Abstract
Drug use disorder, a chronic and relapsing mental disorder, is primarily diagnosed via self-reports of drug-seeking behavioral and psychological conditions, accompanied by psychiatric assessment. Therefore, the identification of peripheral biomarkers that reflect pathological changes caused by such disorders is essential for improving treatment monitoring. Hair possesses great potential as a metabolomic sample for monitoring chronic diseases. This study aimed to investigate metabolic alterations in hair to elucidate a suitable treatment modality for methamphetamine (MA) use disorder. Consequently, both targeted and untargeted metabolomics analyses were performed via mass spectrometry on hair samples obtained from current and former patients with MA use disorder. Healthy subjects (HS), current (CP), and former (FP) patients with this disorder were selected based on psychiatric diagnosis and screening the concentrations of MA in hair. The drug abuse screening questionnaire scores did not differentiate between CP and FP. Moreover, according to both targeted and untargeted metabolomics, clustering was not observed among all three groups. Nevertheless, a model of partial least squares-discriminant analysis was established between HS and CP based on seven metabolites derived from the targeted metabolomics results. Thus, this study demonstrates the promising potential of hair metabolomes for monitoring recovery from drug use disorders in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug use disorder or drug addiction threatens both individuals and society at large. These processes begin with drug-seeking or drug-taking behavior and eventually develop into a chronic and relapsing mental disorder. When transitioning from casual drug use to drug addiction, a combination of positive and negative reinforcement can provide high motivation for compulsive drug use despite the risk and awareness of adverse events. Repeated drug use causes neurobiological changes in the reward circuitry of the brain and leads to the development of physical dependence, tolerance, withdrawal upon discontinuation, craving, and relapse, all of which are characteristics of drug use disorder (Koob 2013; Zaitsu et al. 2016; Kim et al. 2019). Recent studies regarding the treatment of drug use disorders have focused on developing therapeutic drugs against targets in addiction-related neurological systems (Liu and Li 2018) and on the development of vaccines (Xu and Kosten 2020). However, the diagnosis of these disorders is primarily based on self-reports of drug-seeking behavioral and psychological conditions. Therefore, a need exists to identify peripheral biomarkers that reflect pathological changes caused by drug use disorders to further aid in the monitoring of treatments (Bough and Pollock 2018; Ghanbari and Sumner 2018).
Methamphetamine (MA) is a highly addictive psychostimulant, the acute and chronic usage of which induces neurotoxicity in dopaminergic and serotonergic neurons in the brain (Yu et al. 2015; Hwang et al. 2020; Shin et al. 2021). In previous studies, MA addiction or withdrawal in patients has been evaluated by measuring neuronal markers such as N-acetyl aspartate and glutamate through the use of neuroimaging (Howells et al. 2014; Kim et al. 2016; Yang et al. 2018). The relationship between the levels of the neurotrophin brain-derived neurotrophic factor (BDNF) in serum and the different conditions of MA addiction has also been demonstrated (Ren et al. 2016, 2017). Despite this, the diagnosis of MA addiction has been achieved primarily based on clinical symptoms, questionnaires, and interviews, as practical peripheral biomarkers are not yet available (Bough and Pollock 2018).
It is critical to assess the severity of drug abuse for subjects with drug use disorder. The severity of MA abuse is evaluated based on the concentrations of MA and its main metabolite, amphetamine (AM), in hair, as these are considered useful biological indicators of chronic drug exposure (Lee et al. 2009; Yu et al. 2018). However, the analysis of MA and AM in hair is mostly performed in forensic laboratories for illegal MA use determination. Thus, we previously conducted a study regarding the clinical application of hair analysis to monitor the treatment of MA use disorder. The characteristics of Korean patients with MA use disorder were investigated based on drug abuse screening instruments and quantitative analysis of MA and AM in hair. Our previous study demonstrated that by integrating the drug abuse screening tool scores and determined hair drug concentrations, higher performance can be obtained for the diagnosis and prognosis of drug addiction. However, the quantitative results of MA and AM in hair failed to differentiate between healthy subjects (HS) and patients with MA use disorder. Moreover, no correlation was observed between the hair MA concentrations and the scores of drug abuse screening instruments such as the National Institution of Drug Abuse (NIDA)-Modified Alcohol, Smoking and Substance Involvement Screening Test (ASSIST), Drug Use Disorder Identification Test (DUDIT)-Extended and Drug Abuse Screening Test (DAST) in our previous study (Seo et al. 2020). In that regard, the identification of peripheral biomarkers that reflect pathological changes caused by such disorders is essential for improving treatment monitoring.
Recently, hair metabolomic studies have been utilized for monitoring chronic diseases such as cancer and diabetes, and these studies have also been used to assess abnormal conditions during pregnancy due to their potential for long-term and retrospective analysis without dynamic fluctuations in metabolite concentrations that can occur in blood or urine samples (Eisenbeiss et al. 2020). The melanin content in hair and also the lipophilicity and basicity of the compounds under investigation have been suggested to facilitate the incorporation of these compounds into the hair from blood stream. Based on this, lipophilic, basic, and uncharged metabolites can easily cross membranes and exist in hair matrix cells (Pragst and Balikova 2006; Yu et al. 2017). Furthermore, the value of hair as a diagnostic specimen has been emphasized in determining the presence of drug addiction (Jang et al. 2019). In our previous study, hundreds of dysregulated (putative) metabolites were observed in hair obtained from current heavy MA abusers. Furthermore, hair metabolomics demonstrated that the biosynthesis or metabolism of lipids, including glycosphingolipids, sphingolipids, glycophospholipids, and ether lipids, and also the metabolism of amino acids (glycine, serine and threonine metabolism, and cysteine and methionine metabolism) is significantly affected by heavy MA abuse (Kim et al. 2020).
Understanding the metabolic perturbations as well as discovering potential biomarkers in the hair could facilitate the reliable diagnosis and efficient treatment of MA use disorders. To this end, this study aimed to investigate metabolic alterations in hair that could further enhance the treatment of MA use disorder. Consequently, both targeted as well as untargeted metabolomics analyses using mass spectrometry were performed in hair samples obtained from current (CP) and former (FP) patients, who were clinically diagnosed by a psychiatrist.
Materials and methods
Materials and chemicals
Formic acid, 2-aminoanthracene, and 2,3,4,5,6-pentafluorobenzoic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). The AbsoluteIDQ p180 kit for sample preparation for the targeted analysis was purchased from Biocrates Life Sciences AG (Innsbruck, Austria). All solvents were of high-performance liquid chromatography (HPLC) grade. All other chemicals were of reagent grade.
Clinical study
The clinical study was approved by the Institutional Review Board of Bugok National Hospital (Gyeongsangnam-do, Republic of Korea, approval number: BNH-2018-03, approval date: 26 April 2018). All subjects were recruited from the Bugok National Hospital, a national drug addiction treatment hospital in Korea. Subjects were excluded from the study if they presented with a current or previous substance use disorder other than MA and with any general medical condition. The groups of HS (n = 14, male), CP (n = 10, male), and FP (n = 17, male) with MA use disorder were selected from those in the previous study (Seo et al. 2020) based on the concentrations of MA in hair (Supplementary Table 1), the survey results for cigarette, alcohol, and other drug use history, and drug abuse screening questionnaire scores. Prior to the commencement of treatment, the subjects in the CP and FP groups underwent judicial custody for previous illegal MA usage (no other illegal drug use), as confirmed by forensic analysis. Those in the FP group were classified as rehabilitators based on the assessment of their behavioral and psychological conditions by a psychiatrist in the past year. The patients with MA concentrations in hair strands that were higher than 24.5 ng/mg (Lee et al. 2009; Kim et al. 2020) and lower than the limit of quantification were included in the CP and FP groups, respectively. Hair samples from the subjects were collected from three separate areas at the back of the head and stored at room temperature. From each patient, a 3-cm section of hair strands originating from the root was used for analysis.
Targeted metabolomics
Targeted metabolomics using an AbsoluteIDQ p180 kit (Biocrates Life Sciences AG, Innsbruck, Austria) for hair sample preparation was performed as reported in a previous study (Kim et al. 2020). A total of 188 metabolites (40 acylcarnitines, 42 amino acids and biogenic amines, 90 glycerophospholipids, 15 sphingolipids, and the sum of hexoses, including glucose) were simultaneously quantified in hair samples. Hair was washed using methanol and water and then dried and finely cut. Approximately 20 mg portions of hair segments were incubated for 16 h in 2 mL methanol. The extract was evaporated to dryness, and the residue was reconstituted in 100 μL of methanol. Ten microliters of the methanol extract with 10 μL of the solution of internal standards that were composed of 46 isotope-labeled and chemically homologous internal standards was loaded into the kit. Next, 50 μL of a 5% phenylisothiocyanate solution was added to derivatize the amino acids and biogenic amines. The metabolites were extracted using 300 μL of 5 mM ammonium acetate in methanol. Finally, the extract was analyzed using flow injection analysis (FIA)-tandem mass spectrometry (MS/MS) for the determination of amino acids and biogenic amines and to HPLC-MS/MS (AB Sciex 4000 QTrap mass spectrometer, Sciex, Framingham, MA, USA) for the determination of other groups. For the FIA-MS/MS analysis, the sample was run with the solvent provided by the manufacturer at the following rates: 0–1.6 min, 0.03 mL/min; 1.6–2.4 min, 0.2 mL/min; 2.4–2.8 min, 0.2 mL/min; and 2.8–3.0 min, 0.03 mL/min. For the HPLC–MS/MS analysis, a Zorbax Eclipse XDB C18 column (3 × 100 mm, 3.5 μm; Agilent, Santa Clara, CA, USA) was used. The mobile phase was comprised of 0.2% formic acid in water (A) and 0.2% formic acid in acetonitrile (B), and the gradient conditions were as follows: 0–0.5 min, 0% (B); 0.5–5.5 min, 0–95% (B); 5.5–6.5 min, 95% (B); 6.5–7 min, 95–0% (B); and 7–9.5 min, 0% (B). The flow rate was 0.5 mL/min. The quantification of metabolites was performed through the use of multiple reaction monitoring. The kit was validated using MetVal (Biocrates Life Sciences AG, Innsbruck, Austria) software, and the analytical results were processed using Analyst (Sciex, Framingham, MA, USA) and MetVal software. Pooled quality control (QC) samples in which an equal aliquot of each sample was mixed together were analyzed in each kit to assess the reproducibility of the qualitative results of the metabolites. The calibration standards provided in the kit were used for quantitation. A single point calibration standard is used for the reproducible quantitation of the acylcarnitines, glycerophospholipids, and sphingolipids. Therefore, the results for these analytes are described as semi-quantified.
Untargeted metabolomics
Untargeted metabolomics for hair samples was performed as reported in a previous study (Kim et al. 2020). The hair extract was prepared according to the method described above and was then evaporated to dryness. The residue was next reconstituted in 100 μL of a solution of methanol and 0.1% formic acid in water (9:1) before filtering through a 0.45-μm polyvinylidene fluoride microporous membrane. Prior to methanol incubation, 2-aminoanthracene (100 μg/mL, 50 µL) and 2,3,4,5,6-pentafluorobenzoic acid (1 mg/mL, 5 μL) that were used as internal standards for positive and negative electrospray ionization (ESI) modes, respectively, were added to assess analytical reproducibility. Finally, 5 μL was injected into the ultrahigh liquid chromatography coupled with electrospray ionization-quadrupole-time of flight-mass spectrometry (UPLC-ESI-QTOF-MS) system (Agilent 6530 Accurate-Mass Q-TOF LC/MS System with Agilent 1290 Infinity LC, Agilent Technologies, Santa Clara, CA, USA). The guard column (Zorbax SB-C8, 3.5 μm, 2.1 × 30 mm, Agilent Technologies, Santa Clara, CA, USA) and the analytical column (Zorbax SB-Aq, 1.8 μm, 2.1 × 100 mm, Agilent Technologies, Santa Clara, CA, USA) were maintained at 40 °C. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient conditions were as follows: 0–30 min, 1–20% B; 30–40 min, 20–90% B; 40–45 min, 90% B; 45–47 min, 90–1% B; 47–52 min, 1% B, at a flow rate of 400 μL/min. The MS system was operated using ESI in the positive and the negative ionization modes. The optimized MS system conditions for both ionization modes were as follows: drying gas temperature, 300 °C; drying gas flow, 10 L/min; nebulization pressure, 45 psi; sheath gas temperature, 350 °C; sheath gas flow, 10 L/min; capillary voltage, 3500 V; nozzle voltage, 0 V; fragmentor voltage, 175 V; and skimmer voltage, 65 V. The mass range was 50–1700 m/z, and the scan rate was 2.00 spectra/s. Purine (exact mass for [M + H]+ = 121.050873) and 1,2,3,4,5,6-hexakis(2,2,3,3-tetrafluoropropoxy)-1,3,5,2,4,6-triazatriphosphinane (exact mass for [M + H]+ = 922.009798) were used for mass calibration. Pooled QC samples in which an equal aliquot of each sample was mixed together were analyzed concurrently with authentic hair samples.
Data processing and statistical analysis
For targeted metabolomics, data cleaning was performed in accordance with the criteria used in previous studies (Veiga et al. 2018). The analytical results of metabolites in hair samples were excluded if the quantitative values of the QC samples were below the limits of detection (LODs) and their coefficients of variation were above 20%. To calculate fold-changes, 80% frequency in one group and p-values < 0.05 between groups were applied, and values below the LOD in either group were replaced with LOD/2. Fold changes with p-values instead of the quantitative data themselves were used for the comparison between groups, as the quantitative data were not considered to be an accurate indication of the concentration of metabolites in the hair samples given that the calibrators used for quantification were not matrix‑matched.
For data processing in untargeted metabolomics, MassHunter Profinder (version B.06.00) and Mass Profiler Professional (MPP, version B.13.1) software platforms (Agilent Technologies, Santa Clara, CA, USA) were used. The parameters for feature extraction were set as follows: peak height > 300 counts, 80% frequency in all samples, and fold change > 1.2 with p-values < 0.05 among the groups. The data reproducibility of the significantly altered ion features was examined in the QC samples, and those with relative standard deviation (RSD) less than 20% were ultimately selected.
The online MetaboAnalyst 4.0 software (http://www.metaboanalyst.ca/MetaboAnalyst/faces/home.xhtml) was used for statistical analysis of the data obtained from both targeted and untargeted analyses. The data were normalized by the sum by dividing the area of each peak in profile by the total sum of all peaks, and an unpaired t-test was used to determine significant differences in metabolites or ion features. The criteria that were applied included 80% frequency and p-values < 0.05 between groups. The metabolic differences between groups were evaluated using principal component analysis (PCA). The analytical data were modeled using partial least squares-discriminant analysis (PLS-DA) to reveal the differences between the two groups, and the metabolites or ion features contributing to the separation were selected as the variable importance based on projection (VIP) value. Four-component and tenfold cross-validation algorithms were used to prevent overfitting of the PLS-DA model. The R2 and Q2 parameters were used to evaluate the model, mean fitness, and prediction ability. Receiver operator characteristic (ROC) curve analysis was performed to investigate the potential of metabolites as biomarker candidates.
Results
Clinical study
The characteristics of the subjects in the study groups consisting of HS, CP, and FP are shown in Table 1. The age and body mass indices of the three groups were 45.4 ± 7.8, 48.2 ± 5.7, and 49.0 ± 9.6 years and 24.9 ± 2.6, 25.1 ± 3.5, and 25.5 ± 3.4 kg/m2, respectively (mean ± standard deviation). The majority of the subjects in these three groups were current smokers: HS, 64%; CP, 100%; FP, 71%. Alcohol use status was diverse among the groups. The percentages of nondrinkers in the HS, CP, and FP groups were 14%, 70%, and 47%, respectively, and binge drinkers accounted for 79%, 30%, and 35%, respectively. In regard to drug use history, 50% and 24% of the CP and FP groups, respectively, declared that they used only MA. Five subjects in the CP group and fourteen in the FP group reported that they previously used more than two types of drugs, including MA. The majority of CP and FP participants were characterized as high risk according to the NIDA-Modified ASSIST (70% and 94%, respectively) and severe according to the DAST (70% and 84%, respectively). According to the DUDIT-Extended, more than 60% of each group was classified under medium to high motivation.
Results of targeted and untargeted metabolomics
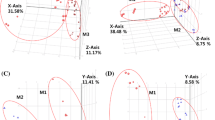
It was previously reported that many functional metabolites of acylcarnitines, amino acids and biogenic amines, glycerophospholipids, and sphingolipids were affected by MA exposure (Zheng et al. 2014; Kim et al. 2019). Therefore, targeted metabolomics analysis of was performed for the simultaneous quantification of 188 metabolites including acylcarnitines, amino acids and biogenic amines, glycerophospholipids, and sphingolipids. Among them, 53 metabolites were (semi-) quantified (Table 2 and Supplementary Table 2). The results of t-tests were considered in the selection of ten metabolites (one within the category of acylcarnitines and nine within the category of amino acids and biogenic amines) that were significantly different among the HS, CP, and FP groups. The levels of glycerophospholipids and sphingolipids did not change among the groups. Since the levels of very limited number of the targeted metabolites were significantly different among the groups, untargeted metabolomics was performed to investigate whether there was any dissimilarity in the levels of other metabolites or ion features. The total ion features extracted in the positive and negative ionization modes of UPLC-ESI-QTOF-MS were 253 and 394, respectively. The PCA score plots of the HS, CP, and FP groups based on 53 metabolites detected in targeted metabolomics and all the ion features detected in untargeted metabolomics are displayed in Fig. 1. In regard to both targeted and untargeted metabolomics, these three groups were not separately clustered.
PCA score plots of the HS (n = 14), CP (n = 10), and FP (n = 17) groups based on 53 metabolites detected in targeted metabolomics (A) and all the ion features detected in the positive (253 ion features, B) and negative (394 ion features, C) ionization modes of UPLC-QTOF-ESI–MS of untargeted metabolomics
The comparison results between the two groups are shown in Fig. 2. The PCA score plots comparing the HS and CP and the HS and FP groups are shown with volcano plots and Venn diagrams that present the significantly changed metabolites/ion features in each metabolomics mode. These results indicate that the HS and CP groups were clearly separated and comprised 50.2% of the first principal component (PC1), while the HS and FP groups were not separated in the targeted metabolomics. In regard to the untargeted metabolomics, these two groups were not separated. Among the 53 metabolites in the targeted metabolomics, seven metabolites differed between hair samples obtained from the HS and CP groups (alanine, glycine, histidine, isoleucine, serine, threonine, and valerylcarnitine) (p < 0.05). Between the HS and FP groups, seven metabolites differed (isoleucine, leucine, phenylalanine, serine, threonine, tyrosine, and valerylcarnitine) (p < 0.05). We obtained three and ten significantly altered ion features from the positive ESI analysis in hair samples collected from the CP and FP groups, respectively, compared to those obtained from the HS group (p < 0.05). From the negative ESI analysis, we observed 24 and 28 significantly altered ion features between the HS and CP groups and between the HS and FP groups, respectively (p < 0.05). However, following the Bonferroni correction for multiple testing, none of the p-values were found to be significant.
Statistical results of the comparison between the groups (HS [n = 14] vs. CP [n = 10], HS [n = 14] vs. FP [n = 17]) analyzed by targeted metabolomics (A) and the positive (B) and negative (C) ionization mode of UPLC-QTOF-ESI-MS of untargeted metabolomics. First column, PCA score plots; Second column, volcano plots (red circle: metabolites or ion features with significance at p < 0.05); third column, Venn diagrams of the number of significantly altered metabolites or ion features
As the classification between the HS and CP groups was satisfactory according to the PCA score plot in the targeted metabolomics, PLS-DA and ROC curve analyses were further performed between the two groups (Fig. 3). From the PLS-DA score plots, a remarkable difference was observed between the two groups. Four components were used to construct the models, and the R2 and Q2 values used were 0.9232 and 0.6182, respectively. Based on this, any variables differing between the groups were filtered using VIP values greater than 1.0, and a total of 20 metabolites were identified for components 1 and 2 each. All of the seven altered metabolites with p < 0.05 between the HS and CP groups comprised the top seven positions in component 1. These metabolites were also identified from 0.757 (serine) to 0.914 (glycine) in the area under the curve of the ROC curve. Compared to the levels in the hair samples from the HS group, the levels of alanine, glycine, threonine, histidine, and serine were higher, whereas those of isoleucine and valerylcarnitine were lower in hair samples obtained from the CP group.
Statistical results of the HS (n = 14) and CP (n = 10) groups analyzed by targeted metabolomics. (A) PLS-DA score plot. (B) VIP score plot. Colored box: relative concentrations in the HS and CP groups. (C) ROC curves of the significantly altered metabolites between the HS and CP groups and the metabolite levels. *:p < 0.05, **:p < 0.01, ***:p < 0.001
Discussion
The clinical diagnosis of drug use disorder is mainly based on self-reported drug-seeking behavioral and psychological conditions using drug abuse screening instruments such as NIDA-Modified ASSIST, DUDIT, and DAST. However, it was not possible to discriminate between the CP and FP groups based solely on their scores obtained in this study. This outcome therefore poses certain limitations with regard to monitoring the treatment process involved in MA use disorder, making the use of alternative diagnostic tools that reflect the different stages of drug dependence a necessity.
The use of human hair as a metabolomics sample enables us to better understand the metabolic consequences encompassing the diverse conditions of drug use disorders (Jang et al. 2019; Kim et al. 2020). Xie et al. (2016) previously reported that sorbitol and cortisol are upregulated while arachidonic acid, glutathione, linoleic acid, and myristic acid are downregulated in the hair of current heroin abusers (heroin-positive in blood testing) analyzed using LC-ion trap-TOF MS, thus establishing the possibility of identifying clinical diagnostic markers for heroin addiction. However, the exclusion of patients with drug use disorder with temporary or prolonged abstinence due to treatment, imprisonment, or education did not allow for a full understanding of metabolism disruption related to drug use disorder. To the best of our knowledge, this is the first report to investigate metabolic alterations related to drug use disorders in the context of different pathological symptoms.
For MS-based metabolomics, two different approaches are often used, namely targeted and untargeted metabolomics, which are complement each other. The former is useful in understanding specific metabolic enzymes and alterations in kinetics, end products, and the known biochemical pathways of the resulting metabolic changes as quantifying specific numbers of known metabolites. On the other hand, the latter deals with large amounts of unknown raw data and facilitates mechanistic studies, hypothesis generation, biomarker discovery, and diagnostics (Jang et al. 2019; Park and Choi 2020). Therefore, both targeted and untargeted metabolomics using different analytical platforms for each were attempted in the present study. Data processing and statistical analysis were performed independently since the normalization of the data vary by each analytical platform.
In the present study, we compared the difference in metabolite levels between current and former patients with MA use disorder and healthy controls. Clustering among the HS, CP, and FP groups was not observed according to both targeted and untargeted metabolomics. In two previous studies comparing the levels of brain dopamine transporters and glucose metabolism between normal controls and MA abusers (n = 5) who were abstinent for a short (less than 6 months) or protracted (12–17 months) period of time, some recoveries were observed as the abstinence time increased (Volkow et al. 2001; Wang et al. 2004). With protracted abstinence, the dopamine transporters were significantly recovered (Volkow et al. 2001) and brain metabolism was partially recovered, and this was accompanied by improvements in motor and verbal memory tests (Wang et al. 2004). The overlap of the HS, CP, and FP groups in PCA in the current study could be attributed to these recoveries in the brain during abstinence. Both the clear separation in PCA and the good classification model in PLS-DA were observed only between the HS and CP groups using targeted metabolomics. The contributing metabolites included six amino acids (alanine, glycine, histidine, isoleucine, serine, and threonine) and valerylcarnitine. In our previous study (Kim et al. 2020), a distinct differentiation was achieved between healthy controls and current heavy MA users using the same targeted and untargeted metabolomics platform as the present study. Significant alterations in the levels of glycerophospholipids and sphingolipids and their related metabolic pathways were observed in hair samples from current heavy MA abusers (Kim et al. 2020). This suggests that the disruption of lipid metabolism caused by heavy MA abuse is substantially recovered during the course of treatment. Overall, this implies that hair metabolomics is a valuable approach for monitoring the progressive changes that occur during the abstinence and treatment periods of MA use disorder.
The main limitation of this study is the relatively small sample size; however, this was compensated by the strict selection of samples according to psychiatric diagnosis followed by cutoff sampling by the results of quantitative analysis of hair for drug detection. In particular, no bias was observed among the three groups for cigarette and alcohol use, as shown in Table 1. The majority of the subjects in the three groups were current smokers: HS, 64%; CP, 100%; FP, 71%. Alcohol use status was diverse among the groups. The percentages of non-drinkers in the HS, CP, and FP groups were 14%, 70%, and 47%, respectively, and binge drinkers accounted for 79%, 30%, and 35%, respectively. Additionally, the hair samples were all black and had not been subjected to any treatments such as dyeing, perming, and/or bleaching. The hair used for this study as proximal hair that was 3 cm in length and obtained from males that presented with a MA use history of 3 months prior to sampling. These sampling criteria were used to exclude the effects of previous MA use severity and melanin content on the levels of the hair metabolomes.
In the present study, both targeted and untargeted metabolomics were employed to investigate metabolic alterations for monitoring MA use disorder. The PLS-DA model differentiated healthy subjects from current patients based on six amino acids and valerylcarnitine; however, it was impossible to differentiate between healthy subjects and former patients or between current and former patients. Despite the relatively small sample size, our findings merit further confirmation via larger human studies in the future. This study demonstrates that hair metabolomes possess promising potential for monitoring recovery from drug use disorders in clinical practice.
References
Bough KJ, Pollock JD (2018) Defining substance use disorders: the need for peripheral biomarkers. Trends Mol Med 24:109–120. https://doi.org/10.1016/j.molmed.2017.12.009
Eisenbeiss L, Binz TM, Baumgartner MR, Kraemer T, Steuer AE (2020) Towards best practice in hair metabolomic studies: systematic investigation on the impact of hair length and color. Metabolites 10:381. https://doi.org/10.3390/metabo10100381
Ghanbari R, Sumner S (2018) Using metabolomics to investigate biomarkers of drug addiction. Trends Mol Med 24:197–205. https://doi.org/10.1016/j.molmed.2017.12.005
Howells FM, Uhlmann A, Temmingh H, Sinclair H, Meintjes E, Wilson D, Stein DJ (2014) (1)H-magnetic resonance spectroscopy ((1)H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophr Res 153:122–128. https://doi.org/10.1016/j.schres.2014.01.029
Hwang JS, Cha EH, Park B, Ha E, Seo JH (2020) PBN inhibits a detrimental effect of methamphetamine on brain endothelial cells by alleviating the generation of reactive oxygen species. Arch Pharm Res 43:1347–1355. https://doi.org/10.1007/s12272-020-01284-5
Jang WJ, Choi JY, Park B, Seo JH, Seo YH, Lee S, Jeong CH, Lee S (2019) Hair metabolomics in animal studies and clinical settings. Molecules 24:2195. https://doi.org/10.3390/molecules24122195
Kim JE, Kim GH, Hwang J, Kim JY, Renshaw PF, Yurgelun-Todd DA, Kim B, Kang I, Jeon S, Ma J, Lyoo IK, Yoon S (2016) Metabolic alterations in the anterior cingulate cortex and related cognitive deficits in late adolescent methamphetamine users. Addict Biol 23:327–336. https://doi.org/10.1111/adb.12473
Kim S, Jang WJ, Yu H, Ryu IS, Jeong CH, Lee S (2019) Integrated non-targeted and targeted metabolomics uncovers dynamic metabolic effects during short-term abstinence in methamphetamine self-administering rats. J Proteome Res 18:3913–3925. https://doi.org/10.1021/acs.jproteome.9b00363
Kim S, Jang WJ, Yu H, Kim J, Lee SK, Jeong CH, Lee S (2020) Revealing metabolic perturbation following heavy methamphetamine abuse by human hair metabolomics and network analysis. Int J Mol Sci 21:6041. https://doi.org/10.3390/ijms21176041
Koob GF (2013) Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry 4:72. https://doi.org/10.3389/fpsyt.2013.00072
Lee S, Han E, Park Y, Choi H, Chung H (2009) Distribution of methamphetamine and amphetamine in drug abusers’ head hair. Forensic Sci Int 190:16–18. https://doi.org/10.1016/j.forsciint.2009.05.004
Liu JF, Li JX (2018) Drug addiction: a curable mental disorder? Acta Pharmacol Sin 39:1823–1829. https://doi.org/10.1038/s41401-018-0180-x
Park SJ, Choi JW (2020) Brain energy metabolism and multiple sclerosis: progress and prospects. Arch Pharm Res 43:1017–1030. https://doi.org/10.1007/s12272-020-01278-3
Pragst F, Balikova MA (2006) State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 370:17–49. https://doi.org/10.1016/j.cca.2006.02.019
Ren W, Tao J, Wei Y, Su H, Zhang J, Xie Y, Guo J, Zhang X, Zhang H, He J (2016) Time-dependent serum brain-derived neurotrophic factor decline during methamphetamine withdrawal. Medicine 95:e2604. https://doi.org/10.1097/MD.0000000000002604
Ren W, Luan X, Zhang J, Gutteea P, Cai Y, Zhao J, Gu Y, Wu C, Su H, Tao J, Xie Y, Lv D, Feng L, He J (2017) Brain-derived neurotrophic factor levels and depression during methamphetamine withdrawal. J Affect Disord 221:165–171. https://doi.org/10.1016/j.jad.2017.06.017
Seo MJ, Song SH, Kim S, Jang WJ, Jeong CH, Lee S (2020) Characteristics of Korean patients with methamphetamine use disorder based on the quantitative analysis of methamphetamine and amphetamine in hair. Arch Pharm Res 43:798–807. https://doi.org/10.1007/s12272-020-01259-6
Shin EJ, Jeong JH, Hwang Y, Sharma N, Dang DK, Nguyen BT, Nah SY, Jang CG, Bing G, Nabeshima T, Kim HC (2021) Methamphetamine-induced dopaminergic neurotoxicity as a model of Parkinson’s disease. Arch Pharm Res 44:668–688. https://doi.org/10.1007/s12272-021-01341-7
Veiga S, Wahrheit J, Rodriguez-Martin A, Sonntag D (2018) Quantitative metabolomics in Alzheimer’s disease: technical considerations for improved reproducibility. Methods Mol Biol 1779:463–470. https://doi.org/10.1007/978-1-4939-7816-8_28
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J (2001) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418. https://doi.org/10.1523/JNEUROSCI.21-23-09414.2001
Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS (2004) Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry 161:242–248. https://doi.org/10.1176/appi.ajp.161.2.242
Xie P, Wang TJ, Yin G, Yan Y, Xiao LH, Li Q, Bi KS (2016) Metabonomic study of biochemical changes in human hair of heroin abusers by liquid chromatography coupled with ion trap-time of flight mass spectrometry. J Mol Neurosci 58:93–101. https://doi.org/10.1007/s12031-015-0655-x
Xu A, Kosten TR (2020) Current status of immunotherapies for addiction. Ann N Y Acad Sci 1489:3–16. https://doi.org/10.1111/nyas.14329
Yang W, Yang R, Luo J, He L, Liu J, Zhang J (2018) Increased absolute glutamate concentrations and glutamate-to-creatine ratios in patients with methamphetamine use disorders. Front Psychiatry 9:368. https://doi.org/10.3389/fpsyt.2018.00368
Yu SB, Zhu L, Shen Q, Bai X, Di XH (2015) Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav Neurol 2015:103969. https://doi.org/10.1155/2015/103969
Yu H, Jang WJ, Jang JH, Park B, Seo YH, Jeong CH, Lee S (2017) Role of hair pigmentation in drug incorporation into hair. Forensic Sci Int 281:171–175. https://doi.org/10.1016/j.forsciint.2017.11.004
Yu H, Choi M, Jang JH, Park B, Seo YH, Jeong CH, Bae JW, Lee S (2018) Development of a column-switching LC-MS/MS method of tramadol and its metabolites in hair and application to a pharmacogenetic study. Arch Pharm Res 41:554–563. https://doi.org/10.1007/s12272-018-1013-7
Zaitsu K, Hayashi Y, Kusano M, Tsuchihashi H, Ishii A (2016) Application of metabolomics to toxicology of drugs of abuse: a mini review of metabolomics approach to acute and chronic toxicity studies. Drug Metab Pharmacokinet 31:21–26. https://doi.org/10.1016/j.dmpk.2015.10.002
Zheng T, Liu L, Shi J, Yu X, Xiao W, Sun R, Zhou Y, Aa J, Wang G (2014) The metabolic impact of methamphetamine on the systemic metabolism of rats and potential markers of methamphetamine abuse. Mol Biosyst 10:1968–1977. https://doi.org/10.1039/c4mb00158c
Acknowledgements
This work was supported by the Basic Science Research Program (NRF-2016R1A6A1A03011325 and NRF-2018R1D1A1B07048159) through the National Research Foundation funded by the Ministry of Education in Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seo, M.J., Song, SH., Kim, S. et al. Mass spectrometry-based metabolomics in hair from current and former patients with methamphetamine use disorder. Arch. Pharm. Res. 44, 890–901 (2021). https://doi.org/10.1007/s12272-021-01353-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-021-01353-3