Abstract
Identification of bioactive natural products with anticancer activity as well as alleviating effects on chemotherapy-induced side effects has significant implications for cancer treatment. Betula platyphylla var. japonica, commonly known as Asian white birch, has been used in Chinese traditional medicine for a variety of purposes. In this study, the medicinal properties of betulin from B. platyphylla var. japonica useful for cancer management were investigated. LC/MS analysis revealed that betulin is a main chemical component of the EtOH extract of B. platyphylla var. japonica bark, and betulin was isolated from EtOH extract using an LC/MS-guided isolation method. Its structure was identified with 1H and 13C NMR spectroscopic data and LC/MS analysis and then compared to the previously reported spectroscopic and physical data. We first verified the cytotoxicity of betulin against three human lung adenocarcinoma cell lines, A549, H1264, and Calu-6, with IC50 values ranging from 18.7 to 39.6 μM. Regarding alleviation of side effects associated with anticancer chemotherapy, betulin ameliorated cisplatin-induced renal cell damage to 80% of the control value from the concentration of 5 μM. In addition, betulin showed anti-gastritis activity against ethanol-induced gastric damage in rats and notably reduced the gastric damage index compared to control in a concentration-dependent manner. These findings provide the first experimental evidence for potential use of B. platyphylla var. japonica as a functional food for cancer treatment that simultaneously alleviates the side effects of chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer, a lethal disease involving deregulated proliferation of abnormal cells followed by invasion to surrounding tissues and metastasis to other organ sites, is now the second leading cause of death worldwide (Hanahan and Weinberg 2011; Fitzmaurice et al. 2017). Since alkaloids isolated from Vinca rosea L. were found to have potent cytotoxic properties against cancer cells in 1959, natural products derived from various species, such as plants, microbes, and marine organisms, have been demonstrated as plentiful sources of lead compounds for therapeutic intervention in cancer (Johnson et al. 1959; Mann 2002; Bhanot et al. 2011; Du and Tang 2014). Indeed, numerous lead compounds with anticancer activity have been identified from natural resources, and drugs based on lead, such as vincristine, paclitaxel, doxorubicin, and rapamycin, are currently used to treat cancer or are being evaluated in preclinical trials (Mann 2002; Bhanot et al. 2011; Du and Tang 2014).
Chemotherapy using cytotoxic drugs to treat cancer has been shown to be effective in reducing tumor burden and removing residual cancer cells after surgery in cancer patients (Chabner and Roberts 2005). However, since those cytotoxic drugs were designed to target rapidly growing cells, they can also exert harmful effects on normal healthy cells and tissues depending on their proliferation rates (De Angelis 2008; Liu et al. 2015). Hence, cytotoxic chemotherapy is usually accompanied by various severe side effects, including fatigue, hair loss, bleeding, infection, nephropathy, and gastrointestinal pain (De Angelis 2008; Liu et al. 2015). In particular, nephrotoxicity and gastrointestinal toxicity of chemotherapy are serious dose-limiting adverse effects, resulting in high rates of therapy discontinuation and unsuccessful outcomes (Boussios et al. 2012; Brami et al. 2016).
Interestingly, a recently published study has demonstrated that PHY906, an herb mixture containing Glycyrrhiza uralensis Fisch, Paeonia lactiflora Pall, Scutellaria baicalensis Georgi, and Ziziphus jujuba Mill, reduces the gastrointestinal toxicity of capecitabine in patients with advanced pancreatic and gastrointestinal malignancies (Saif et al. 2010). In addition, Goshajinkigan, an herbal formula consisting of 10 natural ingredients, has been shown to alleviate nephrotoxic side effects of chemotherapy with paclitaxel combined with carboplatin in patients with ovarian and endometrial cancer (Kaku et al. 2012). This accumulating evidence suggests that natural products and their ingredients have enormous potential to provide clinical benefits to cancer patients by increasing the therapeutic index of chemotherapy and reducing its adverse effects. Therefore, screening of natural products with alleviating effects on chemotherapy-induced side effects as well as anticancer activity and identifying their bioactive compounds will have significant implications in cancer treatment.
Betula platyphylla var. japonica (Miquel) Hara (Betulaceae), well known as “Asian white birch,” is an endemic species widely distributed in the northern temperate zone mainly, Japan, China, and Korea (Matsuda et al. 1998; Huh et al. 2011). B. platyphylla var. japonica has been used in folk medicine, and its bark has been used in Chinese traditional medicine to treat a wide range of inflammatory diseases, including pneumonia, choloplania, nephritis, and chronic bronchitis, as well as cancer (Heo 1980; Matsuda et al. 1998; Ju et al. 2004; Huh et al. 2011). As part of a continuing search for bioactive compounds from natural sources (Kang et al. 2016; Lee et al. 2016; Yu et al. 2016; Beemelmanns et al. 2017; Yu et al. 2017), we have taken an interest in bioactive compounds from the bark of B. platyphylla var. japonica (Eom et al. 2016, 2017). In our recent studies on B. platyphylla var. japonica bark, we reported isolation of antioxidant triterpenoids combined with a phenylpropanoid unit (Eom et al. 2016) and cytotoxic triterpenoids against several human tumor cells (Eom et al. 2017).
In this study, medicinal properties of betulin identified from B. platyphylla var. japonica useful for cancer treatment were investigated. LC/MS analysis of the EtOH extract of B. platyphylla var. japonica bark revealed betulin as a main chemical component. An LC/MS guided isolation technique was applied to separate betulin and effectively reduce analysis time. In the present study, we report LC/MS-guided isolation and structural elucidation of betulin and evaluation of its medicinal properties useful for cancer management.
Materials and methods
General experimental procedures
Optical rotations were measured on a Jasco P-1020 polarimeter (Jasco, Easton, MD, USA). Infrared (IR) spectra were recorded on a Bruker IFS-66/S FT-IR spectrometer (Bruker, Karlsruhe, Germany). Ultraviolet (UV) spectra were acquired on an Agilent 8453 UV–visible spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE III 700 NMR spectrometer operating at 700 MHz (1H) and 175 MHz (13C), with chemical shifts given in ppm (δ) (Bruker). Semi-preparative high-performance liquid chromatography (HPLC) used a Shimadzu Prominence HPLC System with SPD-20A/20AV Series Prominence HPLC UV–Vis Detectors (Shimadzu, Tokyo, Japan). LC/MS analysis was performed on an Agilent 1200 Series HPLC system equipped with a diode array detector and a 6130 Series ESI mass spectrometer using an analytical Kinetex® 5 µm C18 100 Å column (5 μm, 2.1 × 100 mm, Phenomenex, Torrance, CA, USA). Column chromatography was performed with silica gel 60 (Merck, Darmstadt, Germany; 230–400 mesh) and RP-C18 silica gel (Merck, 230–400 mesh). Merck pre-coated silica gel F254 plates and reverse-phase (RP)-18 F254s plates (Merck) were used for thin-layer chromatography (TLC). Spots were detected on TLC under UV light or by heating after spraying with anisaldehyde–sulfuric acid.
Plant materials
The bark of B. platyphylla var. japonica was collected from Danyang, Chungcheongbuk-do, Korea, in October 2014. The material was identified by one of the authors (K. H. Kim). A voucher specimen (NM-14-063) was deposited in the herbarium of the Natural Medicine Research Center of Richwood Pharmaceutical Company, Ltd., Seoul, Korea.
Extraction and isolation
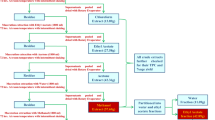
Dried bark of B. platyphylla var. japonica (4.1 kg) was extracted with 80% EtOH (18 L × 1 day × three times) at room temperature and filtered. The resultant was evaporated under reduced pressure using a rotavapor to obtain EtOH extract (351 g). The EtOH extract was dissolved in sterile distilled water, and a small aliquot of the EtOH extract was sequentially injected into LC/MS and eluted with a gradient solvent system of MeOH/H2O (1:9–1:0, flow rate of 0.3 mL/min, UV 210 nm), which revealed the presence of betulin with a molecular ion peak at m/z 465.4 [M + Na]+ in positive ESI mode by comparison with our house-built UV library in LC/MS as a main chemical composition. The EtOH extract in distilled water was successively solvent-partitioned with CHCl3, EtOAc, and n-BuOH, yielding residues weighing 274 g, 25 g, and 30 g, respectively. All the fractions were subjected to LC/MS and eluted with a gradient solvent system of MeOH/H2O (1:9–1:0, flow rate of 0.3 mL/min, UV 210 nm) to identify the target constituent, betulin. Based on LC/MS data, one major peak of betulin was detected in the CHCl3-soluble fraction. The CHCl3-soluble fraction (25 g) was separated by silica gel column chromatography using a solvent system of CH2Cl2–MeOH–H2O (9:3:0.1) to provide seven fractions (I–VII). All seven fractions were subjected to LC/MS prior to purification for target isolation of betulin, which revealed that betulin was detected in fraction II (5.3 g), which was fractionated by reverse-phase (RP)-C18 column chromatography with 70% MeOH and 100% MeOH to give six sub-fractions (II-1–II-6). The desired betulin peak was identified in faction II-5 (1.5 g) according to the LC/MS analysis and the fraction was further subjected to silica column chromatography using a gradient solvent system of CH2Cl2–MeOH (15:1, 3:1, 1:1) to obtain 9 sub-fractions [II-5(1)–II-5(9)]. Finally, LC/MS-guided isolation led to purification of betulin (250 mg) from fraction II-5(3) (380 mg) by semi-preparative RP HPLC with a gradient solvent system of MeOH-H2O (93% MeOH–85% MeOH in 30 min) using a Phenomenex Luna C18(2) column (250 mm × 10 mm i.d., 10 μm).
Betulin: white needles, [α]+22.6 (c 0.08, CHCl3); IR (KBr) νmax: 3300 ~ 3600, 2944, 1458, 1374, 1038 cm−1; 1H NMR (700 MHz, CDCl3) δ: 0.76, 0.82, 0.97, 0.98, 1.02 (3H each, s, 5 × CH3), 1.66 (3H, s, H–30), 3.14 (1H, dd, J = 10.5, 5.0 Hz, H–3), 3.33 (1H, d, J = 11.0 Hz, H–28), 3.80 (1H, d, J = 11.0 Hz, H–28), 4.58 (1H, br s, H–29), 4.68 (1H, br s, H–29); 13C NMR (175 MHz, CDCl3) δ: 14.9 (C–27), 15.5 (C–24), 16.2 (C–25), 16.3 (C–26), 18.5 (C–6), 19.3 (C–30), 21.0 (C–11), 25.4 (C–12), 27.2 (C–15), 27.6 (C–2), 28.2 (C–23), 29.4 (C–16), 29.9 (C–21), 34.2 (C–22), 34.4 (C–7), 37.4 (C–13), 37.5 (C–10), 38.9 (C–4), 39.1 (C–1), 41.1 (C–8), 42.9 (C–14), 48.0 (C–18), 48.0 (C–17), 49.0 (C–19), 50.6 (C–9), 55.5 (C–5), 60.8 (C–28), 79.2 (C–3), 109.9 (C–29), 150.7 (C–20); ESI–MS m/z 465.4 [M + Na]+.
Chemicals and reagents
Cisplatin was purchased from Sigma-Aldrich (Seoul, South Korea). Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Cellgro (Manassas, VA, USA). Fetal bovine serum (FBS) was purchased from Invitrogen Co. (Grand Island, NY, USA).
Cell culture
The human lung cancer cell lines A549, H1264, and Calu-6 were kindly provided by Dr. Steven M. Albelda (University of Pennsylvania School of Medicine, Philadelphia, PA, USA) and maintained in RPMI-1640 medium (WelGENE, Seoul, Korea) supplemented with 10% fetal bovine serum (FBS, Gemini Bio-Products, West Sacramento, CA, USA), 2 mM l-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin (WelGENE). LLC-PK1 cells, a pig kidney epithelial cell line, were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in DMEM (WelGENE) containing 10% FBS, 4 mM l-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin.
Cell viability analysis
Human lung cancer cells were seeded at a density of 5 × 103 cells per well in a 96 well tissue culture plate (Thermo Scientific, Waltham, MA, USA) and treated with betulin isolated from the bark of B. platyphylla var. japonica or equivalent amounts of DMSO as vehicle control for 48 h. Cell viability was then assessed using the WST-1 cell proliferation assay according to the manufacturer’s instructions (Daeil Lab Service, Seoul, Korea). Briefly, 10 μL of the WST-1 reagent was added to each well, and the cells were further incubated for 1 h at 37 °C. Then the absorbance of each well at 450 nm was measured using a scanning multi-well spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) and the cell viability was determined as a percentage of vehicle controls. IC50 values of betulin in human lung cancer cells were determined by non-linear regression analysis of the dose–response curve using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA).
Renoprotective effect against cisplatin-induced kidney cell damage
The renoprotective effect against oxidative renal cell damage was evaluated using LLC-PK1 cells. Cells were seeded in 96-well culture plates at 1 × 104 cells per/well and allowed to adhere for 2 h. Thereafter, the test sample and/or 25 μM cisplatin were added to the culture medium and incubated for 24 h. Then, medium containing the test sample and/or radical donor was removed, and the cells were incubated with serum-free medium (90 µL/well) and Ez-Cytox reagent (10 µL/well) for 2 h at 37 °C. Cell viability was measured by absorbance at 450 nm using a microplate reader (PowerWave XS; Bio-Tek Instruments, Winooski, VT, USA).
Ethanol-induced gastric mucosal injury in rats
All procedures involving the use of live animals described in this study were approved in November 2015 by the Institutional Animal Care and Use Committee of Gachon University (approval number: GIACUC- R2015011), and NIH guidelines were strictly followed for humane treatment of animals. Male Wistar rats weighing 200–220 g were used to evaluate the protective effect of betulin against ethanol-induced mucosal injury in rats. The rats were deprived of food but had free access to water for 24 h before ulcer induction. Gastric mucosal lesions were induced by oral administration of 1 mL of a 60% ethanolic solution containing 0.15 M HCl. Rats were divided into 4 groups based on body weight and treated with either ethanol or ethanol + betulin:
-
Group 1: Normal controls (n = 3), received water only.
-
Group 2: Ethanol (n = 3), received ethanol only.
-
Group 3: Ethanol + betulin 11 (n = 3), orally treated with ethanol and betulin (11 mg/mL) in aqueous solution.
-
Group 4: Ethanol + betulin 22 (n = 3), orally treated with ethanol and betulin (22 mg/mL) in aqueous solution.
All animals were pretreated with water or betulin orally 1 h before the ethanol treatment. After 6 h, the rats were sacrificed, and their stomachs were immediately removed.
Determination of mucosal lesion level
Rat stomachs were removed and opened along the greater curvature to determine the extent of lesion damage. To perform quantitative analysis of mucosal lesions, pictures of stomach tissue were captured and analyzed using the image analysis software Leica Application Suite V3.8 (Leica, Seoul, Korea).
Statistical analysis
All data were presented as the average value and standard deviation (SD). Multiple comparison tests for different dose groups were conducted. The Kruskall-Wallis one-way analysis of variance and data found significant were further analyzed using Mann–Whitney U test. The SPSS statistical package was used for all analyses (IBM SPSS statistics version 21, Boston, Mass). Statistical significance was considered at p values lower than 0.05.
Results and discussion
LC/MS-guided isolation and structural elucidation of betulin
B. platyphylla var. japonica bark was extracted with 80% EtOH and then filtered. After evaporation of the filtrate, the resultant EtOH extract was obtained. By comparison with our house-built UV library, LC/MS analysis of the extract indicated the presence of betulin as a main chemical component with a molecular ion peak at m/z 465.4 [M + Na]+ in positive ESI mode. The high sensitivity and selectivity of the LC/MS-guided isolation method selectively reduced analysis time and enabled fast isolation of target compounds. The target compound, betulin, was isolated by open-column chromatography and semi-preparative HPLC monitored by LC/MS analysis, which led to identification of fractions containing the desired compound. Final LC/MS analysis of fraction II-5(3) depicted betulin with a molecular ion peak at m/z 465.4 [M + Na]+ and UV absorptions indicative of triterpenoids, and it was isolated by semi-preparative HPLC. The structure of betulin was identified by spectroscopic methods of 1H and 13C NMR and LC/MS analysis and comparison of spectroscopic and physical data with previously reported data (Fig. 1) (Kao et al. 2004).
Cytotoxic effects of betulin isolated from B. platyphylla var. japonica bark on human lung cancer cell lines
Betulinic acid, a naturally occurring pentacyclic triterpene found in the bark of several plant species, and its derivatives have shown cytotoxic activity against a broad spectrum of cancer cells through induction of apoptosis (Periasamy et al. 2014). Betulin isolated from B. platyphylla var. japonica bark by LC/MS-guided isolation was structurally related to betulinic acid, in which the C-28 hydroxymethyl of betulin is oxidized to a carboxyl group (Fig. 1). Therefore, we first verified the cytotoxicity of the isolated betulin in human lung adenocarcinoma cells A549, H1264, and Calu-6 (Fig. 2). Consistent with previously published studies (Periasamy et al. 2014), treatment with betulin dramatically decreased cell viability in a dose-dependent manner in all human lung cancer cell lines tested, with IC50 values ranging from 18.7 to 39.6 μM (Fig. 2a). The lung cancer cells treated with betulin showed typical morphological changes observed in apoptotic cells (Elmore 2007), such as cell rounding, cell shrinkage, and membrane blebbing (Fig. 2b). Taken together, these observations demonstrate that betulin isolated from B. platyphylla var. japonica bark also possesses cytotoxic activity toward human cancer cells.
Betulin isolated from B. platyphylla var. japonica bark reduces cell viability in human lung adenocarcinoma cell lines. a Cell viability assessed by WST-1 assay in human lung adenocarcinoma cells A549, H1264, and Calu-6. b Representative bright-field images of human lung adenocarcinoma cells treated with betulin at indicated concentrations. Data are presented as mean ± SEM. Scale bar: 100 μm
Evaluation of renoprotective effects of betulin in anticancer drug-induced nephrotoxicity
Cis-diamminedichloroplatinum (II), also known as cisplatin, is a platinum-based chemotherapeutic drug widely used to treat a broad spectrum of malignancies, including bladder, cervical, ovarian, breast, head and neck, small cell lung cancer, and testicular cancers (Florea and Büsselberg 2011; Dasari and Tchounwou 2014). However, use of cisplatin is often limited by its nephrotoxicity (Yao et al. 2007). Numerous attempts to mitigate cisplatin-induced nephrotoxicity have failed to identify an appropriate treatment. In addition to cisplatin, nephrotoxicity is a serious dose-limiting adverse effect for many other anticancer drugs (Boussios et al. 2012; Brami et al. 2016).
In traditional Chinese medicine, B. platyphylla var. japonica bark has been used to protect kidney function and to treat renal damage such as nephritis. To explore the additional medicinal properties of betulin from the bark of B. platyphylla var. japonica, we evaluated its nephroprotective potential by assessing its effect on cisplatin-induced renal cell damage in LCC-PK1 cells. As shown in Fig. 3, betulin ameliorated cisplatin-induced renal cell damage to 80% of the control value from the concentration of 5 μM. Consequently, we propose that betulin exerts protective effects against anticancer drug-induced renal cell damage.
Our previous study reported that lupane triterpenes (betulinic acid, 29-oxobetulinic acid, betulin 3-acetate, and lupeol) from Cornus walteri ameliorate cisplatin-induced renal cell damage (Lee et al. 2015). Therefore, cisplatin-induced renal cell damage could be prevented by lupane-type triterpenes, and their biological activity may help protect cells from damage caused by cisplatin.
To evaluate the effect of betulin on the cytotoxicity of cisplatin, the effects of co-treatment of betulin and cisplatin on the MCF-7 breast cancer cell viability were determined by MTT assay (Peng et al. 2016; Taher et al. 2016; Aayadi et al. 2017; Jung et al. 2017) since cisplatin as a single agent is recognized as first-line treatment in breast cancer (Vassilomanolakis et al. 2005). The results showed that betulin did not affect therapeutic efficiency of cisplatin (Supplementary material Fig. S5).
Anti-gastritis activity of betulin against ethanol-induced gastric damage in rats
Bacterial infections such as Helicobacter pylori, use of pain relievers, excessive use of alcohols and stress are common causes of gastritis (Tol et al. 2008; Jeon et al. 2014). Gastrointestinal toxicity such as gastritis is also a serious dose-limiting adverse effect induced by chemotherapy. Although there are several gastric damage models using various inducing agents including H. pylori, stress and aspirin for the screening of gastro-protective drugs (Auguste et al. 1990; Hamlet et al. 1998; Verma and Kumar 2018), betulin was further tested for anti-gastritis activity against ethanol-induced gastric damage in rats because ethanol is regarded as a major contributor to mucosal lesion formation and ulcer in the gastric mucosa (Jeon et al. 2014; Kim et al. 2017) and chemotherapy-induced gastrointestinal toxicity is typically associated with mucosal lesions and gastric ulcer (Mori et al. 1995; Tol et al. 2008; Fukuhara et al. 2011). The severity of gastric mucosal injury in rats induced by ethanol is shown in Fig. 4. The model group showed macroscopic morphological changes including glandular area hyperemia and mucosal edema accompanied by dot and linear hemorrhage necrosis. Figure 4 shows that, when gastric lesions were treated with betulin, ulcer formation was significantly reduced in a dose-dependent manner at doses of 11 and 22 mg/mL. Based on this result, we confirmed that betulin is effective for mitigation of ethanol-induced mucosal injury.
Exposure to ethanol in vivo induces ulcers and produces necrotic injury in the gastric mucosa (Jeon et al. 2014; Kim et al. 2017). Although the pathological factors of ethanol-induced gastric damage are complex, this damage may be associated with inflammation and apoptosis. The factors are correlated to oxidative stress caused by an imbalance between oxidant and antioxidant cellular processes (Jeon et al. 2014; Liang et al. 2018). The previous studies have demonstrated that betulin has antioxidant effects (Ma and Long 2016; Chunhua et al. 2017). Ethanol-induced gastric damage might be prevented by betulin, which may help protect cells from damage caused by oxidative stress.
In conclusion, betulin was isolated as a main component from the bark of B. platyphylla var. japonica using an LC/MS-guided isolation technique in the current study. To identify bioactive natural products with alleviating effects on chemotherapy-induced side effects and anticancer activity, medicinal properties of betulin from B. platyphylla var. japonica useful for cancer management were investigated. In parallel with previous studies (Periasamy et al. 2014), we also found that betulin derived from the bark of B. platyphylla var. japonica also exhibits cytotoxic activity against human lung cancer cells in vitro. In addition, of note, betulin was shown to protect renal cells from cisplatin-induced cell death in vitro and reduce ethanol-induced gastric mucosal injury in vivo. These data provide the first experimental evidence for potential use of B. platyphylla var. japonica as a functional food for alleviation of adverse effects of chemotherapy as well as for therapeutic intervention in cancer.
References
Aayadi H, Mittal SPK, Deshpande A, Gore M, Ghaskadbi SS (2017) Cytoprotective effect exerted by geraniin in HepG2 cells is through microRNA mediated regulation of BACH-1 and HO-1. BMB Rep 50:560–565
Auguste LJ, Lackner R, Ratner L, Stein TA, Bailey B (1990) Prevention of stress-induced erosive gastritis by parenteral administration of arachidonic acid. JPEN J Parenter Enteral Nutr 14:615–617
Beemelmanns C, Ramadhar TR, Kim KH, Klassen JL, Cao S, Wyche TP, Hou Y, Poulsen M, Bugni TS, Currie CR (2017) Macrotermycins A-D, glycosylated macrolactams from a termite-associated Amycolatopsis sp. M39. Org Lett 19:1000–1003
Bhanot A, Sharma R, Noolvi MN (2011) Natural sources as potential anti-cancer agents: a review. Int J Phytomed 3:09
Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N (2012) Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol 25:106
Brami C, Bao T, Deng G (2016) Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol 98:325–334
Chabner BA, Roberts TG Jr (2005) Chemotherapy and the war on cancer. Nat Rev Cancer 5:65
Chunhua M, Long H, Zhu W, Liu Z, Jie R, Zhang Y, Wang Y (2017) Betulin inhibited cigarette smoke-induced COPD in mice. Biomed Pharmacother 85:679–686
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
De Angelis C (2008) Side effects related to systemic cancer treatment: are we changing the Promethean experience with molecularly targeted therapies? Curr Oncol 15:198
Du J, Tang XL (2014) Natural products against cancer: a comprehensive bibliometric study of the research projects, publications, patents and drugs. J Can Res Ther 10:27
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Eom HJ, Kang HR, Kim HK, Jung EB, Park HB, Kang KS, Kim KH (2016) Bioactivity-guided isolation of antioxidant triterpenoids from Betula platyphylla var. japonica bark. Bioorg Chem 66:97–101
Eom HJ, Kang HR, Choi SU, Kim KH (2017) Cytotoxic Triterpenoids from the Barks of Betula platyphylla var. japonica. Chem Biodivers 14:e1600400
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed Orchir O, Dandona R, Dandona L (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 3:524–548
Florea AM, Büsselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers 3:1351–1371
Fukuhara K, Terakura M, Katsuragi K (2011) Severe gastric and duodenal ulcer after chemotherapy of mFOLFOX6 and bevacizumab. Gan to kagaku ryoho Cancer chemother 38:457–459
Hamlet A, Lindholm C, Nilsson O, Olbe L (1998) Aspirin-induced gastritis, like Helicobacter pylori-induced gastritis disinhibits acid secretion in humans: relation to cytokine expression. Scand J Gastroenterol 33:346–356
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Heo J (1980) Donguibogam. Namsadang, Seoul
Huh JE, Hong JM, Baek YH, Lee JD, Choi DY, Park DS (2011) Anti-inflammatory and anti-nociceptive effect of Betula platyphylla var. japonica in human interleukin-1β-stimulated fibroblast-like synoviocytes and in experimental animal models. J Ethnopharmacol 135:126–134
Jeon WY, Shin IS, Shin HK, Lee MY (2014) Gastroprotective effect of the traditional herbal medicine, Sipjeondaebo-tang water extract, against ethanol-induced gastric mucosal injury. BMC Complement Altern Med 14:373
Johnson I, Wright H, Svoboda G (1959) Experimental basis for clinical evaluation of anti tumor principles derived from Vinca-Rosea Linn. J Lab Clin Med 54:830
Ju EM, Lee SE, Hwang HJ, Kim JH (2004) Antioxidant and anticancer activity of extract from Betula platyphylla var. japonica. Life Sci 74:1013–1026
Jung EJ, Chung KH, Kim CW (2017) Identification of simvastatin-regulated targets associated with JNK activation in DU145 human prostate cancer cell death signaling. BMB Rep 50:466–471
Kaku H, Kumagai S, Onoue H, Takada A, Shoji T, Miura F, Yoshizaki A, Sato S, Kigawa J, Arai T (2012) Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: a multicenter collaborative study. Exp Ther Med 3:60–65
Kang HR, Lee D, Benndorf R, Jung WH, Beemelmanns C, Kang KS, Kim KH (2016) Termisoflavones A-C, Isoflavonoid Glycosides from Termite-Associated Streptomyces sp. RB1. J Nat Prod 79:3072–3078
Kao KC, Ho YL, Lin I, Ho LK, Chang YS (2004) Flavone glycosides from Strobilanthes formosanus. J Chin Chem Soc 51:199–204
Kim JI, Park SW, Lim JJ, Sohn SI, Shin JS, Park SC, Jang YP, Chung EK, Lee HW, Lee KT (2017) Gastroprotective effects of the isopropanol extract of Artemisia princeps and its gastroretentive floating tablets on gastric mucosal injury. Acta Pharm 67:479–494
Lee S, Jung K, Lee D, Lee SR, Lee KR, Kang KS, Kim KH (2015) Protective effect and mechanism of action of lupane triterpenes from Cornus walteri in cisplatin-induced nephrotoxicity. Bioorg Med Chem Lett 25:5613–5618
Lee SR, Park JY, Yu JS, Lee SO, Ryu JY, Choi SZ, Kang KS, Yamabe N, Kim KH (2016) Odisolane, a novel oxolane derivative, and antiangiogenic constituents from the fruits of mulberry (Morus alba L.). J Agric Food Chem 64:3804–3809
Liang J, Dou Y, Wu X, Li H, Wu J, Huang Q, Luo D, Yi T, Liu Y, Su Z, Chen J (2018) Prophylactic efficacy of patchoulene epoxide against ethanol-induced gastric ulcer in rats: influence on oxidative stress, inflammation and apoptosis. Chem Biol Interact 283:30–37
Liu B, Ezeogu L, Zellmer L, Yu B, Xu N, Joshua Liao D (2015) Protecting the normal in order to better kill the cancer. Cancer Med 4:1394–1403
Ma C, Long H (2016) Protective effect of betulin on cognitive decline in streptozotocin (STZ)-induced diabetic rats. Neurotoxicology 57:104–111
Mann J (2002) Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer 2:143
Matsuda H, Ishikado A, Nishida N, Ninomiya K, Fujiwara H, Kobayashi Y, Yoshikawa M (1998) Hepatoprotective, superoxide scavenging, and antioxidative activities of aromatic constituents from the bark of Betula platyphylla var. japonica. Bioorg Med Chem Lett 8:2939–2944
Mori K, Tominaga K, Yokoyama K, Suga Y, Kishiro I, Tsurui M (1995) Efficacy of famotidine in patients with acute gastric mucosal injury after continuous infusion of cisplatin plus vindesine. J Cancer Res Clin Oncol 121:367–370
Peng Y, Zhong Y, Li G (2016) Tubeimoside-1 suppresses breast cancer metastasis through downregulation of CXCR4 chemokine receptor expression. BMB Rep 49:502–507
Periasamy G, Teketelew G, Gebrelibanos M, Sintayehu B, Gebrehiwot M, Karim A, Geremedhin G (2014) Betulinic acid and its derivatives as anti-cancer agent: a review. Arch Appl Sci Res 6:47–58
Saif MW, Lansigan F, Ruta S, Lamb L, Mezes M, Elligers K, Grant N, Jiang Z, Liu S, Cheng Y (2010) Phase I study of the botanical formulation PHY906 with capecitabine in advanced pancreatic and other gastrointestinal malignancies. Phytomedicine 17:161–169
Taher M, Aminuddin A, Susanti D, Aminudin NI, On S, Ahmad F, Hamidon H (2016) Cytotoxic, anti-Inflammatory and adipogenic effects of inophyllum D, calanone, isocordato-oblongic acid, and morelloflavone on cell lines. Nat Prod Sci 22:122–128
Tol J, Cats A, Mol L, Koopman M, Bos MMEM, Van Der Hoeven JJM, Antonini NF, Van Krieken JHJM, Punt CJA (2008) Gastrointestinal ulceration as a possible side effect of bevacizumab which may herald perforation. Invest New Drugs 26:393–397
Vassilomanolakis M, Koumakis G, Barbounis V, Demiri M, Panopoulos C, Chrissohoou M, Apostolikas N, Efremidis AP (2005) First-line chemotherapy with docetaxel and cisplatin in metastatic breast cancer. Breast 14:136–141
Verma S, Kumar VL (2018) Artesunate affords protection against aspirin-induced gastric injury by targeting oxidative stress and proinflammatory signaling. Pharmacol Rep 70:390–397
Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334:115–124
Yu JS, Baek J, Park HB, Moon E, Kim SY, Choi SU, Kim KH (2016) A new rearranged eudesmane sesquiterpene and bioactive sesquiterpenes from the twigs of Lindera glauca (Sieb. et Zucc.) Blume. Arch Pharm Res 39:1628–1634
Yu JS, Moon E, Kim KH (2017) A new cerebroside from the twigs of Lindera glauca (Sieb. et Zucc.) Blume. Bioorg Chem 74:122–125
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1C1A1A02037383) and by the Ministry of Education (NRF-2012R1A5A2A28671860).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
So, H.M., Eom, H.J., Lee, D. et al. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharm. Res. 41, 815–822 (2018). https://doi.org/10.1007/s12272-018-1064-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1064-9