao J, Liang ZS (2016) Effect of Fuzheng Jiedu granule on immunological function and level of immune-related cytokines in immune-suppressed mice. J Integr Agric 15:650–657. https://doi.org/10.1016/S2095-3119(14)60971-0
Yu J, Ji HY, Liu AJ (2018) Alcohol-soluble polysaccharide from Astragalus membranaceus: preparation, characteristics and antitumor activity. Int J Biol Macromol 118:2057–2064. https://doi.org/10.1016/j.ijbiomac.2018.07.073
Yu J, Dong XD, Jiao JS, Ji HY, Liu AJ (2021) Antitumor and immunoregulatory activities of a novel polysaccharide from Astragalus membranaceus on S180 tumor-bearing mice. Int J Biol Macromol 189:930–938. https://doi.org/10.1016/j.ijbiomac.2021.08.099
Zahran E, Risha E, Abdelhamid F, Mahgoub HA, Ibrahim T (2014) Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 38:149–157. https://doi.org/10.1016/j.fsi.2014.03.002
Zhai QQ, Li J, Feng YY, Ge QQ (2019) Evaluation of combination effects of Astragalus polysaccharides and florfenicol against acute hepatopancreatic necrosis disease-causing strain of Vibrio parahaemolyticus in Litopenaeus vannamei. Fish Shellfish Immunol 86:374–383. https://doi.org/10.1016/j.fsi.2018.11.065
Zhang W, Ma K (2020) Altered frequency of NK cells and treg cells by Astragalus polysaccharide combined with budesonide in asthma model mice. Advances in Polymer Technology 220:1–6. https://doi.org/10.1155/2020/1763245
Zhang PP, Meng ZT, Wang LC, Guo LM, Li K (2015) Astragalus polysaccharide promotes the release of mature granulocytes through the L-selectin signaling pathway. Chin Med 10:17. https://doi.org/10.1186/s13020-015-0043-z
Zhang PJ, Wang J, Wang WX, Liu XH, Liu HY, Li XT, Wu XH (2017) Astragalus polysaccharides enhance the immune response to avian infectious bronchitis virus vaccination in chickens. Microb Pathog 111:81–85. https://doi.org/10.1016/j.micpath.2017.08.023
Zhang YM, Liu YQ, Liu DL, Zhang LY, Qin L, Zhang ZM, Su Y, Yan CL, Luo YL, Li JT, Xie XD, Guan QL (2019a) The effects of Astragalus polysaccharide on bone marrow-derived mesenchymal stem cell proliferation and morphology induced by A549 lung cancer cells. Med Sci Monit 25:4110–4121. https://doi.org/10.12659/msm.914219
Zhang ZX, Zhang L, Xu HS (2019b) Effect of Astragalus polysaccharide in treatment of diabetes mellitus: a narrative review. J Tradit Chin Med 39:133–138. https://doi.org/10.19852/j.cnki.jtcm.2019.01.017
Zhang WN, Zhang MX, Cheng AY, Hao ET, Huang XH, Chen XH (2020a) Immunomodulatory and antioxidant effects of Astragalus polysaccharide liposome in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol 100:126–136. https://doi.org/10.1016/j.fsi.2020.03.004
Zhang YM, Zhang LY, Zhou H, Li YY, Wei KX, Li CH, Zhou T, Wang JF, Wei WJ, Hua JR, He Y, Hong T, Liu YQ (2020b) Astragalus polysaccharide inhibits radiation-induced bystander effects by regulating apoptosis in Bone Mesenchymal Stem Cells (BMSCs). Cell Cycle 19:3195–3207. https://doi.org/10.1080/15384101.2020.1838793
Zhang CH, Yang X, Wei JR, Chen NMH, Xu JP, Bi YQ, Yang M, Gong X, Li ZY, Ren K, Han QH, Zhang L, Li X, Ji MY, Wang CC, Li MH (2021) Ethnopharmacology, Phytochemistry, Pharmacology, Toxicology and Clinical Applications of Radix Astragali. Chin J Integr Med 27:229–240. https://doi.org/10.1007/s11655-019-3032-8
Zhao LH, Ma ZX, Zhu J, Yu XH, Weng DP (2011) Characterization of polysaccharide from Astragalus radix as the macrophage stimulator. Cell Immunol 271:329–334. https://doi.org/10.1016/j.cellimm.2011.07.011
Zhao ED, Xu HB, Wang L, Kryczek I, Wu K, Hu Y, Wang GB, Zou WP (2012) Bone marrow and the control of immunity. Cell Mol Immunol 9:11–19. https://doi.org/10.1038/cmi.2011.47
Zhao WX, Chen LJ, Cui N, Ji XM, Jiang HQ, Deng TT, Wang SJ (2019) Polysaccharides from radix astragali exert immunostimulatory effects to attenuate the dampness stagnancy due to spleen deficiency syndrome. Pharmacogn Mag 15:500–506. https://doi.org/10.4103/pm.pm_503_18
Zheng Y, Ren W, Zhang L, Zhang Y, Liu D, Liu Y (2020) A review of the pharmacological action of Astragalus polysaccharide. Front Pharmacol 11:349. https://doi.org/10.3389/fphar.2020.00349
Zhou X, Xu Y, Yang G, Li F (2011) Increased galectin-1 expression in muscle of Astragalus polysaccharide-treated Type 1 diabetic mice. J Nat Med 65:500–507. https://doi.org/10.1007/s11418-011-0527-9
Zhou L, Liu Z, Wang Z, Yu S, Long T, Zhou X, Bao Y (2017) Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci Rep. https://doi.org/10.1038/srep44822
Zhou X, Liu Z, Long T, Zhou L, Bao Y (2018) Immunomodulatory effects of herbal formula of Astragalus polysaccharide (APS) and polysaccharopeptide (PSP) in mice with lung cancer. Int J Biol Macromol 106:596–601. https://doi.org/10.1016/j.ijbiomac.2017.08.054
Zhou YM, Zong YH, Liu ZH, Zhao HH, Zhao XS, Wang J (2021) Astragalus polysaccharides enhance the immune response to OVA antigen in BALB/c mice. Biomed Res Int. https://doi.org/10.1155/2021/9976079
Zhu N, Lv X, Wang Y, Li J, Liu Y, Lu W, Yang L, Zhao J, Wang F, Zhang LW (2016) Comparison of immunoregulatory effects of polysaccharides from three natural herbs and cellular uptake in dendritic cells. Int J Biol Macromol 93:940–951. https://doi.org/10.1016/j.ijbiomac.2016.09.064
Zhu J, Zhang YY, Fan FH, Wu GY, Xiao Z, Zhou HQ (2018) Tumor necrosis factor-alpha-induced protein 8-like-2 is involved in the activation of macrophages by Astragalus polysaccharides in vitro. Mol Med Rep 17:7428–7434. https://doi.org/10.3892/mmr.2018.8730
Zhu WT, Zhang YQ, Zhang JC, Yuan GL, Liu XL, Ai TS, Su JG (2019) Astragalus polysaccharides, chitosan and poly(I:C) obviously enhance inactivated Edwardsiella ictaluri vaccine potency in yellow catfish Pelteobagrus fulvidraco. Fish Shellfish Immunol 87:379–385. https://doi.org/10.1016/j.fsi.2019.01.033
Zhuge ZY, Dong YP, Li LA, Jin TM (2017) Effects of Astragalus polysaccharide on the adhesion-related immune response of endothelial cells stimulated with CSFV in vitro. PeerJ 5:e3862–e3862. https://doi.org/10.7717/peerj.3862
Acknowledgements
This work was supported by the Sichuan Provincial Administration of Traditional Chinese Medicine (Grant No. 2018QN019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chun-xiao Li, Ying Liu and Yu-zhen Zhang contributed equally to this study and should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Li, Cx., Liu, Y., Zhang, Yz. et al. Astragalus polysaccharide: a review of its immunomodulatory effect. Arch. Pharm. Res. 45, 367–389 (2022). https://doi.org/10.1007/s12272-022-01393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-022-01393-3