Abstract
Adiponectin, an adipose tissue-derived hormone, exhibits a modulatory effect on cell death/survival and possesses potent anti-inflammatory properties. However, the underlying molecular mechanisms remain elusive. Sestrin2, a stress-inducible metabolic protein, has shown cytoprotective and inflammation-modulatory effects under stressful conditions. In this study, we examined the role of sestrin2 signaling in the modulation of cell survival and inflammatory responses by globular adiponectin (gAcrp) in macrophages. We observed that gAcrp induced a significant increase in sestrin2 expression in both RAW 264.7 murine macrophages and primary murine macrophages. Notably, gAcrp treatment markedly increased expression of hypoxia inducible factor-1 α (HIF-1α) and gene silencing of HIF-1α blocked sestrin2 induction by gAcrp. In addition, pretreatment with a pharmacological inhibitor of ERK or PI3K abrogated both sestrin2 and HIF-1α expression by gAcrp, indicating that ERK/PI3K-mediated HIF-1α signaling pathway plays a critical role in sestrin2 induction by gAcrp. Furthermore, sestrin2 induction is implicated in autophagy activation, and knockdown of sestrin2 prevented enhanced cell viability by gAcrp. Moreover, gene silencing of sestrin2 caused restoration of gAcrp-induced expression of anti-inflammatory genes in a gene-selective manner. Taken together, these results indicate that sestrin2 induction critically contributes to cell survival and anti-inflammatory responses by gAcrp in macrophages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose tissue has long been considered an organ for excessive fat storage. However, accumulating evidence indicates that adipose tissue acts as a dynamic endocrine organ, which plays significant roles in the modulation of systemic metabolism (Coelho et al. 2013). The metabolic and endocrine effects of adipose tissue are mediated via the secretion of various hormones, collectively called adipokines or adipocytokines (Tsai et al. 2020; Stern et al. 2016). While adipokines were originally reported to play decisive roles in the modulation of lipid metabolism, insulin resistance, and other metabolic syndrome (Stern et al. 2016), a growing body of recent evidence has suggested that they also modulate various other physiological functions in a paracrine or endocrine manner, including regulation of inflammation (Ouchi et al. 2011) and development and/or progression of various types of cancers (Dalamaga et al. 2012). Importantly, imbalance in the secretion of adipokines or dysregulation of these hormones are accompanied with a number of pathological conditions (Deng and Scherer 2010).
Of the various adipokines, adiponectin is the most abundant adipokine in the circulation, accounting for approximately 0.01% of total serum proteins (Arita et al. 1999). In many cases, adiponectin exhibits beneficial metabolic effects, including enhanced insulin sensitivity (Berg et al. 2001), and stimulating glucose utilization and fatty acid oxidation (Yanai and Yoshida 2019). Notably, low levels of plasma adiponectin correlate to the development of various metabolic syndromes, such as type 2 diabetes and obesity (Osei et al. 2005; Sepilian and Nagamani 2005). Increasing recent evidence has also demonstrated that adiponectin possesses potent anti-inflammatory properties. A number of previous studies have extensively studied the inhibitory effects of adiponectin on inflammatory cytokines production, which are mediated via activation of AMPK and heme oxygenase-1 (HO-1) signaling, inhibition of pro-inflammatory NF-κB and AP-1 signaling (Wulster-Radcliffe et al. 2004; Jian et al. 2019), and autophagy induction (Park 2018). In addition, adiponectin induces increases in various anti-inflammatory mediators, such as IL-10, IL-1RA, and PPAR-γ (Wolf et al. 2004; Ishtiaq et al. 2019). AMPK and HO-1 signaling pathways have been shown to play critical roles in the suppression of pro-inflammatory mediators and other metabolic effects of adiponectin. However, the mechanisms by which adiponectin induces expression of anti-inflammatory mediators are not clearly understood, and the cell survival effects in macrophages remain elusive. Globular adiponectin (gAcrp) treatment enhances the viability of macrophages (Oh et al. 2020), in addition to its beneficial metabolic effects and anti-inflammatory properties. While mild ER stress induction, sestrin2 induction, and autophagy activation have been shown to act as potential mechanisms for the survival effect of adiponectin in macrophages, detailed molecular mechanisms need to be further elucidated.
Sestrins, a highly conserved family of stress-inducible proteins, modulate various biological responses, including antioxidant responses, aging, and inflammation (Ho et al. 2016). Of the three isoforms identified to date, sestrin2 is the best-characterized for its protective roles against a variety of stressful conditions and noxious stimuli, including hypoxia, nitric oxide exposure, and lipopolysaccharide (LPS), through various mechanisms (Pasha et al. 2017). Sestrin2 induction in response to stressful conditions is mediated via diverse signaling cascades depending on experimental conditions. For instance, p53 (Budanov and Karin 2008; Chen et al. 2019), HIF-1α (Essler et al. 2009; Shi et al. 2016), and Nrf2 (Shin et al. 2012) have been found to induce transcriptional activation of sestrin. In addition, mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signaling are also implicated in sestrin2 induction (Yi et al. 2014; Zhang et al. 2013). Once sestrin2 is induced, it activates diverse signaling cascades and modulates various biological functions as indicated, which ultimately contributes to maintenance of cellular homeostasis. Of the diverse signaling cascades, AMPK/mTORC1 axis plays a pivotal role in mediating the biological responses of sestrin2. AMPK/mTORC1 signaling critically contributes to the prevention of reactive oxygen species (ROS) accumulation by sestrin2 and controls redox homeostasis (Kim et al. 2015a), and is also crucial for sestrin2-mediated regulation of inflammatory responses in macrophages (Yang et al. 2015). Sestrin2 has been considered a promising therapeutic target for many pathological conditions. For instance, overexpression of sestrin2 decreases the mortality rate in sepsis by attenuating inflammation and detoxifying ROS accumulation (Kim et al. 2016). It also alleviates insulin resistance via autophagy induction (Li et al. 2017) and regulates many metabolic dysfunctions by modulating the AMPK/mTORC signaling (Hwang et al. 2017, 2018).
Based on previous reports, it is well established that adiponectin and sestrin2 play important roles in the regulation of cellular homeostasis and are potential targets to modulate metabolic systems. However, the role of sestrin2 signaling in the physiological functions of adiponectin and other potential relationships between adiponectin and sestrin2 are largely unknown. In the present study, we have elucidated the molecular mechanisms underlying sestrin2 induction by globular adiponectin and further demonstrated the crucial role of sestrin2 signaling in the modulation of anti-inflammatory responses and cell survival by adiponectin in macrophages.
Materials and methods
Materials
All the reagents for cell culture were obtained from Hyclone Laboratories (South Logan, UT, USA). Recombinant human globular adiponectin was acquired from Peprotech Inc. (Rocky Hill, NJ, USA). Primary antibodies against LC3I/II, SQSTM1/P62, and total/phosphor-specific AMPKα were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA); Sestrin2, HIF-1α, and β-actin were obtained from Protein Tech (Chicago, IL, USA), Abcam (Cambridge, United Kingdom), and Thermo Scientific, Inc. (Rockford, IL, USA), respectively. Secondary antibodies conjugated with horseradish peroxidase were obtained from Thermo Scientific, Inc. (Rockford, IL, USA). CM2-H2DCFDA and Hank’s Balanced Salts (HBSS) for ROS detection were obtained from Thermo Scientific, Inc. (Rockford, IL, USA), and Sigma Aldrich (St Louis, MO, USA), respectively. SP600125, SB203580, and Dorsomorphin Dihydrochloride (Compound C) were obtained from Tocris Bioscience (Tocris House, IO Centre, Bristol, UK). U0126 and LY294002 were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA) and Sigma Aldrich (St Louis, MO, USA), respectively.
Cell culture
RAW 264.7 murine macrophage cell line was acquired from the Korean Cell Line Bank (Seoul, Korea). Cells were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin-Streptomycin (P/S) at 37 °C in an incubator with 5% CO2. THP-1 and U937 monocytes were acquired from Korean Cell Line Bank (Seoul, Korea). Cells were cultured in RPMI 1640 media supplemented with 10% FBS and 1% penicillin/streptomycin. For differentiation into macrophages, THP-1 and U937 monocytes were incubated with phorbol 12-myristate 13-acetate (PMA, 100 nM) for 72 h followed by a rest period in PMA-free media for at least 24 h before any experiment.
Isolation and culture of murine peritoneal macrophages
All the animal experiments were performed in accordance with the guidelines of the Yeungnam University Institutional Animal Care and Use Committee (IACUC). The experimental protocols were reviewed and approved by the Yeungnam University IACUC (Approval number: 2020-008). The murine macrophages were isolated as described previously (Tilija Pun and Park 2018). In brief, 4% Brewer thioglycollate medium (1 ml) was injected into the peritoneum region of 5- to 6-week-old male C57BL/6 mice. Three days after injection, primary cells were extracted with 10 ml of cold-HBSS without calcium and magnesium and collected by centrifugation at 1500 rpm for 3 min. Cells were then incubated with RBC lysis buffer for 4 min to get rid of red blood cells and mixed with RPMI-1640 media containing 10% Fetal Calf Serum (FCS) and 1% P/S and cultured in an incubator with 5% CO2 at 37 °C.
Transient gene silencing by transfection with small interfering RNAs (siRNA)
RAW 264.7 macrophages were plated in 35-mm dishes at a density of 6 × 105 cells/dish. After overnight incubation, cells were transfected with siRNA targeting sestrin2 or scrambled control siRNA using Hiperfect Transfection Reagent (Qiagen) according to the manufacturer’s instructions. After 24 h of incubation, efficiency of gene silencing was determined by western blot analysis. The siRNA duplexes used in this study were purchased from Bioneer (Daejeon, South Korea). The sequences of the siRNA are shown in Table 1.
Western blot analysis
Cells were seeded in 35-mm dishes at a density of 1 × 106 cells/dish. After treatments with gAcrp or inhibitors as indicated, cells were lysed with RIPA lysis buffer containing Halt protease and phosphatase inhibitor cocktail. Total cellular extracts were used for protein quantitation using a BSA protein assay kit (Thermo Scientific). Proteins (20–30 µg) were loaded onto SDS-PAGE (7–13%), separated by electrophoresis, and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were then incubated with 5% skim milk (in PBS/T) for 1 h to remove non-specific binding, treated with appropriate primary antibody overnight at 4 °C, and finally were incubated with the secondary antibodies for 1 h at room temperature. The blots were incubated with chemiluminescence substrate solution and the images were acquired using the LAS-4000 mini system (Fujifilm, Tokyo, Japan).
RNA isolation, reverse transcription (RT), and quantitative PCR (qPCR)
Expression levels of mRNA of the target genes were measured by reverse transcription-polymerase chain reaction (RT-PCR) essentially as described previously (Pham et al. 2021). Briefly, for total RNA extraction, cells were lysed with Qiagen lysis reagent (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. To synthesize complementary DNA (cDNA), RNA (1 µg) was reverse transcribed using the Go script reverse transcription system (Promega Corporation, Madison, WI, USA). cDNAs were amplified by quantitative real-time PCR using a Roche Light Cycler 1.5 and Absolute SYBR capillary mix system. PCR amplification was carried out for 40 cycles of 95 °C for 15 s, 56 °C for 30 s, and 72 °C for 45 s. Expression levels of the RNAs were analyzed by the comparative threshold method and normalized to the value of glyceraldehyde-3 phosphate dehydrogenase (GAPDH) mRNA. The sequence of the primers used in the PCR reaction are listed in Table 2.
Cell viability assay (MTS assay)
The viability of the macrophages was determined using the CellTiter 96 Aqueous One kit (Promega Corporation, Madison, WI, USA) as previously indicated (Raut and Park 2020). In brief, cells were seeded in 96-well plate at a density of 3 × 104 cells/well. After treatments as indicated, cells were incubated with 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2 H-tetrazolium (MTS, 20 µl) for 2 h at 37 °C. The number of viable cells was determined by measuring the absorbance at 490 nm using a SPECTROstar Nano microplate reader (BMG Labtech Inc., Ortenberg, Germany).
Measurement of reactive oxygen species (ROS) accumulation
Intracellular ROS production was determined by measuring the fluorescence of the chloromethyl derivative of H2DCFDA (CM-H2DCFDA) as described previously (Raut et al. 2019). In brief, cells were seeded in 96-well black plates at a density of 3 × 04 cells/well. After overnight incubation, cells were incubated with 5µM of CM-H2DCFDA dissolved in Hank’s Balanced Salts (HBSS) in the dark for 30 min. After washing with HBSS to remove excess dye, ROS accumulation was assessed by fluorescence intensity using a Flurostar Optima Fluorometer (BMG Labtech, Ortenberg, Germany).
Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM) from at least three independent experiments. Statistical analysis was performed by one way analysis of variance (ANOVA) and Tukey’s multiple comparison tests using the GraphPad Prisms software version 5.01 (LA Jolla, CA, USA). Differences between groups were considered to be significant at P < 0.05.
Results
Globular adiponectin induces increase in sestrin2 expression in macrophages
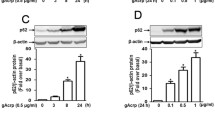
To investigate the role of sestrin2 signaling in the anti-inflammatory and cell survival effects of adiponectin, we first examined the effect of globular adiponectin (gAcrp) on sestrin2 expression in macrophages. As shown in Fig. 1, gAcrp treatment significantly increased sestrin2 expression in a time- and dose-dependent manner in RAW 264.7 macrophages (Fig. 1A, B). In addition, essentially similar effects were observed in primary macrophages isolated from the murine peritoneum (Fig. 1C, D). Moreover, gAcrp markedly enhanced the mRNA level of sestrin2 in RAW 264.7 macrophages (Fig. 1E). We further examined the sestrin2 inducing effect of gAcrp in different types of immune cells and observed that gAcrp also increased sestrin2 expression in U937 and THP-1 human monocytic cell lines in a time-dependent manner (Supplementary Fig. S1). All these results clearly demonstrated that gAcrp induces an increase in sestrin2 expression in macrophages.
Effect of globular adiponectin on sestrin2 expression in RAW 264.7 macrophages and primary peritoneal macrophages. A and B RAW 264.7 macrophages were treated with gAcrp (0.5 µg/ml) for the indicated duration (A) or different concentrations for 24 h (B). C and D Macrophages were isolated from peritoneum of C57BL/6 mice and stimulated with gAcrp (0.5 µg/ml) for the indicated time periods (C) or concentrations for 24 h (D). Sestrin2 protein expression levels were determined by western blot analysis along with β-actin as a loading control. E RAW 264.7 macrophages were treated with gAcrp (0.5 µg/ml) for the indicated time duration. Sestrin2 mRNA levels were analyzed by qRT-PCR and normalized to the level of GAPDH. In western blot analysis, sstrin2 expression levels were quantified by densitometric analysis and are presented in the lower panel of each image. Representative images are presented from three independent experiments. Values are presented as fold change compared to the untreated control cells and are expressed as means ± SEM, n = 3. *Indicates P < 0.05 compared to the control cells
ERK/HIF-1α and PI3K/HIF-1α axis play a critical role in sestrin2 induction by globular adiponectin in RAW 264.7 macrophages
Sestrin2 expression is regulated through various signaling mechanisms depending on experimental conditions. We next elucidated the molecular mechanisms underlying sestrin2 induction by gAcrp. Sestrin2 expression can be determined at the transcriptional level and protein stability (Kim et al. 2015b; Hu et al. 2015). Treatment with gAcrp previously induced an increase in sestrin2 mRNA expression in RAW 264.7 macrophages (Fig. 1E), implying that sestrin2 induction by gAcrp would be determined at transcriptional level, rather than protein stability. In a series of experiments to identify the transcription factor involved in sestrin2 induction, gAcrp induces an increases in HIF-1α expression in a time-dependent manner (Fig. 2A). Moreover, gene silencing of HIF-1α abrogated gAcrp-induced sestrin2 expression at both protein and mRNA levels (Fig. 2B, C), indicating a functional role of HIF-1α in sestrin2 expression. Previous studies have demonstrated that various transcription factors, including p53 and Nrf2, can induce transcriptional activation of sestrin2 depending on the experimental conditions (Maiuri et al. 2009; Kim et al. 2021). Furthermore, we found that gAcrp induced significant increases in expression of Nrf2 and p53 (Supplementary Fig. S2A and 2B). However, blockage of Nrf2 and p53 did not significantly affect sestrin2 expression (Supplementary Fig. 2 C and 2D). These results collectively suggest that HIF-1α induction critically contributes to sestrin2 induction by gAcrp. To further elucidate upstream signaling mechanisms, we investigated the involvement of MAPK signaling and found that treatment with a pharmacological inhibitor of MEK (U0126) prominently blocked sestrin2 induction, while no significant effect was observed by pretreatment with inhibitors of JNK or p38 MAPK (Fig. 2D, F). In addition, pretreatment with LY294002, a pharmacological inhibitor of PI3K, also markedly abolished sestrin2 induction by gAcrp (Fig. 2G). Furthermore, treatment with U0126 or LY294002 also abrogated gAcrp-induced HIF-1α expression (Fig. 2H, I), suggesting that both ERK and PI3K signaling are implicated in gAcrp-induced HIF-1α activation. Taken together, these results indicate that sestrin2 induction by gAcrp is mediated via ERK/HIF-1α and PI3K/HIF-1α axis.
Involvement of HIF-1α, ERK, and PI3K signaling in sestrin2 induction by globular adiponectin in RAW 264.7 macrophages. A Cells were treated with gAcrp (0.5 µg/ml) for the indicated time duration. HIF-1α expression levels were determined by western blot analysis. B and C Cells were transfected with siRNA targeting HIF-1α or scrambled control siRNA for 24 h and were further stimulated with gAcrp (0.5 µg/ml) for additional 8 h. Sestrin2 protein and mRNA expression levels were measured by western blot analysis (B) or RT-PCR (C), respectively. Gene silencing efficiency was monitored after 24 h transfection by western blot analysis and presented in the upper panel of Fig. 2B. D–G Cells were pretreated with inhibitor of MEK (U0126, D), JNK (SP600125, E), p38MAPK (SB203580, F), or PI3K (LY294002, G) for 1 h, followed by further treatment with gAcrp (0.5 µg/ml) for 24 h. Sestrin2 expression levels were determined by western blot analysis. H and I Cells were pretreated with U0126 or LY294002 for 1 h and were treated with gAcrp (0.5 µg/ml) for additional 8 h. HIF-1α expression levels were detected by western blot analysis. In western blot analysis, β-actin was used as a loading control. Values represent fold changes compared with untreated control cells and are expressed as means ± SEM, n = 3. *Denotes P < 0.05 compared with control cells; #Indicates P < 0.05 compared with the cells treated with only gAcrp
Sestrin2 induction contributes to enhanced cell survival by globular adiponectin via autophagy induction in RAW 264.7 macrophages
Previous studies have reported that sestrin2 signaling is implicated in the modulation of cell survival under various experimental conditions. In this study, we explored the possible implication of the sestrin2 signaling in adiponectin-enhanced cell viability in macrophages. For this, we first confirmed that gAcrp enhanced the viability of macrophages in a time- and dose-dependent manner (Supplementary Fig. 3A, B), consistent with previous report. Interestingly, enhanced cell viability by gAcrp was almost completely restored to the normal level by gene silencing of sestrin2 without significant effect by transfection with scrambled control siRNA (Fig. 3A). Essentially similar results were also observed in U937 cells (Supplementary Fig. S4), suggesting a crucial role of the sestrin2 signaling in gAcrp-induced survival of macrophages. Autophagy, an intracellular self-digestive process, modulates cell death and survival in a complex manner. To further examine the mechanisms by which sestrin2 induction contributes to enhanced cell viability by gAcrp, we explored whether sestrin2 signaling is implicated in autophagy activation by adiponectin. As shown in Fig. 3, gene silencing of sestrin2 significantly suppressed gAcrp-induced expression of the genes related with autophagy, including p62 and conversion of LC3II (Fig. 3B, C). Moreover, it was confirmed that inhibition of the autophagic process suppressed gAcrp-induced enhanced viability of macrophages under our experimental conditions (Supplementary Fig. S3C). Collectively, sestrin2 signaling contributes to gAcrp-induced survival of macrophages via autophagy induction.
Role of sestrin2 induction in enhanced cell viability and autophagy induction by globular adiponectin in RAW 264.7 macrophages. A Cells were transfected with siRNA targeting sestrin2 or scrambled control siRNA for 24 h, followed by stimulation with gAcrp (0.5 µg/ml) for additional 24 h. (Upper panel) Gene silencing efficiency was monitored after 24 h transfection by western blot analysis. (Lower panel) Cell viability was determined by MTS assay as indicated in the methods. B and C Cells were transfected with sestrin2 siRNA for 24 h and further stimulated with gAcrp for additional 24 h. Expression levels of LC3 I/II (B) and p62 (C). D Cells were treated with gAcrp (0.5 µg/ml) for the indicated time periods. Phosphor- and total AMPK levels were analyzed by western blot analysis. E Cells were transfected with sestrin2 siRNA for 24 h and further treated with gAcrp (0.5 µg/ml) for 24 h. Phosphor- and total AMPK levels were measured by western blot analysis. F Cells were with pretreated with compound C, a pharmacological inhibitor of AMPK, for 2 h and further treated with gAcrp (0.5 µg/ml) for 24 h. LC3 I/II and p62 expression levels were detected by western blot analysis. For all western blot analyses, β-actin was used as a loading control. Expression levels of the target genes were quantified by densitometric analysis and are presented in the lower panel of the image. Values are presented as the fold change compared with control cells and represented as means ± SEM, n = 3 or 4. *Denotes P < 0.05 compared with control cells; #Indicates P < 0.05 compared with the cells treated with only gAcrp
In following experiments to further elucidate the mechanisms by which sestrin2 induction leads to autophagy activation, we observed that gAcrp induced phosphorylation of AMPK (Fig. 3D) and pretreatment with a pharmacological inhibitor of AMPK (Compound C) prevented autophagy induction, as determined by measurement of LC3II conversion and p62 expression (Fig. 3E, F), suggesting a pivotal role of the AMPK signaling in autophagy induction by gAcrp. Importantly, knockdown of sestrin2 dese-dependently suppressed gAcrp-induced AMPK phosphorylation (Fig. 3G). Taken together, these results suggest that sestrin2 signaling leads to autophagy induction via AMPK signaling in macrophages treated with adiponectin.
Sestrin2 signaling is implicated in increase of anti-inflammatory genes expression by globular adiponectin in RAW 264.7 macrophages
Adiponectin and sestrin2 exhibit potent anti-inflammatory properties (Ouchi and Walsh 2007; Yang et al. 2017). To investigate the potential role of the sestrin2 signaling in anti-inflammatory responses by adiponectin, we examined the possible implication of the sestrin2 signaling in the expression of anti-inflammatory genes by gAcrp. We first examined the effects of gAcrp on expression of various anti-inflammatory genes in macrophages and found that gAcrp treatment induced significant increases in an array of anti-inflammatory genes, including Arg-1, CD163, IL-1RA, IL-4, IL-10, and TGF-β, while CD206 and Mgl1 expression were not up-regulated (Fig. 4A–H). We next examined the functional role of sestrin2 in the expression of these anti-inflammatory genes by gAcrp. As indicated in Fig. 5, gAcrp-induced increases in Arg-1, CD163, IL-1RA, and IL-10 expression were prominently alleviated by gene silencing of sestrin2 (Fig. 5A–D), while no significant effects on IL-4 and TGF-β expression were observed (Fig. 5E, F). The contribution of sestrin2 signaling in gAcrp-induced increases in the expression of Arg-1, CD163, IL-1RA, and IL-10 were further confirmed in U937 human monocytes (Supplementary Fig. S5). These results imply that sestrin2 signaling plays a critical role in the up-regulation of anti-inflammatory genes by gAcrp in a gene-selective manner.
Effects of globular adiponectin on the expression of anti-inflammatory genes in RAW 264.7 macrophages. Cells were treated with gAcrp (0.5 µg/ml) for the indicated time periods. Expression levels of the anti-inflammatory target genes were analyzed by qRT-PCR and normalized to the level of GAPHD as indicated in the methods. Values present fold change compared with the control cells and are expressed as means ± SEM, n = 3 or 4. *Indicates P < 0.05 compared with untreated control cells
Role of sestrin2 signaling in globular adiponectin-induced expression of anti-inflammatory genes in RAW 264.7 macrophages. Cells were transfected with siRNA targeting sestrin2 siRNA for 24 h and further stimulated with gAcrp (0.5 µg/ml). Messenger RNA expression levels of anti-inflammatory genes were determined by qRT-PCR as indicated in the methods. Values present fold change compared with control cells and are represented as means ± SEM, n = 3 or 4. *Denotes P < 0.05 compared to untreated cells; #indicates P < 0.05 compared with the cells treated with only gAcrp
Sestrin2 induction is required for the inhibition of ROS accumulation by globular adiponectin in RAW 264.7 macrophages
Reactive oxygen species (ROS) act as upstream signaling molecules that induce inflammatory responses, and adiponectin exhibits potent antioxidant properties. In this study, we further examined whether sestrin2 induction contributes to the suppression of ROS production by adiponectin. As shown in Fig. 6A, gAcrp significantly suppressed lipopolysaccharide (LPS)-stimulated ROS accumulation under our experimental conditions (Fig. 6A), consistent with previous reports. Gene silencing of sestrin2 resulted in the restoration of LPS-stimulated ROS accumulation suppressed by gAcrp (Fig. 6B), implying the involvement of sestrin2 in the regulation of ROS production by gAcrp.
Role of sestrin2 signaling in the suppression of LPS-stimulated ROS production by globular adiponectin in RAW 264.7 macrophages. A Cells were pretreated with gAcrp (0.5 µg/ml) for 24 h and further stimulated with LPS (100 ng/ml) for 18 h. B Cells were transfected with sestrin2 siRNA or control scrambled siRNA for 24 h followed by treatment with gAcrp (0.5 µg/ml) for 24 h. Finally, cells were stimulated with LPS (100 ng/ml) for additional 18 h. Intracellular ROS levels were assessed by measurement of CM2-DCFDA fluorescence as indicated in the methods. Values present fold change compared to the cells with LPS and are expressed as mean ± SEM, n = 3–6. *Indicates P < 0.05 compared with control cells; # denotes P < 0.05 compared with the cells treated with LPS only; $denotes P < 0.05 compared with the cells treated with LPS and gAcrp
Discussion
Adiponectin, a key fat-derived hormone, is the most abundant adipokine in plasma and acts as a critical modulator in a variety of physiological and pathological responses. In particular, adiponectin possesses potent anti-inflammatory properties and modulate cell death/survival in a complex manner (Pham and Park 2020). Adiponectin treatment causes cell death in various types of cancer cells (Libby et al. 2014; Hebbard and Ranscht 2014). In contrast, globular adiponectin causes the survival of macrophages, implying that the modulatory effects of adiponectin on cell death and survival depend on cell type and other contexts. While autophagy induction and regulation of inflammatory transcription factors have been proposed for the anti-inflammatory responses and cell survival effects of adiponectin, detailed underlying mechanisms need to be further elucidated. Sestrin2 plays a cytoprotective role by maintaining cellular homeostasis and inhibits the expression of inflammatory mediators, such as nitric oxide and ROS (Wang et al. 2019a). Although adiponectin and sestrin2 are critically implicated in cellular responses for the modulation of inflammation and maintenance of cellular homeostasis, the physiological relationships between adiponectin and sestrin2 are largely unknown. In this study, we have examined the role of the sestrin2 signaling in anti-inflammatory responses and cell survival effects of adiponectin, and have shown that globular adiponectin (gAcrp) induces a significant increase in sestrin2 expression in macrophages. Furthermore, we have demonstrated for the first time that sestrin2 induction critically contributes to enhanced cell viability and expression of anti-inflammatory mediators by gAcrp.
There is a growing appreciation that sestrin2 signaling generates cytoprotective responses under various stressful conditions, such as hypoxia and DNA damage, mainly via the AMPK/mTORC signaling pathway and suppression of ROS accumulation (Pan et al. 2021; Shi et al. 2017). Sestrin2 signaling also regulates metabolic homeostasis by inducing anti-oxidative and anti-inflammatory responses (Ren et al. 2020). More specifically, overexpression of sestrin2 alleviates sepsis-induced cardiac dysfunction by inhibiting the p-S6K and p-AMPK pathways (Wang et al. 2019b) and protects liver cells from acetaminophen by modulating ER stress (Kim et al. 2017). As indicated previously, adiponectin also regulates inflammatory responses and plays important roles in maintaining cellular homeostasis. Driven by these considerations, we examined whether sestrin2 signaling plays a role in mediating the biological responses of adiponectin. Indeed, we have previously shown that gAcrp induces sestrin2 expression via mild ER stress and sestrin2 signaling is potentially implicated in autophagy induction by gAcrp in RAW 264.7 macrophages (Oh et al. 2020). In this study, we have identified the molecular mechanisms underlying sestrin2 induction in macrophages and further demonstrated the crucial role of sestrin2 signaling in the expression of anti-inflammatory genes and cell survival effects by gAcrp. Sestrin2 expression can be determined at the transcriptional level and regulation of protein stability (Seo et al. 2016; Kim et al. 2015b). Herein, we found that gAcrp prominently increased sestrin2 mRNA levels (Fig. 1E), suggesting that gAcrp may induce sestrin2 expression at the transcriptional level, rather than by regulating protein stability. Various transcription factors and signaling mechanisms have been proposed for the transcriptional activation of sestrin2. We have conducted a series of experiments to elucidate the signaling mechanisms underlying sestrin2 expression and found that gAcrp increases expression of many transcription factors, including Nrf2, p53, and HIF-1α (Fig. 2 and Supplementary Fig. S2). While transfection with siRNA targeting HIF-1α prominently suppressed gAcrp-enhanced sestrin2 expression at both mRNA and protein levels (Fig. 2), knockdown of Nrf2 and p53 did not significantly affect gAcrp-induced sestrin2 expression (Supplementary Fig. S2), clearly suggesting that gAcrp-induced sestrin2 induction is mediated via HIF-1α. In addition, diverse upstream signaling mechanisms, such as ERK, JNK, p38MAPK, and PI3K, are implicated in sestrin2 expression in a context-dependent manner (Chai et al. 2015; Lee et al. 2010). We observed that pretreatment with pharmacological inhibitors of JNK and PI3K abolished gAcrp-induced sestrin2 expression, while no significant effects were observed by inhibitors of ERK and p38 MAPK (Fig. 2), indicating the involvement of JNK and PI3K signaling in sestrin2 expression by gAcrp. Moreover, treatment with a PI3K or ERK inhibitor also substantially blocked HIF-1α expression. These results clearly indicate that gAcrp induces sestrin2 expression via ERK/HIF-1α and PI3K/HIF-1α axes in macrophages.
The physiological role of adiponectin in cell death/survival might be determined in a context-dependent manner, particularly depending on the cell type. For example, while adiponectin potently induces cell death in various types of cancer cells, gAcrp induces enhanced cell viability in macrophages under our experimental conditions. The survival effect of gAcrp in macrophages was eliminated by gene silencing of sestrin2 (Fig. 3A), and sestrin2 induction also contributed to autophagy induction (Fig. 3B, C), demonstrating that the crucial role of sestrin2 signaling in gAcrp-enhanced viability of macrophages is exerted via autophagy induction. Autophagy, a self-digestive cellular process that removes dysfunctional and damaged cellular components, is important to maintain cellular homeostasis (Glick et al. 2010). While autophagy was originally reported as a type of cell death distinct from apoptosis, emerging recent evidence indicates that autophagy may act as a cytoprotective process and is a vital mechanism for cell survival (Kim and Lee 2014). Interestingly, autophagy induction is a critical event in mediating various adiponectin-induced biological responses. For example, autophagy activation is required for gAcrp-mediated suppression of inflammatory responses via ZFP36L1 and AUF induction (Shrestha et al. 2018), Beclin-1 phosphorylation and Bcl-2 mRNA destabilization (Tilija Pun and Park 2018), and activation of the SIRT1/FoxO3a axis (Pun et al. 2015). More importantly, autophagy activation critically contributes to the enhanced viability of macrophages induced by adiponectin under our experimental conditions (Supplementary Fig. S3). These results collectively indicate that enhanced cell viability by setrin2 induction is mediated through autophagy activation.
It has been well documented that adiponectin treatment improves the degree of inflammatory responses (Fantuzzi 2013). In this study, we examined the potential role of sestrin2 signaling in the modulation of pro- and anti-inflammatory gene expression by gAcrp. We observed that knockdown of sestrin2 did not significantly affect gAcrp-suppression of pro-inflammatory gene expression, including TNF-α and IL-1β (data not shown). In contrast, sestrin2 signaling is critically implicated in the gAcrp-induced anti-inflammatory gene expression in a gene-selective manner (Fig. 5). In the present study, downstream signaling mechanisms by which sestrin2 signaling leads to increase in anti-inflammatory genes expression are not fully elucidated. Each anti-inflammatory gene expression is regulated in its own unique way. Considering gene selective regulation, sestrin2 signaling activates signals that induce the expression of specific anti-inflammatory genes, but does not appear to be involved in signaling pathways involved in the expression of other certain genes. Investigation of the signaling mechanisms, including identification of the transcription factors and signaling pathways, which are implicated in sestrin2-modulation of anti-inflammatory gene expression, would be an interesting topic for the future study.
It is interesting to note that macrophages exist in two distinct functional phenotypes depending on the specific microenvironment. M1 macrophages produce pro-inflammatory cytokines and initiate an innate immune response, which represents the classical activated phenotype, while M2 macrophages have anti-inflammatory and immune-regulatory properties, which are closely associated with wound healing and tissue repair (Funes et al. 2018). Adiponectin has been shown to suppress M1 macrophages activation and promotes M2 polarization via adiponectin receptor type 2-IL-4-STAT6 dependent signaling (Mandal et al. 2011). Moreover, adiponectin inhibits M1 macrophage proliferation, while it induces M2 macrophage proliferation (Ohashi et al. 2010; Hui et al. 2015). Sestrin2 has been shown to play a role in the enhanced viability of macrophages and production of anti-inflammatory M2 markers, raising a possibility of the potential role of the sestrin2 signaling in gAcrp-induced M2 polarization. In the present study, we mostly focused on the role of sestrin2 in the regulation of cell viability and expression of anti-inflammatory genes. Further studies will be required for providing the better insights into the role of the sestrin2 signaling in changing phenotype of the macrophages.
In conclusion, the data presented in this study demonstrated that globular adiponectin induces increase in sestrin2 expression in macrophages via ERK/HIF-1α and PI3K/HIF-1α axis. Sestrin2 induction plays a crucial role in globular adiponectin-induced anti-inflammatory genes expression, enhanced cell viability, and regulation of ROS production. Given that adiponectin exhibits the various other biological responses, such as potent anti-tumor activity and beneficial metabolic effects, sestrin2 would be a promising target for the treatment of pathological conditions associated with adiponectin. In this study, although we clearly indicate the involvement of the sestrin2 signaling in the modulation of inflammatory responses and enhanced viability, further studies to unravel the detailed underlying mechanisms and in vivo studies for validating in vitro observations are required.
References
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953
Budanov AV, Karin M (2008) p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134:451–460
Chai D, Wang G, Zhou Z, Yang H, Yu Z (2015) Insulin increases sestrin 2 content by reducing its degradation through the PI 3 K/mTOR signaling pathway. Int J Endocrinol 2015:505849
Chen T, Li T, Wang J (2019) p53 mediates PEDF-induced autophagy in human umbilical vein endothelial cells through sestrin2 signaling. Mol Med Rep 20:1443–1450
Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. AMS 9:191–200
Dalamaga M, Diakopoulos KN, Mantzoros CS (2012) The role of adiponectin in cancer: a review of current evidence. Endocr Rev 33:547–594
Deng Y, Scherer PE (2010) Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci 1212:E1–E19
Essler S, Dehne N, Brüne B (2009) Role of sestrin2 in peroxide signaling in macrophages. FEBS Lett 583:3531–5
Fantuzzi G (2013) Adiponectin in inflammatory and immune-mediated diseases. Cytokine 64:1–10
Funes SC, Rios M, Escobar-Vera J, Kalergis AM (2018) Implications of macrophage polarization in autoimmunity. Immunology 154:186–195
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12
Hebbard L, Ranscht B (2014) Multifaceted roles of adiponectin in cancer. Best practice & research. Clin Endocrinol Metab 28:59–69
Ho A, Cho CS, Namkoong S, Cho US, Lee JH (2016) Biochemical basis of sestrin physiological activities. Trends Biochem Sci 41:621–632
Hu HJ, Shi ZY, Lin XL, Chen SM, Wang QY, Tang SY (2015) Upregulation of Sestrin2 expression protects against macrophage apoptosis induced by oxidized low-density lipoprotein. DNA Cell Biol 34:296–302
Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, Feng T, Zhong C, Wang Y, Lam KS, Xu A (2015) Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metabol 22:279–290
Hwang HJ, Jung TW, Choi JH, Lee HJ, Chung HS, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH, Yoo HJ (2017) Knockdown of sestrin2 increases pro-inflammatory reactions and ER stress in the endothelium via an AMPK dependent mechanism. Biochim Biophys Acta Mol Basis Dis 1863:1436–1444
Hwang HJ, Kim JW, Chung HS, Seo JA, Kim SG, Kim NH, Choi KM, Baik SH, Yoo HJ (2018) Knockdown of sestrin2 increases lipopolysaccharide-induced oxidative stress, apoptosis, and fibrotic reactions in H9c2 cells and heart tissues of mice via an AMPK-dependent mechanism. Mediat Inflamm 2018:6209140
Ishtiaq SM, Rashid H, Hussain Z, Arshad MI, Khan JA (2019) Adiponectin and PPAR: a setup for intricate crosstalk between obesity and non-alcoholic fatty liver disease. Rev Endocr Metab Disord 20:253–261
Jian M, Kwan JS, Bunting M, Ng RC, Chan KH (2019) Adiponectin suppresses amyloid-β oligomer (AβO)-induced inflammatory response of microglia via AdipoR1-AMPK-NF-κB signaling pathway. J Neuroinflamm 16:110
Kim KH, Lee MS (2014) Autophagy—a key player in cellular and body metabolism. Nat Rev Endocrinol 10:322–337
Kim H, An S, Ro SH, Teixeira F, Park GJ, Kim C, Cho CS, Kim JS, Jakob U, Lee JH, Cho US (2015a) Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat Commun 6:10025
Kim MG, Yang JH, Kim KM, Jang CH, Jung JY, Cho IJ, Shin SM, Ki SH (2015b) Regulation of Toll-like receptor-mediated Sestrin2 induction by AP-1, Nrf2, and the ubiquitin-proteasome system in macrophages. Toxicol Sci 144:425–435
Kim MJ, Bae SH, Ryu JC, Kwon Y, Oh JH, Kwon J, Moon JS, Kim K, Miyawaki A, Lee MG, Shin J, Kim YS, Kim CH, Ryter SW, Choi AM, Rhee SG, Ryu JH, Yoon JH (2016) SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy 12:1272–1291
Kim SJ, Kim KM, Yang JH, Cho SS, Kim JY, Park SJ, Lee SK, Ku SK, Cho IJ, Ki SH (2017) Sestrin2 protects against acetaminophen-induced liver injury. Chem Biol Interact 269:50–58
Kim M, Kowalsky AH, Lee JH (2021) Sestrins in physiological stress responses. Annu Rev Physiol 83:381–403
Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M (2010) Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies, vol 327. Science, New York, pp 1223–1228
Li H, Liu S, Yuan H, Niu Y, Fu L (2017) Sestrin 2 induces autophagy and attenuates insulin resistance by regulating AMPK signaling in C2C12 myotubes. Exp Cell Res 354:18–24
Libby EF, Frost AR, Demark-Wahnefried W, Hurst DR (2014) Linking adiponectin and autophagy in the regulation of breast cancer metastasis. J Mol Med 92:1015–1023
Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, Carnuccio R, Kroemer G (2009) Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle (Georgetown. Tex) 8:1571–1576
Mandal P, Pratt BT, Barnes M, Mcmullen MR, Nagy LE (2011) Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem 286:13460–13469
Oh HJ, Lee S, Park PH (2020) ER stress contributes to autophagy induction by adiponectin in macrophages: Implication in cell survival and suppression of inflammatory response. Cytokine 127:154959
Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K (2010) Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285:6153–6160
Osei K, Gaillard T, Schuster D (2005) Plasma adiponectin levels in high risk African-Americans with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes. Obes Res 13:179–185
Ouchi N, Walsh K (2007) Adiponectin as an anti-inflammatory factor. Clin Chim Acta 380:24–30
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85–97
Pan C, Chen Z, Li C, Han T, Liu H, Wang X (2021) Sestrin2 as a gatekeeper of cellular homeostasis: physiological effects for the regulation of hypoxia-related diseases. J Cell Mol Med 25:5341–5350
Park PH (2018) Autophagy induction: a critical event for the modulation of cell death/survival and inflammatory responses by adipokines. Arch Pharm Res 41:1062–1073
Pasha M, Eid AH, Eid AA, Gorin Y, Munusamy S (2017) Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid Med Cell Longev 2017:3296294
Pham DV, Park PH (2020) Recent insights on modulation of inflammasomes by adipokines: a critical event for the pathogenesis of obesity and metabolism-associated diseases. Arch Pharm Res 43:997–1016
Pham DV, Tilija Pun N, Park PH (2021) Autophagy activation and SREBP-1 induction contribute to fatty acid metabolic reprogramming by leptin in breast cancer cells. Mol Oncol 15:657–678
Pun NT, Subedi A, Kim MJ, Park PH (2015) Globular adiponectin causes tolerance to LPS-induced TNF-α expression via autophagy induction in RAW 264.7 macrophages: involvement of SIRT1/FoxO3A axis. PLoS ONE 10:e0124636
Raut PK, Park PH (2020) Globular adiponectin antagonizes leptin-induced growth of cancer cells by modulating inflammasomes activation: critical role of HO-1 signaling. Biochem Pharmacol 180:114186
Raut PK, Kim SH, Choi DY, Jeong GS, Park PH (2019) Growth of breast cancer cells by leptin is mediated via activation of the inflammasome: critical roles of estrogen receptor signaling and reactive oxygen species production. Biochem Pharmacol 161:73–88
Ren D, Quan N, Fedorova J, Zhang J, He Z, Li J (2020) Sestrin2 modulates cardiac inflammatory response through maintaining redox homeostasis during ischemia and reperfusion. Redox Biol 34:101556
Seo K, Seo S, Ki SH, Shin SM (2016) Compound C increases sestrin2 expression via mitochondria-dependent ROS production. Biol Pharm Bull 39:799–806
Sepilian V, Nagamani M (2005) Adiponectin levels in women with polycystic ovary syndrome and severe insulin resistance. J Soc Gynecol Investig 12:129–134
Shi X, Doycheva DM, Xu L, Tang J, Yan M, Zhang JH (2016) Sestrin2 induced by hypoxia inducible factor1 alpha protects the blood-brain barrier via inhibiting VEGF after severe hypoxic-ischemic injury in neonatal rats. Neurobiol Dis 95:111–121
Shi X, Xu L, Doycheva DM, Tang J, Yan M, Zhang JH (2017) Sestrin2, as a negative feedback regulator of mTOR, provides neuroprotection by activation AMPK phosphorylation in neonatal hypoxic-ischemic encephalopathy in rat pups. J Cereb Blood Flow Metab 37:1447–1460
Shin BY, Jin SH, Cho IJ, Ki SH (2012) Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic Biol Med 53:834–841
Shrestha A, Pun NT, Park PH (2018) ZFP36L1 and AUF1 induction contribute to the suppression of inflammatory mediators expression by globular adiponectin via autophagy induction in macrophages. Biomol Therap 26:446–457
Stern JH, Rutkowski JM, Scherer PE (2016) Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metabol 23:770–784
Tilija Pun N, Park PH (2018) Adiponectin inhibits inflammatory cytokines production by Beclin-1 phosphorylation and B-cell lymphoma 2 mRNA destabilization: role for autophagy induction. Br J Pharmacol 175:1066–1084
Tsai YW, Fu SH, Dong JL, Chien MW, Liu YW, Hsu CY, Sytwu HK (2020) Adipokine-modulated immunological homeostasis shapes the pathophysiology of inflammatory bowel disease. Int J Mol Sci 21
Wang LX, Zhu XM, Yao YM (2019a) Sestrin2: its potential role and regulatory mechanism in host immune response in diseases. Front Immunol 10:2797
Wang Z, Bu L, Yang P, Feng S, Xu F (2019b) Alleviation of sepsis-induced cardiac dysfunction by overexpression of Sestrin2 is associated with inhibition of p-S6K and activation of the p-AMPK pathway. Mol Med Rep 20:2511–2518
Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H (2004) Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 323:630–5
Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME (2004) Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316:924–9
Yanai H, Yoshida H (2019) Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci 20
Yang JH, Kim KM, Kim MG, Seo KH, Han JY, Ka SO, Park BH, Shin SM, Ku SK, Cho IJ, Ki SH (2015) Role of sestrin2 in the regulation of proinflammatory signaling in macrophages. Free Radic Biol Med 78:156–167
Yang K, Xu C, Zhang Y, He S, Li D (2017) Sestrin2 suppresses classically activated macrophages-mediated inflammatory response in myocardial infarction through inhibition of mTORC1 signaling. Front Immunol 8:728
Yi L, Li F, Yong Y, Jianting D, Liting Z, Xuansheng H, Fei L, Jiewen L (2014) Upregulation of sestrin-2 expression protects against endothelial toxicity of angiotensin II. Cell Biol Toxicol 30:147–156
Zhang XY, Wu XQ, Deng R, Sun T, Feng GK, Zhu XF (2013) Upregulation of sestrin 2 expression via JNK pathway activation contributes to autophagy induction in cancer cells. Cell Signal 25:150–158
Acknowledgements
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2021R1A2C1013132) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03044512). The authors thank the Core Research Support Center for Natural Products and Medical Materials (CRCNM) for the technical support regarding the confocal microscopic analysis.
Author information
Authors and Affiliations
Contributions
PHP; designed the study. SL and DP; performed the experiments. PHP, SL, and DP; analyzed the data. PHP and SL; wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.





Rights and permissions
About this article
Cite this article
Lee, S., Pham, DV. & Park, PH. Sestrin2 induction contributes to anti-inflammatory responses and cell survival by globular adiponectin in macrophages. Arch. Pharm. Res. 45, 38–50 (2022). https://doi.org/10.1007/s12272-021-01364-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-021-01364-0