Abstract
Exercise has beneficial effects in ameliorating metabolic disorders, and a combined therapeutic regimen of regular exercise and pharmaceutical treatment is often recommended. Exercise biology is complex and it involves various metabolic and molecular changes that translate into changes in substrate utilization, enzyme activation, and alternatively, improvement in exercise performance. Besides the effect of exercise on muscle metabolism, it has recently been discovered that contracting muscle can induce secretion of molecules called myokines. In the past few decades, a number of myokines have been discovered, such as interleukin-6, irisin, myostatin, interleukin-15, brain-derived neurotrophic factor, β-aminoisobutyric acid, meteorin-like, leukemia inhibitory factor, and secreted protein acidic and rich in cysteine, through secretome analysis. The existence of myokines has enhanced our understanding of how muscles communicate with other organs such as adipose tissue, liver, bone, and brain to exert beneficial effects of exercise at the whole body level. In this review, we focus on the role of these myokines in regulating local muscle metabolism as well as systemic metabolism in an autocrine/paracrine/endocrine fashion. The therapeutic potential of myokines and the natural or synthetic compounds known to date that regulate myokines are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise is by far an effective way to improve health. In contrast, physical inactivity is associated with development of various diseases such as type 2 diabetes mellitus (T2DM), sarcopenia, osteoporosis, cardiovascular disease, and cancer (Tuomilehto et al. 2001; Monninkhof et al. 2007; Nocon et al. 2008; Wolin et al. 2009; Naseeb and Volpe 2017). Moreover, exercise on a regular basis exerts beneficial effects on metabolic health through not only modifying the traditional risk factors, such as blood glucose and lipid levels, but also by directly regulating glucose transport, insulin utilization, endothelial function, autonomic nervous system etc. (Goodyear and Kahn 1998; Joyner and Green 2009). Therefore, studying the exercise modality can help us discover biomarkers and therapeutic molecules which could underpin numerous physical inactivity-related disorders. However, it is difficult to dissect the mechanisms underlying exercise-induced changes since exercise is a highly complex process which simultaneously involves integrative and adaptive responses in multiple tissues and organs at the cellular and systemic level. Studies have been performed during the past few decades in an effort to elucidate the cellular and molecular mechanisms of acute and chronic exercise, but the majority of exercise biology still remains poorly understood. Anatomically, skeletal muscle is the largest organ which constitutes about 40% of the total body mass, and therefore, it plays a major role in regulation of metabolism. Along with the local effects of skeletal muscle on metabolism, it has recently been discovered that, similar to adipocytes, skeletal muscle is a secretory organ responsible for the production of several hundreds of peptides classified as ‘myokines’ (Bortoluzzi et al. 2006; Yoon et al. 2009; Henningsen et al. 2010). The discovery of myokines has opened a new door for understanding the biology of exercise, providing evidence that muscles are able to communicate with other organs, such as bone, liver, adipose tissue, brain, etc. In this review, we focus on providing an update on some of the well-known myokines as well as the newly discovered myokines, and study their role in mediating the beneficial effects of exercise on metabolism through either an autocrine, paracrine, or endocrine mechanism.
Exercise physiology
Adaptation to exercise is a complex process as it involves diverse changes in transcriptional and translational responses, mitochondrial function, metabolic regulation, and signaling pathways that govern these changes (Egan and Zierath 2013). In simple terms, the molecular and metabolic responses to exercise can be first categorized into acute exercise (single bout) and chronic exercise training. Exercise training leads to molecular adaptations and these responses can be further classified as adaptation to aerobic (endurance) and resistance exercise. Acute exercise can alter the expression of various genes (Yang et al. 2005) and phosphorylation of proteins (Hoffman et al. 2015) to stimulate the muscle adaptation. However, a transient response to acute exercise is insufficient to alter the muscle phenotype. Rather, phenotypic adaptation in response to chronic exercise training involves accumulation of repeated single bout exercise-induced stimulation. Chronic exercise causes changes in the protein content and subsequently the enzyme function, resulting in improved exercise performance. During acute exercise, the metabolic pathway which provides the energy source is mostly determined by the relative duration and intensity of exercise. If exercise is performed at a low or moderate intensity, glucose derived from the liver or from oral ingestion (Coker and Kjaer 2005), and free fatty acids (FFA) from adipose tissue (Horowitz 2003) primarily provide the fuel needed to the skeletal muscle. If the intensity of exercise is increased, the contribution of circulating FFA is modestly declined while the use of circulating glucose is extensively upregulated (van Loon et al. 2001). If the exercise is continued for more than 1 h at a fixed intensity, the use of energy from lipid oxidation inclines (Romijn et al. 1993). In the case of aerobic exercise, mitochondrial biogenesis is one of the well-known molecular adaptation processes (Howald et al. 1985). Increased mitochondrial ATP production, glucose transport, utilization of fatty acids, and antioxidant capacity all reflect the enhancement of intrinsic oxidative capacity of the muscle after endurance training (Holloszy and Coyle 1984; Powers et al. 1994; Perseghin et al. 1996; Talanian et al. 2010). Among various regulators of skeletal muscle phenotype, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) is a well-defined transcription factor responsible for mitochondrial biogenesis, transformation of muscle fiber type, and regulation of skeletal muscle metabolism (Wu et al. 1999; Lin et al. 2005). On the other hand, resistance exercise is an efficient exercise intervention to improve muscle function in terms of its strength, power, and size through morphological and neurological adaptations (Booth and Thomason 1991; Folland and Williams 2007). The major pathway related to resistance exercise-induced muscle hypertrophy involves p70S6K and mTOR signaling. These pathways combine the nutrient and metabolic stimuli to induce cellular growth and proliferation (Baar and Esser 1999; Bodine et al. 2001). Also, anabolic hormones such as insulin-like growth factor (IGF)-1 can induce mTOR activation and thus adaptive hypertrophy (Adams and McCue 1998). Further details on the molecular mechanisms related to exercise-induced skeletal muscle adaptation have been described elsewhere (Egan and Zierath 2013).
The skeletal muscle as an endocrine organ
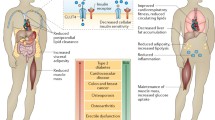
More than 50 years ago, there was a notion that skeletal muscle may secrete humoral factors. This was hypothesized based on the fact that when a muscle contracts, the physiology and metabolism of other organs are affected (Goldstein 1961). Later through secretome profiling, numerous myokines were discovered. Myokines are molecules that are expressed, produced, and released by muscle fibers which exert autocrine, paracrine, or endocrine effects (Pedersen et al. 2003). The autocrine and paracrine effects of myokines are mostly involved in the regulation of muscle physiology, such as muscle growth or lipid metabolism, which can provide a feedback loop for the muscle to adapt to exercise training. In contrast, the endocrine effect of myokines is important in mediating the whole-body effect of exercise. To date, the muscle is known to crosstalk with adipose tissue, liver, pancreas, bone, and brain. Among these interactions, the crosstalk with adipose tissue is interesting as adipose tissues are also recently discovered to exert an endocrine effect through secretion of adipokines (Maury and Brichard 2010). During physical inactivity, adipose tissue secretes adipokines, which are mostly pro-inflammatory cytokines, to mediate the pathological process (Fig. 1). It is now well recognized that adipose tissue inflammation can lead to development of metabolic diseases, such as T2DM and atherosclerosis (Iyer et al. 2010). In contrast, myokines are produced during exercise to mediate the health benefits of exercise (Pedersen and Febbraio 2012). Therefore, it is hypothesized that myokines may counteract the harmful effects of pro-inflammatory adipokines and maintain the whole body homeostasis. In the following section, we will focus on some of the roles of myokines that have been discovered to date.
Relationship between adipose tissue derived adipokines and skeletal muscle derived myokines. In the state of sedentary lifestyle, nutrient overload results in accumulation of fat and subsequent disturbance in adipocyte metabolism, which results in secretion of adipokines which are primarily proinflammatory cytokines. In contrast, contracting muscles in response to exercise secretes myokines, which are suggested to counteract the effects of proinflammatory adipokines. Therefore, the metabolic homeostasis is regulated by balance between adipokines and myokines, and are critical in development of metabolic diseases
Interleukin-6
Interleukin-6 (IL-6) is known as the prototypical myokine induced by contracting skeletal muscle during exercise. During exercise, the circulating IL-6 levels derived from the muscle fibers are elevated up to 100-fold and is correlated with the duration and intensity of exercise (Pedersen and Febbraio 2008; Raschke and Eckel 2013). As early as after 30 min of acute exercise, IL-6 transcription is increased (Fischer 2006), which contributes to the increase in IL-6 secretion. It is confusing that IL-6 is generally classified as a pro-inflammatory cytokine, while as a myokine it is involved in the anti-inflammatory effect of exercise. Specifically, exercise-induced IL-6 is reported to inhibit the production of pro-inflammatory cytokines such as TNFα and IL-1β (Steinbacher and Eckl 2015). Along with its anti-inflammatory effect, myotube-produced IL-6 regulates satellite cell-mediated hypertrophic muscle growth (Serrano et al. 2008), induces glycogen breakdown and lipolysis via AMPK (Kelly et al. 2009), and enhances GLUT4 expression and insulin sensitivity which are canceled by injection of the IL-6 neutralizing antibody before exercise (Ikeda et al. 2016). IL-6 seems to play a dual role in insulin action in myotubes, where short-term insulin exposure shows an additive effect with IL-6 and chronic exposure produces insulin resistance (Nieto-Vazquez et al. 2008). Exercise-induced IL-6 is not only capable of regulating local muscle metabolism but it also exerts beneficial effects on systemic glucose homeostasis and lipid metabolism (Steinbacher and Eckl 2015). Of note, it has been proposed that the skeletal muscle-adipose tissue axis is important for the systemic effects of IL-6 (Pedersen and Febbraio 2012). In humans, IL-6 increases lipolysis and FFA oxidation in adipocytes, which suggests that IL-6 plays a critical role in regulation of fat metabolism (van Hall et al. 2003). Interestingly, IL-6 is involved in exercise training-induced uncoupling protein 1 (UCP1) expression in murine inguinal white adipose tissue (WAT) and thus it participates in adipocyte browning (Knudsen et al. 2014). It has also been recently reported that exercise-induced IL-6 plays a role in protection against myocardial ischemia reperfusion injury (McGinnis et al. 2015). Although numerous studies have discovered that exercise-induced IL-6 has a beneficial role in the regulation of metabolism, understanding IL-6 physiology is still a complex process due to its pro-inflammatory nature in general (Pal et al. 2014; Almuraikhy et al. 2016).
Irisin/FNDC5
Irisin is a PGC1α-dependent myokine suggested to mediate the effect of exercise on adipocyte browning by increasing the expression of UCP1 (Bostrom et al. 2012). In mice overexpressing PGC1α specifically in muscle, PGC1α induces the expression of a membrane protein fibronectin type III domain-containing protein 5 (FNDC5), and exercise triggers the cleavage of FNDC5 to secrete irisin into the bloodstream, which subsequently elevates energy expenditure in the subcutaneous adipose tissue through adipocyte browning (Bostrom et al. 2012). While discovery of irisin has received attention as a candidate for an exercise mimetic, numerous studies that thereafter investigated irisin came to somewhat controversial results, especially with respect to the circulating levels of irisin post-exercise (Bostrom et al. 2012; Huh et al. 2012; Ellefsen et al. 2014; Norheim et al. 2014; Albrecht et al. 2015; Jedrychowski et al. 2015). One possible reason for this discrepancy is the technique used to measure the plasma or serum irisin level. The concern was that human irisin antibodies used in some of the commercial ELISA kits were not able to accurately detect irisin, which may have caused inaccurate measurement or false-positive/false-negative results regarding exercise-induced circulating irisin levels (Perakakis et al. 2017). Recently, circulating human irisin was quantified using mass spectrometry in an antibody-independent manner. Through this technique, circulating irisin levels were detected and were increased by both acute and chronic exercise (Daskalopoulou et al. 2014; Jedrychowski et al. 2015), which concluded the discussion on whether human irisin exists in the circulation and whether it is regulated by exercise. Despite controversies over the effect of exercise on circulating irisin levels, the therapeutic potential of irisin has been proved in numerous reports. The beneficial role of irisin on skeletal muscle metabolism has been proposed by our group and others, and it was shown that irisin stimulates glucose uptake and lipid metabolism via activation of AMPK (Huh et al. 2014a, b; Lee et al. 2015; Rodriguez et al. 2015). Irisin is also involved in muscle growth through induction of IGF-1 and suppression of myostatin (Huh et al. 2014b). In addition to its effects on muscle, exogenous administration of irisin in mice induces adipocyte browning in subcutaneous fat through p38 MAPK and ERK1/2 activation (Zhang et al. 2014). In addition, FNDC5 overexpression in mice stimulates lipolysis via the cAMP-PKA-perilipin/HSL pathway in adipocytes, leading to reduced serum lipid levels (Xiong et al. 2015). In the liver, irisin stimulates glycogenesis while it reduces gluconeogenesis and lipogenesis through regulating GSK3, FOXO1, and SREBP2 (Liu et al. 2015; Xin et al. 2015; Tang et al. 2016). Interestingly, recent reports have suggested that irisin is not only a myokine but also an adipokine, although expressed to a lesser extent (Moreno-Navarrete et al. 2013; Roca-Rivada et al. 2013). Whether the expression of irisin in adipocytes contributes to the local adipocyte or whole body metabolism needs to be further examined. Although the effect of irisin has been implicated the most often in insulin-sensitive tissues, its beneficial effects on other organs such as bone, heart, and blood vessel are being reported (Xie et al. 2015; Fu et al. 2016; Colaianni et al. 2017).
Myostatin
Myostatin is a myokine primarily expressed and secreted by muscle fibers. It is unique in that myostatin is the only myokine reduced in response to exercise (McPherron et al. 1997). Myostatin inhibits satellite cell proliferation and differentiation in an autocrine and paracrine manner, and conversely, genetic deletion of myostatin leads to muscle hypertrophy in humans and mice (McPherron et al. 1997; Lee and McPherron 2001; Schuelke et al. 2004; Rodgers and Garikipati 2008; Relizani et al. 2014). While myostatin activation negatively regulates muscle growth, myostatin expression is downregulated after endurance as well as resistance exercise (Allen et al. 2011). Therefore, it has been proposed that the means of myostatin blockade (antibodies, soluble decoy activin receptor type II B, propeptides) could serve as a therapeutic target for treatment of patients with muscle dystrophies (Lebrasseur 2012). In addition to its local effects on muscle atrophy, myostatin can also modulate metabolic homeostasis through regulation of adipose tissue function (Zhao et al. 2005; Feldman et al. 2006; Guo et al. 2009). In mice fed a high-fat diet, it has been reported that inhibition of myostatin using soluble decoy activin receptor type II B ameliorates the development of obesity and insulin resistance, through mechanisms associated with lipolysis and mitochondrial lipid oxidation in adipose tissue and liver (Zhang et al. 2012). Interestingly, myostatin gene knockout mice show signs of fat browning in the WAT and this effect is thought to be mediated by AMPK activation in skeletal muscle and subsequent induction of PGC1α, FNDC5, and irisin (Zhang et al. 2012; Shan et al. 2013; Dong et al. 2016). On the other hand, in vitro studies have provided evidence that irisin downregulates myostatin gene expression in cultured mouse myocytes and human primary myotubes, suggesting a bidirectional regulation between myostatin and irisin in modulation of muscle growth (Huh et al. 2014a; Rodriguez et al. 2015). These findings highlight the myostatin-irisin pathway as a potential therapeutic target against obesity through adipocyte browning and subsequent induction of energy expenditure. Apart from the effect of myostatin on muscle and fat, myostatin also strongly accelerates osteoclast formation through SMAD2 and its absence ameliorates rheumatoid arthritis in mice (Camporez et al. 2016). Of note, follistatin is an endogenous inhibitor of myostatin. Follistatin is a hepatokine, which suggests a possible muscle-liver crosstalk in exercise physiology (Hansen et al. 2011). Recently, a phase II clinical trial has been completed using humanized monoclonal myostatin antibody (LY2495655), and it showed improvements such as increase in appendicular lean body mass in patients undergoing elective total hip arthroplasty (Woodhouse et al. 2016) and increased muscle power in older weak fallers (Becker et al. 2015). In addition, the antibody has shown promising results in preclinical models of tumor-induced muscle wasting (Smith et al. 2015).
Interleukin-15
Interleukin-15 (IL-15) belongs to the IL-2 superfamily and is expressed in human skeletal muscle (Quinn et al. 1995). IL-15 is primarily known for its anabolic effects on skeletal muscle. Specifically, it is known to stimulate the accumulation of contractile proteins in differentiated myocytes and muscle fibers (Quinn et al. 1995). IL-15 also modulates glucose uptake in cultured myocytes in vitro and in isolated skeletal muscle ex vivo through activation of the JAK3/STAT3 signaling pathway (Busquets et al. 2005; Krolopp et al. 2016). In addition, IL-15 exerts protective effect against H2O2-mediated oxidative stress (Li et al. 2014) and enhances mitochondrial activity through the PPARδ-dependent mechanism in skeletal muscle cells (Thornton et al. 2016). In addition to its effects on muscle, IL-15 downregulates the accumulation of lipids in preadipocytes and reduces the WAT mass, partly through stimulation of adiponectin secretion (Carbo et al. 2001; Quinn et al. 2005), which suggests that IL-15 mediates the exercise-induced muscle-fat crosstalk. Although numerous studies have demonstrated that exercise alters the IL-15 concentration in serum (Riechman et al. 2004; Tamura et al. 2011), there are somewhat conflicting data on the effect of exercise on IL-15 protein expression and secretion from skeletal muscle, which needs to be further studied in the future.
Brain-derived neurotrophic factor
Brain-derived neurotrophic factor (BDNF) is primarily known to be released from the hypothalamus and is a key element in the regulation of neuronal development, plasticity and energy homeostasis (Lapchak and Hefti 1992). In a meta-analysis, blood concentrations of BDNF were increased by acute exercise as well as aerobic exercise training, but not by resistance exercise training (Dinoff et al. 2016, 2017). It is interesting to note that the gene and protein expressions of BDNF are upregulated in human skeletal muscle after exercise, whereas this effect does not seem to translate into its secretion (Pedersen et al. 2009). Therefore, it remains to be elucidated whether skeletal muscle directly contributes to the increased circulating BDNF level. It has recently been reported that exercise induces hypothalamic BDNF and subcutaneous fat browning in mice (Cao et al. 2011). In line with this report, overexpression of FNDC5 using an adenoviral vector in mice upregulated circulating irisin levels, increased hippocampal BDNF expression, and induced subcutaneous fat browning (Wrann et al. 2013), suggesting that there exists an exercise-induced PGC1α/FNDC5/BDNF pathway, which serves as an evidence that irisin mediates the effect of exercise on muscle to brain. In relation to learning and memory, exercise-induced BDNF was shown to reduce the production of toxic amyloid beta peptides, which could be valuable in the treatment of Alzheimer’s disease (Nigam et al. 2017). In contrast to the beneficial effect of BDNF in the brain, the roles of BDNF in the periphery are not yet well characterized. Nevertheless, in addition to its role in the regulation of central metabolic pathways, studies have suggested that BDNF may act as a metabolic regulator of skeletal muscle. Specifically, BDNF has been shown to increase the phosphorylation of AMPK and ACC and thus enhance fatty acid oxidation and glucose utilization in skeletal muscle, in an autocrine and paracrine fashion (Matthews et al. 2009). Also, BDNF has been shown to ameliorate insulin resistance in several diabetic mouse models (Tonra et al. 1999; Tsuchida et al. 2001; Yamanaka et al. 2006).
β-Aminoisobutyric acid
β-Aminoisobutyric acid (BAIBA) is formed by the catabolism of thymine, and it has recently been identified in the culture media of myocytes overexpressing PGC1α, through metabolite screening (Roberts et al. 2014). Circulating BAIBA levels have been reported to be significantly increased by 3 weeks of voluntary running exercise training in mice and also by 20 weeks of supervised submaximal aerobic exercise training in humans (Roberts et al. 2014). BAIBA exerts various beneficial effects on muscle metabolism in an autocrine/paracrine manner. First, BAIBA increases mitochondrial FFA oxidation leading to amelioration of insulin signaling, especially the IRS-1/Akt pathway. In addition, BAIBA protects against inflammation in vivo through AMPK-PPARδ-dependent mechanisms (Roberts et al. 2014; Jung et al. 2015). Similar to its effects on muscle, the endocrine effect of BAIBA includes upregulation of mitochondrial FFA oxidation in adipocytes, resulting in reduced fat accumulation in mice (Maisonneuve et al. 2004; Begriche et al. 2008). BAIBA also interacts with liver, where it reduces hepatic de novo lipogenesis through PPARα activation (Roberts et al. 2014). Also, BAIBA attenuates hepatic ER stress and apoptosis via AMPK, leading to improvement in glucose/lipid metabolic disturbance in mice with T2DM (Shi et al. 2016). Similar to other myokines, BAIBA treatment has shown to induce fat browning through upregulation of thermogenic gene expression in murine WAT (Roberts et al. 2014). Recently, the therapeutic role of BAIBA in renal fibrosis has also been demonstrated, where BAIBA attenuates angiotensin II-induced fibroblast activation and extracellular matrix deposition (Wang et al. 2017).
Meteorin-like
A novel form of PGC1α has been recently discovered, which results from alternative promoter usage and splicing, and was named as PGC1α4. PGC1α4 does not seem to exert most of the known effects of PGC1α, such as regulation of mitochondrial oxidation, but rather is upregulated after resistance exercise, mediating the effect of exercise on muscle hypertrophy and strength in mice and humans (Ruas et al. 2012). Interestingly, mice with muscle-specific overexpression of PGC1α4 produce and secrete a hormone called meteorin-like (also known as subfatin) (Rao et al. 2014). In mice, acute exercise results in upregulation of meteorin-like mRNA expression in muscle after 6 h and circulating meteorin-like levels after 24 h (Rao et al. 2014). Consistently, a single bout of combined resistance and aerobic exercise in young healthy male subjects increases circulating meteorin-like levels at both 1 and 4 h after exercise (Rao et al. 2014). Meteorin-like induced by exercise stimulates upregulation of genes related to adipocyte browning and mitochondrial oxidation as well as anti-inflammatory cytokines. It is interesting to note that whereas other myokines directly induce adipocyte browning through upregulation of thermogenic genes such as UCP1 in adipocytes, meteorin-like has an indirect effect on adipocyte browning through regulation of immune cells. Specifically, meteorin-like stimulates the eosinophils to secrete IL-4 and IL-13, and promotes alternative activation of adipose tissue macrophages which are required for upregulation of thermogenic gene expression as well as anti-inflammatory gene expression in WAT (Rao et al. 2014). A recent study has shown that meteorin-like is not only a myokine, but also an adipokine. However, studies have shown contradicting results regarding its role on adipocytes. One study showed that meteorin-like promotes adipogenesis and controls insulin sensitivity in adipocytes through the PPARγ pathway in mice (Li et al. 2015). On the other hand, another study showed that meteorin-like expression was higher in stromal vascular fraction compared to adipocytes in humans, and that overexpression of meteorin-like inhibits human adipocyte differentiation (Loffler et al. 2017). Therefore, the role of meteorin-like as an adipokine/myokine has yet to be explored.
Leukemia inhibitory factor
Leukemia inhibitory factor (LIF) has previously been reported to have multiple biological functions in platelets, bone, neurons, and liver (Metcalf 2003). Since LIF mRNA expression is increased in human skeletal muscle after resistance exercise and LIF protein is secreted when human cultured myotubes are electrically stimulated (Broholm et al. 2008; Broholm et al. 2011), LIF is classified as a contraction-induced myokine. It is known that LIF plays an important role in skeletal muscle hypertrophy and regeneration by enhancing cell proliferation through the JAK/STAT and PI3K signaling pathway (Alter et al. 2008; Diao et al. 2009). Along with its effects on muscle hypertrophy, LIF acutely increases muscle glucose uptake through the PI3K/mTORC2/Akt pathway (Brandt et al. 2015), suggesting that LIF exerts local effects in muscle in an autocrine and/or paracrine manner. Even before it was classified as a myokine, LIF was shown to stimulate osteoblast differentiation while it was found to inhibit adipocyte differentiation (Aubert et al. 1999; Sims and Johnson 2012). Whether exercise-induced LIF mediates these processes are unclear and yet to be discovered. In terms of measuring post-exercise levels, it is difficult to detect circulating levels of LIF protein, since LIF has a very short half-life of 6-8 min in serum (Hilton et al. 1991). Therefore, the expression and secretion levels of LIF protein after exercise are not well characterized.
Secreted protein acidic and rich in cysteine
Secreted protein acidic and rich in cysteine (SPARC) was initially identified in the bone as osteonectin, but recent studies have shown that it is also found in the muscle, where its level increases during muscle development and regeneration (Termine et al. 1981; Kupprion et al. 1998). SPARC is a matricellular glycoprotein which modulates the interaction between cells and the extracellular matrix (ECM) proteins such as collagen and vitronectin (Bradshaw 2012). Interestingly, it has recently been shown that SPARC directly interacts with actin and plays a critical role in skeletal muscle tissue remodeling (Jorgensen et al. 2017). The ability of SPARC to regulate tissue remodeling also seems to play an important role in adipocyte differentiation and adipose tissue turnover. SPARC inhibits adipogenesis by activating the Wnt/β-catenin pathway (Nie and Sage 2009), whereas higher expression of SPARC in obesity limits the ability of adipose tissue to accumulate lipids (Tartare-Deckert et al. 2001; Kos et al. 2009), leading to metabolic dysregulation in obesity. Distinct from the role of SPARC in regulating the ECM, it has been reported that SPARC directly interacts with AMPK and is involved in glucose metabolism in myocytes (Nie and Sage 2009; Song et al. 2010). Therefore, the relationship between SPARC and metabolic disease is of current interest, which needs to be further examined in detail. Recently, it was discovered that exercise-induced SPARC can also inhibit progression of colon tumor through inducing colon cell apoptosis in mice, suggesting its role in amelioration of cancer (Aoi et al. 2013).
Other myokines
Apart from the myokines discussed above, exercise-responsive myokines are continuously being discovered through global mRNA sequencing and secretome analysis. Apelin is a well-known adipokine upregulated in obese individuals undergoing an 8 week endurance training, and thus, it is identified as a novel exercise-regulated myokine and is suggested to improve muscle metabolism and function (Besse-Patin et al. 2014). IGF-1 and FGF-2 are two well-known osteogenic factors, which are found to be abundant in homogenized muscle tissue and are also secreted from cultured myotubes in vitro (Hamrick 2011), suggesting a muscle-bone crosstalk by exercise. Chitinase-3-like protein 1 (CHI3L1) is another myokine whose gene expression is increased after a single bout of strength and aerobic exercise (Gorgens et al. 2016). Recent evidence suggests that CHI3L1 acts in an autocrine/paracrine manner to stimulate myoblast proliferation and inhibit pro-inflammatory signaling pathways (Gorgens et al. 2014, 2016). CXCL1 (fractalkine) and CCL2 (MCP-1) are well-known chemokines which were induced in muscle by acute exercise (Catoire et al. 2014). Since infiltration of macrophages is important for exercise-induced hypertrophy, CXCL1 and CCL2 are believed to play a role in this process.
The role of myokines in regulating local and systemic metabolism and their therapeutic potential
The identified roles of myokines have proven that myokines are involved in various processes of exercise adaptation, primarily muscle growth and substrate mobilization through regulation of whole body glucose/lipid metabolism. The local effect of myokines on skeletal muscle is summarized in Fig. 2 and Table 1. Many of the discovered myokines mediate exercise-induced muscle growth (IL-6, IL-15, irisin, myostatin, LIF), which implies that these myokines stimulate muscle protein synthesis. Activation of Akt-mTOR-p70S6 K signaling is critical for mRNA translation, ribosomal biogenesis, and nutrient metabolism (Coffey and Hawley 2007; Drummond et al. 2009), and therefore, it is likely that similar pathways are associated with these myokines. Myostatin is unique as it induces muscle atrophy which may counterbalance the other anabolic myokines. Myokines also regulate muscle metabolism through enhancing muscle insulin sensitivity, either by stimulating glucose uptake (IL-6, IL-15, irisin, BDNF, LIF) or lipid metabolism (IL-6, irisin, BDNF, BAIBA). This is in line with the fact that during exercise, ATP synthesis is rapidly activated through substrate utilization (Gaitanos et al. 1993; Parolin et al. 1999), and release of myokines could be a response mechanism against increased glucose demand during contraction.
The local effect of myokines on skeletal muscle. The exercise-induced myokines can regulate muscle physiology in an autocrine and paracrine manner. The figure summarizes the specific roles of each myokines on muscle metabolism and muscle growth. In some cases where the downstream mechanism is known, the signaling pathways which mediate the effect of myokine is shown in the grey box
The mobilization of extramuscular substrates is also critical for maintaining skeletal muscle metabolism during prolonged exercise (van Loon et al. 2005; Wasserman 2009). Therefore, the main target of the secreted myokines in terms of their endocrine effects are insulin-sensitive tissues, such as liver and adipose tissue (Fig. 3 and Table 1). Irisin and BAIBA regulate liver glycogenesis and gluconeogenesis, and a number of myokines have an effect on lipolysis and FFA oxidation in adipocytes (IL-6, IL-15, irisin, myostatin, BAIBA). These effects on adipocytes and liver would potentially enhance whole body insulin sensitivity, which would be beneficial for the treatment of metabolic diseases. The discovery of irisin received attention as it was suggested to mediate the effect of exercise on adipocyte browning. Indeed, the effects of other myokines on adipocyte browning were also shown to be dependent on the action of irisin (BDNF, myostatin). Meteorin-like, BAIBA, and IL-6 can also induce adipocyte browning, but whether this is independent of irisin needs to be investigated further. The myokines that stimulate lipolysis and FFA oxidation in adipocytes usually have an effect on adipocyte browning. However, in terms of myokine-induced adipocyte browning, it is still not known why exercise would induce a process that would reduce the storage of energy. A potential explanation is that overall metabolism is increased to produce energy, but this point needs to be discussed further in future studies.
The endocrine effect of myokines on brain, bone, adipose tissue, and liver. The exercise-induced myokines are capable of mediating the beneficial effect of exercise from muscle to other organs. Among various organs, the crosstalk with the adipose tissue exerts multiple actions including adipocyte browning and inhibition of adipocyte differentiation. Myostatin and LIF have opposite actions on bone. In the liver, irisin and BAIBA modulates glucose and lipid metabolism. Of note, muscle-derived irisin is known to induce BDNF expression in the brain which subsequently results in adipocyte browning
Although the identified myokines share a common role in regulating metabolism, how each myokine works and how these myokines work together still remain to be elucidated. It is also important to note that myokines seem to regulate each other, as in the case of myostatin-irisin and irisin-BDNF axis, which implies that myokines may work synergistically to effectively regulate exercise-induced adaptation. The role of myokines in mediating exercise-induced adaptation opens a new door to their pharmaceutical application, where myokines could be used to mimic exercise-induced muscle hypertrophy and substrate mobilization. Understanding the mechanism on how the muscle communicates with other organs will advance the discovery and development of pharmaceutical therapies to support certain disease groups wherein the patients are unable to exercise. Especially, age-related muscle disorders such as sarcopenia could benefit from the myokine-derived drugs. Also, development of anti-obesity and anti-diabetic drugs seems rational based on the metabolic effects of myokines on adipocytes and liver.
Regulation of myokine synthesis and secretion by natural or synthetic compounds
Based on the therapeutic potential of the identified myokines described above, it is important to understand how these myokines are regulated in terms of their expression and secretion. Moreover, it would be valuable to develop natural products or small compounds that regulate the myokines, independent of physical activity. So far, a number of natural or synthetic compounds have been reported to regulate myokines (Table 1). PDX ((10S,17S)-dihydroxydocosa-(4Z,7Z,11E,13Z,15E,19Z)-hexaenoic acid) is produced via sequential lipoxygenation of docosahexaenoic acid and is reported to stimulate the release of IL-6 from skeletal muscle (White et al. 2014). Elocalcitol (a non-hypercalcemic VDR agonist), ionomycin (Ca2+ ionophore), and calcineurin (Ca2+-calmodulin–dependent serine/threonine protein phosphatase) also stimulate IL-6 expression or secretion (Holmes et al. 2004; Allen et al. 2010; Antinozzi et al. 2017). AMPK activators AICAR and metformin have been implicated in the upregulation of various myokines including IL-6 (Lauritzen et al. 2013), irisin (Yang et al. 2015), and BDNF (Guerrieri and van Praag 2015). This implies that activation of AMPK signaling is critical to the mechanism of action of myokines in regulating metabolic homeostasis. Leptin also regulates a number of myokines including IL-6, IL-15, and irisin (Nozhenko et al. 2015; Rodriguez et al. 2015), indicating fat-muscle crosstalk. Regulation of irisin by small compounds has been examined in various studies, and showed that sodium butyrate, azacytidine, and inorganic nitrate upregulate irisin (Kim et al. 2017; Roberts et al. 2017). Interestingly, treatment with glucagon-like peptide-1 (GLP-1) receptor agonist exenatide markedly increased serum irisin levels (Liu et al. 2016), implying a synergistic action of irisin with the anti-diabetic drug. Whether this effect is directly or indirectly associated with muscle irisin needs to be examined further. In addition, natural product dihydromyricetin and ursolic acid stimulate irisin secretion (Bang et al. 2014; Zhou et al. 2015). In line with this finding, ursolic acid was also shown to decrease the expression of myostatin (Yu et al. 2017), implying its role in maintenance of muscle mass. Myostatin is by far the most extensively studied myokine in terms of its regulation. Small molecules and known drugs such as dorsomorphin, LDN-193189, atomoxetine, formoterol, fenofibrate and ghrelin analogues (Castillero et al. 2011; Busquets et al. 2012; Lenk et al. 2013; Jesinkey et al. 2014; Horbelt et al. 2015; Gomez-SanMiguel et al. 2016), and natural products such as magnolol, epigallocatechin-3-gallate, (−)-epicatechin (Gutierrez-Salmean et al. 2014; Chen et al. 2015; Horbelt et al. 2015) all downregulated myostatin expression and/or secretion, leading to a protective effect against muscle atrophy. In addition, myostatin is the only myokine for which a targeted therapeutic molecule has been developed to date. As mentioned above, there are numerous antibodies against myostatin (LY2495655, ACE-031, domagrozumab, MYO-029, BMS-986089, 10B3) and some of them have been successful in human clinical trials and have proved their potential as novel drugs in the treatment of skeletal muscle atrophy and muscle weakness (Becker et al. 2015; Singh et al. 2016; Woodhouse et al. 2016; Bhattacharya et al. 2017; Wurtzel et al. 2017). With respect to BDNF, there are only indirect evidences which show that BDNF upregulation by resveratrol, loganin, rolipram, and taurine improved brain function (Chou et al. 2013; Tseng et al. 2016; Zhong et al. 2016; Wicinski et al. 2017). However, it is not known whether these compounds can specifically induce muscle BDNF expression/secretion. Only inorganic nitrate has been reported to stimulate BAIBA (Roberts et al. 2017), and there are no compounds known to date that regulate meteorin-like, LIF, and SPARC. Evidence from previous studies can help us to not only understand the mechanisms underlying the regulation of myokines but also to provide insights into developing therapeutic molecules that target myokines. Since myostatin antibody has shown a good example of myokine as a drug candidate, development of myokine analogue seems promising.
Conclusion
Skeletal muscle is the major organ contributing to the whole body metabolism, and identification of exercise-induced myokines set a new paradigm in exercise biology and metabolic homeostasis. The fact that muscles produce secretory molecules provides the basis for the crosstalk between skeletal muscle and other organs, such as adipose tissue, bone, liver, kidney, brain, etc. Given the complexity and variability among exercise regimens and responses at the metabolic and molecular level, myokines that are sensitive to exercise could serve as prognostic biomarkers which reflect the improvement of whole body metabolism. In the future, expression profiles of the identified myokines could provide means to coordinate individual exercise programs and to maximize the health-promoting benefits of exercise on metabolism. Moreover, based on the role of myokines in fine tuning the metabolic process associated with exercise, development of exercise mimetics or small compounds derived from myokines is a promising field in the treatment of metabolic diseases.
References
Adams GR, McCue SA (1998) Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84:1716–1722
Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L, Lee S, Brenmoehl J, Thomas S, Drevon CA, Erickson HP, Maak S (2015) Irisin—a myth rather than an exercise-inducible myokine. Sci Rep 5:8889
Allen DL, Uyenishi JJ, Cleary AS, Mehan RS, Lindsay SF, Reed JM (2010) Calcineurin activates interleukin-6 transcription in mouse skeletal muscle in vivo and in C2C12 myotubes in vitro. Am J Physiol Regul Integr Comp Physiol 298:R198–R210
Allen DL, Hittel DS, McPherron AC (2011) Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc 43:1828–1835
Almuraikhy S, Kafienah W, Bashah M, Diboun I, Jaganjac M, Al-Khelaifi F, Abdesselem H, Mazloum NA, Alsayrafi M, Mohamed-Ali V, Elrayess MA (2016) Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia 59:2406–2416
Alter J, Rozentzweig D, Bengal E (2008) Inhibition of myoblast differentiation by tumor necrosis factor alpha is mediated by c-Jun N-terminal kinase 1 and leukemia inhibitory factor. J Biol Chem 283:23224–23234
Antinozzi C, Corinaldesi C, Giordano C, Pisano A, Cerbelli B, Migliaccio S, Di Luigi L, Stefanantoni K, Vannelli GB, Minisola S, Valesini G, Riccieri V, Lenzi A, Crescioli C (2017) Potential role for the VDR agonist elocalcitol in metabolic control: evidences in human skeletal muscle cells. J Steroid Biochem Mol Biol 167:169–181
Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, Koyama R, Wada S, Higashi A, Kokura S, Ichikawa H, Yoshikawa T (2013) A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 62:882–889
Aubert J, Dessolin S, Belmonte N, Li M, McKenzie FR, Staccini L, Villageois P, Barhanin B, Vernallis A, Smith AG, Ailhaud G, Dani C (1999) Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J Biol Chem 274:24965–24972
Baar K, Esser K (1999) Phosphorylation of p70(S6 k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276:C120–C127
Bang HS, Seo DY, Chung YM, Oh KM, Park JJ, Arturo F, Jeong SH, Kim N, Han J (2014) Ursolic acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J Physiol Pharmacol 18:441–446
Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, Recknor CP, Hochberg MC, Ferrari SL, Blain H, Binder EF, Rolland Y, Poiraudeau S, Benson CT, Myers SL, Hu L, Ahmad QI, Pacuch KR, Gomez EV, Benichou O, On behalf of the STEADY Group (2015) Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol 3:948–957
Begriche K, Massart J, Abbey-Toby A, Igoudjil A, Letteron P, Fromenty B (2008) Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity 16:2053–2067
Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, Daviaud D, Mir L, Marques MA, Thalamas C, Valet P, Langin D, Moro C, Viguerie N (2014) Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes 38:707–713
Bhattacharya I, Pawlak S, Marraffino S, Christensen J, Sherlock SP, Alvey C, Morris C, Arkin S, Binks M (2017) Safety, tolerability, pharmacokinetics, and pharmacodynamics of domagrozumab (PF-06252616), an antimyostatin monoclonal antibody, in healthy subjects. Clin Pharmacol Drug Dev. https://doi.org/10.1002/cpdd.386
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Booth FW, Thomason DB (1991) Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev 71:541–585
Bortoluzzi S, Scannapieco P, Cestaro A, Danieli GA, Schiaffino S (2006) Computational reconstruction of the human skeletal muscle secretome. Proteins 62:776–792
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468
Bradshaw AD (2012) Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol 44:480–488
Brandt N, O’Neill HM, Kleinert M, Schjerling P, Vernet E, Steinberg GR, Richter EA, Jorgensen SB (2015) Leukemia inhibitory factor increases glucose uptake in mouse skeletal muscle. Am J Physiol Endocrinol Metab 309:E142–E153
Broholm C, Mortensen OH, Nielsen S, Akerstrom T, Zankari A, Dahl B, Pedersen BK (2008) Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J Physiol 586:2195–2201
Broholm C, Laye MJ, Brandt C, Vadalasetty R, Pilegaard H, Pedersen BK, Scheele C (2011) LIF is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol 111:251–259
Busquets S, Figueras MT, Meijsing S, Carbo N, Quinn LS, Almendro V, Argiles JM, Lopez-Soriano FJ (2005) Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int J Mol Med 16:471–476
Busquets S, Toledo M, Marmonti E, Orpi M, Capdevila E, Betancourt A, Lopez-Soriano FJ, Argiles JM (2012) Formoterol treatment downregulates the myostatin system in skeletal muscle of cachectic tumour-bearing rats. Oncol Lett 3:185–189
Camporez JP, Petersen MC, Abudukadier A, Moreira GV, Jurczak MJ, Friedman G, Haqq CM, Petersen KF, Shulman GI (2016) Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci USA 113:2212–2217
Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ (2011) White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 14:324–338
Carbo N, Lopez-Soriano J, Costelli P, Alvarez B, Busquets S, Baccino FM, Quinn LS, Lopez-Soriano FJ, Argiles JM (2001) Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim Biophys Acta 1526:17–24
Castillero E, Nieto-Bona MP, Fernandez-Galaz C, Martin AI, Lopez-Menduina M, Granado M, Villanua MA, Lopez-Calderon A (2011) Fenofibrate, a PPAR{alpha} agonist, decreases atrogenes and myostatin expression and improves arthritis-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab 300:E790–E799
Catoire M, Mensink M, Kalkhoven E, Schrauwen P, Kersten S (2014) Identification of human exercise-induced myokines using secretome analysis. Physiol Genom 46:256–267
Chen MC, Chen YL, Lee CF, Hung CH, Chou TC (2015) Supplementation of magnolol attenuates skeletal muscle atrophy in bladder cancer-bearing mice undergoing chemotherapy via suppression of FoxO3 activation and induction of IGF-1. PLoS ONE 10:e0143594
Chou CT, Lin WF, Kong ZL, Chen SY, Hwang DF (2013) Taurine prevented cell cycle arrest and restored neurotrophic gene expression in arsenite-treated SH-SY5Y cells. Amino Acids 45:811–819
Coffey VG, Hawley JA (2007) The molecular bases of training adaptation. Sports Med 37:737–763
Coker RH, Kjaer M (2005) Glucoregulation during exercise: the role of the neuroendocrine system. Sports Med 35:575–583
Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, Notarnicola A, Severi I, Passeri G, Mori G, Brunetti G, Moretti B, Tarantino U, Colucci SC, Reseland JE, Vettor R, Cinti S, Grano M (2017) Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep 7:2811
Daskalopoulou SS, Cooke AB, Gomez YH, Mutter AF, Filippaios A, Mesfum ET, Mantzoros CS (2014) Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur J Endocrinol 171:343–352
Diao Y, Wang X, Wu Z (2009) SOCS1, SOCS3, and PIAS1 promote myogenic differentiation by inhibiting the leukemia inhibitory factor-induced JAK1/STAT1/STAT3 pathway. Mol Cell Biol 29:5084–5093
Dinoff A, Herrmann N, Swardfager W, Liu CS, Sherman C, Chan S, Lanctot KL (2016) The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. PLoS ONE 11:e0163037
Dinoff A, Herrmann N, Swardfager W, Lanctot KL (2017) The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor (BDNF) in healthy adults: a meta-analysis. Eur J Neurosci. https://doi.org/10.1111/ejn.13603
Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L (2016) Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes 40:434–442
Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB (2009) Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587:1535–1546
Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17:162–184
Ellefsen S, Vikmoen O, Slettalokken G, Whist JE, Nygaard H, Hollan I, Rauk I, Vegge G, Strand TA, Raastad T, Ronnestad BR (2014) Irisin and FNDC5: effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur J Appl Physiol 114:1875–1888
Feldman BJ, Streeper RS, Farese RV Jr, Yamamoto KR (2006) Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci USA 103:15675–15680
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12:6–33
Folland JP, Williams AG (2007) The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 37:145–168
Fu J, Han Y, Wang J, Liu Y, Zheng S, Zhou L, Jose PA, Zeng C (2016) Irisin lowers blood pressure by improvement of endothelial dysfunction via AMPK-Akt-eNOS-NO pathway in the spontaneously hypertensive rat. J Am Heart Assoc. https://doi.org/10.1161/jaha.116.003433
Gaitanos GC, Williams C, Boobis LH, Brooks S (1993) Human muscle metabolism during intermittent maximal exercise. J Appl Physiol 75:712–719
Goldstein MS (1961) Humoral nature of the hypoglycemic factor of muscular work. Diabetes 10:232–234
Gomez-SanMiguel AB, Gomez-Moreira C, Nieto-Bona MP, Fernandez-Galaz C, Villanua MA, Martin AI, Lopez-Calderon A (2016) Formoterol decreases muscle wasting as well as inflammation in the rat model of rheumatoid arthritis. Am J Physiol Endocrinol Metab 310:E925–E937
Goodyear LJ, Kahn BB (1998) Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49:235–261
Gorgens SW, Eckardt K, Elsen M, Tennagels N, Eckel J (2014) Chitinase-3-like protein 1 protects skeletal muscle from TNFalpha-induced inflammation and insulin resistance. Biochem J 459:479–488
Gorgens SW, Hjorth M, Eckardt K, Wichert S, Norheim F, Holen T, Lee S, Langleite T, Birkeland KI, Stadheim HK, Kolnes KJ, Tangen DS, Kolnes AJ, Jensen J, Drevon CA, Eckel J (2016) The exercise-regulated myokine chitinase-3-like protein 1 stimulates human myocyte proliferation. Acta Physiol 216:330–345
Guerrieri D, van Praag H (2015) Exercise-mimetic AICAR transiently benefits brain function. Oncotarget 6:18293–18313
Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC (2009) Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 4:e4937
Gutierrez-Salmean G, Ciaraldi TP, Nogueira L, Barboza J, Taub PR, Hogan MC, Henry RR, Meaney E, Villarreal F, Ceballos G, Ramirez-Sanchez I (2014) Effects of (-)-epicatechin on molecular modulators of skeletal muscle growth and differentiation. J Nutr Biochem 25:91–94
Hamrick MW (2011) A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev 39:43–47
Hansen J, Brandt C, Nielsen AR, Hojman P, Whitham M, Febbraio MA, Pedersen BK, Plomgaard P (2011) Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology 152:164–171
Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I (2010) Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteom 9:2482–2496
Hilton DJ, Nicola NA, Waring PM, Metcalf D (1991) Clearance and fate of leukemia-inhibitory factor (LIF) after injection into mice. J Cell Physiol 148:430–439
Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stockli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JF, Richter EA, James DE (2015) Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab 22:922–935
Holloszy JO, Coyle EF (1984) Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56:831–838
Holmes AG, Watt MJ, Carey AL, Febbraio MA (2004) Ionomycin, but not physiologic doses of epinephrine, stimulates skeletal muscle interleukin-6 mRNA expression and protein release. Metabolism 53:1492–1495
Horbelt D, Boergermann JH, Chaikuad A, Alfano I, Williams E, Lukonin I, Timmel T, Bullock AN, Knaus P (2015) Small molecules dorsomorphin and LDN-193189 inhibit myostatin/GDF8 signaling and promote functional myoblast differentiation. J Biol Chem 290:3390–3404
Horowitz JF (2003) Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol Metab 14:386–392
Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R (1985) Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch 403:369–376
Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS (2012) FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61:1725–1738
Huh JY, Dincer F, Mesfum E, Mantzoros CS (2014a) Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes 38:1538–1544
Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos II, Filippaios A, Panagiotou G, Park KH, Mantzoros CS (2014b) Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab 99:E2154–E2161
Ikeda SI, Tamura Y, Kakehi S, Sanada H, Kawamori R, Watada H (2016) Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem Biophys Res Commun 473:947–952
Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L (2010) Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol 6:71–82
Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM (2015) Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab 22:734–740
Jesinkey SR, Korrapati MC, Rasbach KA, Beeson CC, Schnellmann RG (2014) Atomoxetine prevents dexamethasone-induced skeletal muscle atrophy in mice. J Pharmacol Exp Ther 351:663–673
Jorgensen LH, Jepsen PL, Boysen A, Dalgaard LB, Hvid LG, Ortenblad N, Ravn D, Sellathurai J, Moller-Jensen J, Lochmuller H, Schroder HD (2017) SPARC interacts with actin in skeletal muscle in vitro and in vivo. Am J Pathol 187:457–474
Joyner MJ, Green DJ (2009) Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587:5551–5558
Jung TW, Hwang HJ, Hong HC, Yoo HJ, Baik SH, Choi KM (2015) BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARdelta-dependent pathway in mice. Diabetologia 58:2096–2105
Kelly M, Gauthier MS, Saha AK, Ruderman NB (2009) Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes 58:1953–1960
Kim HK, Jeong YJ, Song IS, Noh YH, Seo KW, Kim M, Han J (2017) Glucocorticoid receptor positively regulates transcription of FNDC5 in the liver. Sci Rep 7:43296
Knudsen JG, Murholm M, Carey AL, Bienso RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen JB, Pilegaard H (2014) Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS ONE 9:e84910
Kos K, Wong S, Tan B, Gummesson A, Jernas M, Franck N, Kerrigan D, Nystrom FH, Carlsson LM, Randeva HS, Pinkney JH, Wilding JP (2009) Regulation of the fibrosis and angiogenesis promoter SPARC/osteonectin in human adipose tissue by weight change, leptin, insulin, and glucose. Diabetes 58:1780–1788
Krolopp JE, Thornton SM, Abbott MJ (2016) IL-15 activates the Jak3/STAT3 signaling pathway to mediate glucose uptake in skeletal muscle cells. Front Physiol 7:626
Kupprion C, Motamed K, Sage EH (1998) SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem 273:29635–29640
Lapchak PA, Hefti F (1992) BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. NeuroReport 3:405–408
Lauritzen HP, Brandauer J, Schjerling P, Koh HJ, Treebak JT, Hirshman MF, Galbo H, Goodyear LJ (2013) Contraction and AICAR stimulate IL-6 vesicle depletion from skeletal muscle fibers in vivo. Diabetes 62:3081–3092
Lebrasseur NK (2012) Building muscle, browning fat and preventing obesity by inhibiting myostatin. Diabetologia 55:13–17
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311
Lee HJ, Lee JO, Kim N, Kim JK, Kim HI, Lee YW, Kim SJ, Choi JI, Oh Y, Kim JH, Suyeon H, Park SH, Kim HS (2015) Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol 29:873–881
Lenk K, Palus S, Schur R, Datta R, Dong J, Culler MD, Anker S, Springer J, Schuler G, Adams V (2013) Effect of ghrelin and its analogues, BIM-28131 and BIM-28125, on the expression of myostatin in a rat heart failure model. J Cachexia Sarcopenia Muscle 4:63–69
Li F, Li Y, Tang Y, Lin B, Kong X, Oladele OA, Yin Y (2014) Protective effect of myokine IL-15 against H2O2-mediated oxidative stress in skeletal muscle cells. Mol Biol Rep 41:7715–7722
Li ZY, Song J, Zheng SL, Fan MB, Guan YF, Qu Y, Xu J, Wang P, Miao CY (2015) Adipocyte metrnl antagonizes insulin resistance through PPARgamma signaling. Diabetes 64:4011–4022
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370
Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, Chen Q, Li YH, Wang JJ, Kang YM, Zhu GQ (2015) Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3 K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci 129:839–850
Liu J, Hu Y, Zhang H, Xu Y, Wang G (2016) Exenatide treatment increases serum irisin levels in patients with obesity and newly diagnosed type 2 diabetes. J Diabetes Complicat 30:1555–1559
Loffler D, Landgraf K, Rockstroh D, Schwartze JT, Dunzendorfer H, Kiess W, Korner A (2017) METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int J Obes 41:112–119
Maisonneuve C, Igoudjil A, Begriche K, Letteron P, Guimont MC, Bastin J, Laigneau JP, Pessayre D, Fromenty B (2004) Effects of zidovudine, stavudine and beta-aminoisobutyric acid on lipid homeostasis in mice: possible role in human fat wasting. Antivir Ther 9:801–810
Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, Akerstrom T, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Bruunsgaard H, Pedersen BK, Febbraio MA (2009) Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 52:1409–1418
Maury E, Brichard SM (2010) Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 314:1–16
McGinnis GR, Ballmann C, Peters B, Nanayakkara G, Roberts M, Amin R, Quindry JC (2015) Interleukin-6 mediates exercise preconditioning against myocardial ischemia reperfusion injury. Am J Physiol Heart Circ Physiol 308:H1423–H1433
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Metcalf D (2003) The unsolved enigmas of leukemia inhibitory factor. Stem Cells 21:5–14
Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, van Leeuwen FE, Tfpac (2007) Physical activity and breast cancer: a systematic review. Epidemiology 18:137–157
Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, Ricart W, Fernandez-Real JM (2013) Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 98:E769–E778
Naseeb MA, Volpe SL (2017) Protein and exercise in the prevention of sarcopenia and aging. Nutr Res 40:1–20
Nie J, Sage EH (2009) SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem 284:1279–1290
Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Lorenzo M (2008) Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes 57:3211–3221
Nigam SM, Xu S, Kritikou JS, Marosi K, Brodin L, Mattson MP (2017) Exercise and BDNF reduce Abeta production by enhancing alpha-secretase processing of APP. J Neurochem. https://doi.org/10.1111/jnc.14034
Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN (2008) Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil 15:239–246
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA (2014) The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 281:739–749
Nozhenko Y, Rodriguez AM, Palou A (2015) Leptin rapidly induces the expression of metabolic and myokine genes in C2C12 muscle cells to regulate nutrient partition and oxidation. Cell Physiol Biochem 35:92–103
Pal M, Febbraio MA, Whitham M (2014) From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol 92:331–339
Parolin ML, Chesley A, Matsos MP, Spriet LL, Jones NL, Heigenhauser GJ (1999) Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am J Physiol 277:E890–E900
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88:1379–1406
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8:457–465
Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B (2003) Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 24:113–119
Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA (2009) Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol 94:1153–1160
Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS (2017) Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol 13:324–337
Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI (1996) Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335:1357–1362
Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G (1994) Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol 266:R375–R380
Quinn LS, Haugk KL, Grabstein KH (1995) Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology 136:3669–3672
Quinn LS, Strait-Bodey L, Anderson BG, Argiles JM, Havel PJ (2005) Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int 29:449–457
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157:1279–1291
Raschke S, Eckel J (2013) Adipo-myokines: two sides of the same coin–mediators of inflammation and mediators of exercise. Mediat Inflamm 2013:320724
Relizani K, Mouisel E, Giannesini B, Hourde C, Patel K, Morales Gonzalez S, Julich K, Vignaud A, Pietri-Rouxel F, Fortin D, Garcia L, Blot S, Ritvos O, Bendahan D, Ferry A, Ventura-Clapier R, Schuelke M, Amthor H (2014) Blockade of ActRIIB signaling triggers muscle fatigability and metabolic myopathy. Mol Ther 22:1423–1433
Riechman SE, Balasekaran G, Roth SM, Ferrell RE (2004) Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol 97:2214–2219
Roberts LD, Bostrom P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM, Gerszten RE (2014) beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19:96–108
Roberts LD, Ashmore T, McNally BD, Murfitt SA, Fernandez BO, Feelisch M, Lindsay R, Siervo M, Williams EA, Murray AJ, Griffin JL (2017) Inorganic nitrate mimics exercise-stimulated muscular fiber-type switching and myokine and gamma-aminobutyric acid release. Diabetes 66:674–688
Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen Crujeiras A, Seoane LM, Casanueva FF, Pardo M (2013) FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 8:e60563
Rodgers BD, Garikipati DK (2008) Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev 29:513–534
Rodriguez A, Becerril S, Mendez-Gimenez L, Ramirez B, Sainz N, Catalan V, Gomez-Ambrosi J, Fruhbeck G (2015) Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int J Obes (Lond) 39:397–407
Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol 265:E380–E391
Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM (2012) A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151:1319–1331
Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350:2682–2688
Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P (2008) Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7:33–44
Shan T, Liang X, Bi P, Kuang S (2013) Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J 27:1981–1989
Shi CX, Zhao MX, Shu XD, Xiong XQ, Wang JJ, Gao XY, Chen Q, Li YH, Kang YM, Zhu GQ (2016) beta-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci Rep 6:21924
Sims NA, Johnson RW (2012) Leukemia inhibitory factor: a paracrine mediator of bone metabolism. Growth Factors 30:76–87
Singh P, Rong H, Gordi T, Bosley J, Bhattacharya I (2016) Translational pharmacokinetic/pharmacodynamic analysis of MYO-029 antibody for muscular dystrophy. Clin Transl Sci 9:302–310
Smith RC, Cramer MS, Mitchell PJ, Capen A, Huber L, Wang R, Myers L, Jones BE, Eastwood BJ, Ballard D, Hanson J, Credille KM, Wroblewski VJ, Lin BK, Heuer JG (2015) Myostatin neutralization results in preservation of muscle mass and strength in preclinical models of tumor-induced muscle wasting. Mol Cancer Ther 14:1661–1670
Song H, Guan Y, Zhang L, Li K, Dong C (2010) SPARC interacts with AMPK and regulates GLUT4 expression. Biochem Biophys Res Commun 396:961–966
Steinbacher P, Eckl P (2015) Impact of oxidative stress on exercising skeletal muscle. Biomolecules 5:356–377
Talanian JL, Holloway GP, Snook LA, Heigenhauser GJ, Bonen A, Spriet LL (2010) Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am J Physiol Endocrinol Metab 299:E180–E188
Tamura Y, Watanabe K, Kantani T, Hayashi J, Ishida N, Kaneki M (2011) Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J 58:211–215
Tang H, Yu R, Liu S, Huwatibieke B, Li Z, Zhang W (2016) Irisin inhibits hepatic cholesterol synthesis via AMPK-SREBP2 signaling. EBioMedicine 6:139–148
Tartare-Deckert S, Chavey C, Monthouel MN, Gautier N, Van Obberghen E (2001) The matricellular protein SPARC/osteonectin as a newly identified factor up-regulated in obesity. J Biol Chem 276:22231–22237
Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR (1981) Osteonectin, a bone-specific protein linking mineral to collagen. Cell 26:99–105
Thornton SM, Krolopp JE, Abbott MJ (2016) IL-15 mediates mitochondrial activity through a PPARdelta-dependent-PPARalpha-independent mechanism in skeletal muscle cells. PPAR Res 2016:5465804
Tonra JR, Ono M, Liu X, Garcia K, Jackson C, Yancopoulos GD, Wiegand SJ, Wong V (1999) Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes 48:588–594
Tseng YT, Chen CS, Jong YJ, Chang FR, Lo YC (2016) Loganin possesses neuroprotective properties, restores SMN protein and activates protein synthesis positive regulator Akt/mTOR in experimental models of spinal muscular atrophy. Pharmacol Res 111:58–75
Tsuchida A, Nakagawa T, Itakura Y, Ichihara J, Ogawa W, Kasuga M, Taiji M, Noguchi H (2001) The effects of brain-derived neurotrophic factor on insulin signal transduction in the liver of diabetic mice. Diabetologia 44:555–566
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, Finnish Diabetes Prevention Study G (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350
van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK (2003) Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 88:3005–3010
van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ (2001) The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536:295–304
van Loon LJ, Thomason-Hughes M, Constantin-Teodosiu D, Koopman R, Greenhaff PL, Hardie DG, Keizer HA, Saris WH, Wagenmakers AJ (2005) Inhibition of adipose tissue lipolysis increases intramuscular lipid and glycogen use in vivo in humans. Am J Physiol Endocrinol Metab 289:E482–E493
Wang H, Qian J, Zhao X, Xing C, Sun B (2017) beta-Aminoisobutyric acid ameliorates the renal fibrosis in mouse obstructed kidneys via inhibition of renal fibroblast activation and fibrosis. J Pharmacol Sci 133:203–213
Wasserman DH (2009) Four grams of glucose. Am J Physiol Endocrinol Metab 296:E11–E21
White PJ, St-Pierre P, Charbonneau A, Mitchell PL, St-Amand E, Marcotte B, Marette A (2014) Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med 20:664–669
Wicinski M, Malinowski B, Weclewicz MM, Grzesk E, Grzesk G (2017) Resveratrol increases serum BDNF concentrations and reduces vascular smooth muscle cells contractility via a NOS-3-independent mechanism. Biomed Res Int 2017:9202954
Wolin KY, Yan Y, Colditz GA, Lee IM (2009) Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer 100:611–616
Woodhouse L, Gandhi R, Warden SJ, Poiraudeau S, Myers SL, Benson CT, Hu L, Ahmad QI, Linnemeier P, Gomez EV, Benichou O, Study I (2016) A Phase 2 randomized study investigating the efficacy and safety of myostatin antibody LY2495655 versus placebo in patients undergoing elective total hip arthroplasty. J Frailty Aging 5:62–70
Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM (2013) Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab 18:649–659
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Wurtzel CN, Gumucio JP, Grekin JA, Khouri RK Jr, Russell AJ, Bedi A, Mendias CL (2017) Pharmacological inhibition of myostatin protects against skeletal muscle atrophy and weakness after anterior cruciate ligament tear. J Orthop Res. https://doi.org/10.1002/jor.23537
Xie C, Zhang Y, Tran TD, Wang H, Li S, George EV, Zhuang H, Zhang P, Kandel A, Lai Y, Tang D, Reeves WH, Cheng H, Ding Y, Yang LJ (2015) Irisin controls growth, intracellular Ca2+ signals, and mitochondrial thermogenesis in cardiomyoblasts. PLoS ONE 10:e0136816
Xin C, Liu J, Zhang J, Zhu D, Wang H, Xiong L, Lee Y, Ye J, Lian K, Xu C, Zhang L, Wang Q, Liu Y, Tao L (2015) Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes 40(3):443–451
Xiong XQ, Chen D, Sun HJ, Ding L, Wang JJ, Chen Q, Li YH, Zhou YB, Han Y, Zhang F, Gao XY, Kang YM, Zhu GQ (2015) FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta 1852:1867–1875
Yamanaka M, Itakura Y, Inoue T, Tsuchida A, Nakagawa T, Noguchi H, Taiji M (2006) Protective effect of brain-derived neurotrophic factor on pancreatic islets in obese diabetic mice. Metabolism 55:1286–1292
Yang Y, Creer A, Jemiolo B, Trappe S (2005) Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98:1745–1752
Yang Z, Chen X, Chen Y, Zhao Q (2015) PGC-1 mediates the regulation of metformin in muscle irisin expression and function. Am J Transl Res 7:1850–1859
Yoon JH, Yea K, Kim J, Choi YS, Park S, Lee H, Lee CS, Suh PG, Ryu SH (2009) Comparative proteomic analysis of the insulin-induced L6 myotube secretome. Proteomics 9:51–60
Yu R, Chen JA, Xu J, Cao J, Wang Y, Thomas SS, Hu Z (2017) Suppression of muscle wasting by the plant-derived compound ursolic acid in a model of chronic kidney disease. J Cachexia Sarcopenia Muscle 8:327–341
Zhang C, McFarlane C, Lokireddy S, Masuda S, Ge X, Gluckman PD, Sharma M, Kambadur R (2012) Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia 55:183–193
Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang LJ, Tang D (2014) Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 63:514–525
Zhao B, Wall RJ, Yang J (2005) Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun 337:248–255
Zhong Y, Zhu Y, He T, Li W, Yan H, Miao Y (2016) Rolipram-induced improvement of cognitive function correlates with changes in hippocampal CREB phosphorylation, BDNF and Arc protein levels. Neurosci Lett 610:171–176
Zhou Q, Chen K, Liu P, Gao Y, Zou D, Deng H, Huang Y, Zhang Q, Zhu J, Mi M (2015) Dihydromyricetin stimulates irisin secretion partially via the PGC-1alpha pathway. Mol Cell Endocrinol 412:349–357
Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea (No. 2015R1C1A1A02037367) and by Chonnam National University (No. 2014-2215 and No. 2015-3035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest.
Rights and permissions
About this article
Cite this article
Huh, J. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 41, 14–29 (2018). https://doi.org/10.1007/s12272-017-0994-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0994-y