Abstract
This study investigated the interaction among valsartan (VAL), TGF-β pathways, and long non-coding RNA (lncRNA) cardiac hypertrophy-related factor (CHRF) in doxorubicin (DOX)-induced heart failure (HF), and explored their roles in DOX-induced HF progression. HF mice models in vivo were constructed by DOX induction. The expression of CHRF and TGF-β1 in hearts was detected, along with cardiac function, caspase-3 activity, and cell apoptosis. Primary myocardial cells were pretreated with VAL, followed by DOX induction in vitro for functional studies, including the detection of cell apoptosis with terminal deoxynucleotidyl transferase dUTP nick-end labeling and the expression of proteins associated with TGF-β1 pathways. HF models were established in vivo and in vitro. Expression of CHRF and TGF-β1 was up-regulated, and cell apoptosis and caspase-3 activity were increased in the hearts and cells of the HF models. VAL supplementation alleviated the cardiac dysfunction and injury in the HF process. Moreover, overexpressed CHRF up-regulated TGF-β1, promoted myocardial cell apoptosis, and reversed VAL’s cardiac protective effect, while interference of CHRF (si-CHRF) did the opposite. Down-regulation of CHRF reversed the increased expression of TGF-β1 and the downstream proteins induced by pcDNA-TGF-β1 in HL-1 cells, while overexpression of CHRF reversed the VAL’s cardiac protective effect in vivo. In conclusion, VAL regulates TGF-β pathways through lncRNA CHRF to improve DOX-induced HF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is one of the most common chronic diseases in clinic; it causes high morbidity and mortality worldwide. When hearts suffer stress, the “cardiac remodeling” characterized by typical pathological cardiac hypertrophy occurs, resulting in heart failure (HF) (Viereck et al. 2016). Patients with HF experience the progressive deterioration of cardiac function (Sun et al. 2016). Doxorubicin (DOX), an anthracycline antibiotic commonly used as chemotherapy medication, is commonly used to establish cardiomyopathy in animal models (Houser et al. 2012) and acts as an important factor in dilated cardiomyopathy-like congestive HF in clinic (Jain and Kishore 2013; Nakamae et al. 2015). However, this widely applicable and effective anti-cancer drug’s utility is limited partly due to its cardiotoxicity and ability to induce severe HF (Albini et al. 2010; Dhingra et al. 2014). At present, common treatments mainly slow down the functional decline but cannot prevent or reverse the HF reduced by DOX. In addition, although the pathogenic factor of HF has been determined, the complex biology and underlying molecular mechanisms have not yet been fully elucidated.
Long non-coding RNAs (lncRNAs) are RNA transcripts with more than 200 nucleotides and without protein-coding potential (Sun et al. 2015). Numerous studies have confirmed that lncRNAs play a critical role in cell proliferation and differentiation (Cesana et al. 2011), chromatin modification (Kanduri 2011), and also serve as “miRNA sponges” (Liang et al. 2015). Currently, compared with lncRNAs’ well-reported dysregulation in various cancers, their function in heart disease has seldom been investigated. LncRNA myosin heavy chain-associated RNA transcript (MHRT) prevented DOX-induced cardiac apoptosis by positively regulating nuclear factor erythroid 2-related factor (Nrf2) expression (Sui et al. 2016). The mitochondrial lncRNA uc022bqs.1 (LIPCAR) was quickly down-regulated after a myocardial infarction but up-regulated during later stages of myocardial infarction; it acts as a novel biomarker in HF patients (Kumarswamy et al. 2014). Moreover, inhibiting the lncRNA NONRATT021972 may rescue the decreased heart rate variability in diabetic rats in superior cervical ganglia (Xu et al. 2016). A recent report also indicated that a lncRNA cardiac hypertrophy-related factor (CHRF) is directly bound to miR-489 and regulated MyD88 expression in cardiac hypertrophy (Wang et al. 2014), implying that CHRF plays a role in heart diseases.
A growing body of evidences link elevated levels of TGF-β super-family ligands to the progression of HF (Sun et al. 2016). TGF-β1 is a locally generated cytokine that has been implicated as a major contributor to myofibroblast proliferation and tissue fibrosis in diverse organ systems. Numerous reports have demonstrated the functional outcomes of TGF-β1 in HF, and these results have shown that TGF-β antagonism could inhibit fibrotic processes and provide salutary cardiac effects (Ikeuchi et al. 2004). Another study found that cardiac transplantation corrected the dysregulated. A low level of TGF-β1 was associated with HF, which may result from higher consumption of TGF-β1 within the impaired myocardium or antifibrotic functions of natriuretic peptides (Behnes et al. 2011). Recently, CHRF has been shown to regulate MyD88 and Smad3 by targeting miR-489, and overexpression of CHRF led to increased expression of TGF-β1 protein levels in silica-induced pulmonary fibrosis (Wu et al. 2016). Thus, CHRF may also influence the TGF-β1 pathway in HF.
Valsartan (VAL) is a kind of anti-hypertensive drug and licensed for the treatment of patients with symptomatic HF (Chaplin 2016), and it plays an important role in the process of HF injury. For example, compared to a placebo added to a prescribed therapy, VAL slowed progressively deteriorating quality of life in HF patients (Majani et al. 2005). In addition, adding VAL to a prescribed therapy could significantly reduce the incidence of atrial fibrillation (Maggioni et al. 2005). In the PARADIGM-HF trial (Prospective Comparison of angiotensin receptor neprilysin inhibitor (ARNI) with angiotensin converting enzyme inhibitor (ACEI) to determine impact on global mortality and morbidity in heart failure), the risk of death from cardiovascular causes from worsening HF was significantly reduced with VAL compared to the ACE inhibitor enalapril in patients with chronic HF (Keating and McCormack 2016). Therefore, VAL represents effect approach in the treatment of HF; it is commonly used in clinical practice and is known to have heart–lung protective effects (Jasmin et al. 2003).
The current study sought to determine the expression and function of CHRF and TGF-β1 pathways in the tissues and cells of patients with HF. It also sought to detecte the protective effect of VAL in regulating HF malignant processes and the underlying mechanisms involved.
Materials and methods
Ethics statement
The present study was approved by the Institutional Animal Care and Use Committee (IACUC number: 2014-0072) of the First Affiliated Hospital of Zhengzhou University, and the protocols of the animal experiment were conducted according to the Guide for the Care and Use of Laboratory Animals.
Animals and treatment
To construct the mouse model of HF, eighteen C57BL/6 male mice aged 8 weeks were purchased from Henan Research Center of Laboratory Animals (Zhengzhou, China). These mice were randomly divided into three groups (n = 6 in each group) : Group 1 was used as a control and mice were treated with an equivalent volume of sterile saline solution; mice in Group 2 were intraperitoneally injected with 2.5 mg/kg of DOX six times in 2 weeks; animals in Group 3 were also intraperitoneally injected with 2.5 mg/kg of DOX six times in 2 weeks, but then were treated with 30 mg/kg/days of VAL for 4 weeks by oral gavage. All mice were housed in appropriately sized cages and allowed food and water ad libitum. After treatment, these mice were sacrificed under anesthesia to relieve pain. Heart tissues were excised and immediately stored at − 80 °C for further analysis.
Injection of adenovirus vector with CHRF overexpressed
Twenty-four C57BL/6 mice were randomly divided into four groups (n = 6 in each group): control, VAL, VAL + Ad-control, and VAL + Ad-CHRF. The adenoviral vector (Ad-control or Ad-CHRF) injections were based on previous research (Wang et al. 2015). Briefly, after entering the chest through a small left anterior thoracotomy, the pericardial sac was removed. Then 2 × 1011 multiplicity of infection (moi) adenoviruses of Ad-CHRF or equal amounts of Ad-control were injected with a catheter from the apex of the left ventricle into the aortic root while the aorta and pulmonary arteries were cross-clamped. The clamp was maintained for 20 s while the heart pumped against a closed system. After removing air and blood, the chest was closed, and the mice were returned to their cages for recovery. The VAL treatment method was the same as described above. After the VAL treatment, the mice were sacrificed, and the hearts were isolated and stored at − 80 °C.
Cell culture and transfection
Primary cardiomyocyte cells were isolated, and the mouse myocardial cell lines HL-1 used in this study were obtained from American Type Culture Collection (USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA), 100 U/mL of penicillin, and 100 μg/mL of streptomycin. The cell lines were cultured in a humidified atmosphere containing 5% CO2 at 37 °C. Cells in different groups were transfected or co-transfected with pc-DNA-CHRF, si-CHRF, pc-DNA TGFβ1, or the corresponding controls using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNA from the cultured cells and mouse tissues was extracted using Trizol (Life Technologies, USA) according to the manufacturer’s protocol. The concentration and quality of the RNA were confirmed using a Thermo Scientific NanoDrop 2000 spectrophotometer. To quantify the CHRF, 500 ng of total RNA was reverse-transcribed using random primers in a 10 μl reaction system. The cDNA was obtained from the RNA via reverse transcription by using a PrimeScript RT reagent kit. The SYBR Premix Ex Taq II kit (TaKaRa Bio Inc, Japan) was used for CHRF amplification. Quantitative real-time PCR analysis was performed using an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, USA). CHRF expression was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The relative mRNA expression levels were analyzed using the \( 2^{{ - \Delta \Delta \text{Ct} }} \) method.
Western blot analysis
The proteins in the heart tissues and cultured cells were determined by western blot analysis. Protein concentration was determined via a bicinchoninic acid (BCA) protein assay kit (Beyotime, Shanghai, China). A total of 80 μg of protein extracts were electrophoresed on 12.5% polyacrylamide gradient gels and then transferred onto a poly vinylidene fluoride (PVDF) membrane. The membranes were incubated in a blocking buffer (5% non-fat milk) prior to incubation with primary antibodies at 4 °C overnight. Antibodies specific to total Smad3, p-Smad3, total Smad2, p-Smad2, p38, p-p38, TGF-β1, and β-actin were obtained from Cell Signaling Technology, Inc. After incubation with the secondary antibody for 1 h, the blots were visualized with a PowerOpti-ECL kit according to the recommended procedure, and protein bands were quantified using NIH ImageJ software. β-actin was used as an internal control.
TUNEL assay
Cell apoptosis was determined via a (TUNEL) staining assay. Cells were stained with 3, 3-diaminobenzidine (DAB) as a substrate for the peroxidase at room temperature for 10 min. For each section, 10 different fields were randomly selected for counting a minimum of 150 cells from at least 3 separate experiments per condition. The number of TUNEL-positive cells was analyzed using a light microscope system at ×400 magnification in a blinded manner. Positive apoptotic cells were stained with claybank (Wang et al. 2013).
Caspase-3 activity assay
Caspase-3 is closely related to cell apoptosis, and caspase-3 activity is usually used to reflect apoptosis status (Porter and Janicke 1999). The caspase-3 activity was determined by using a caspase-3 activity kit (Beyotime Institute of Biotechnology, Nanjing, China) per the manufacturer’s protocol. To evaluate the caspase-3 activity, primary cardiomyocyte cell lysates were prepared subsequent to various designated treatments. Assays were performed on 96-well plates by incubating 10 μL of cell lysate protein per sample in 80 μL of reaction buffer that contained 10 μL of caspase-3 substrate. Lysates were incubated at 37 °C for 4 h. Samples were measured with an enzyme-linked immunosorbent assay (ELISA) reader at an absorbance of 405 nm.
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM) of three independent experiments. Results were analyzed with independent-samples of student t-tests between two groups and one-way analysis of variance (ANOVA) for more groups with Dunnett’s test. A value of P < 0.05 was considered significant.
Results
The expression of CHRF and TGF-β1 in the hearts of DOX-induced HF models
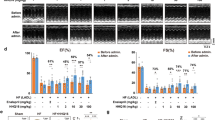
Compared with the control mice (Group 1, n = 6), the levels of left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and ± dp/dt max parameters were aberrant in the HF mice (Group 2, n = 6) (Fig. 1a), implying seriously dysregulated cardiac function. In addition, the activity of caspase-3 and cleaved caspase-3 were significantly increased in the HF mice compared to the control group (Group 1, n = 6) (Fig. 1b). Compared with the control group (Group 1, n = 6), CHRF expression was greatly boosted in the hearts of the HF mice (Fig. 1c). Western blotting results showed that TGF-β1 expression was also markedly increased in the heart tissues of the HF mice (Group 2, n = 6) compared to the control mice (Group 1, n = 6) (Fig. 1d). However, VAL at a dosage of 30 mg/kg/d (Group 3, n = 6) significantly mitigated the adverse DOX-induced effects noted above (Fig. 1a, b, c, d). These data indicate that while CHRF and TGF-β1 were abnormally expressed in the process of HF, VAL relieved those effects.
Effects of VAL on the expression of CHRF, TGF-β1, and caspase-3 activity in DOX-induced HF mice. Mice were randomly divided into three groups (n = 6 in each group): Group 1 was used as a control and treated with an equivalent volume of sterile saline solution; mice in Group 2 were intraperitoneally injected with 2.5 of mg/kg DOX six times in 2 weeks; animals in Group 3 were intraperitoneally injected with 2.5 mg/kg of DOX six times in 2 weeks, and then given 30 mg/kg/d of VAL for 4 weeks by oral gavage. a Cardiac function was evaluated via LVSP, LVEDP, and ± dp/dt max parameters detection; b Caspase-3 activity was detected to assess cell apoptosis, and the cleaved caspase-3 was analyzed by western blot; c Relative CHRF expression was determined by qRT-PCR; d The level of TGF-β1 protein was analyzed by western blot. All data were expressed as the mean ± SEM of three independent experiments. β-actin was used as an internal control in the western blot, and GAPDH acted as the control in qRT-PCR. *P < 0.05 versus Group 1; #P < 0.05 versus Group 2
The expression of CHRF and TGF-β1 in mice primary myocardial cells
To confirm the dysregulation of CHRF, TGF-β1, and caspase-3 activity in the HF mice, the primary myocardial cells were pretreated with 1 μM of VAL for 12 h, followed by 2 μM of DOX. As shown in Fig. 2a, compared with the control, CHRF was significantly up-regulated in the DOX group. In addition, the protein level of TGF-β1 was higher in the DOX treatment group than in the control group (Fig. 2b), and the activity of caspase-3 and cleaved caspase-3 were markedly increased in the DOX group compared to the control group (Fig. 2c). TUNEL assay results also indicated that DOX treatment significantly promoted the apoptosis of primary myocardial cells (Fig. 2d). Importantly, all these DOX-induced changes were effectively reversed by VAL (Fig. 2a, b, c, d). These data indicate that VAL, CHRF, TGF-β1, and cell apoptosis might be related to the development of HF.
Effects of VAL on the expression of CHRF, TGF-β1 and caspase-3 activity in primary myocardial cells. Cells were pretreated with 1 μM of VAL for 12 h, followed by 2 μM of DOX to establish a HF model in vitro. a The expression of CHRF was determined by qRT-PCR; b The protein expression of TGF-β1 was analyzed by western blot; c Caspase-3 activity was detected to assess cell apoptosis, and the cleaved caspase-3 was analyzed by western blot; d Cell apoptosis was examined by TUNEL. All data were expressed as the mean ± SEM of three independent experiments. β-actin was used as the internal control in the western blot and GAPDH was acted as the control in the qRT-PCR. *P < 0.05 versus control; #P < 0.05 versus DOX
The effect of CHRF on the expression of TGF-β1 in primary myocardial cells
Primary myocardial cells were transfected with si-CHRF, and the levels of TGF-β1 and caspase-3 activity were determined. As shown in Fig. 3a, si-CHRF transfection partly reversed the DOX-induced up-regulation of CHRF. As shown in Fig. 3b and c, the TGF-β1 protein level and activity of caspase-3 and cleaved caspase-3 clearly decreased in the cells transfected with si-CHRF compared with the si-control group. In addition, the number of apoptosis cells was also synchronously reduced in the CHRF interference group compared to the cells transfected with the si-control (Fig. 3d). However, overexpression of CHRF (with pcDNA-CHRF transfected) reversed the VAL’s inhibitory effect on CHRF (Fig. 3e). While treatment with VAL reduced the DOX-induced up-regulation of the TGF-β1 protein level, this effect was reversed by pcDNA-CHRF transfection (Fig. 3f). Furthermore, pcDNA-CHRF transfection significantly increased the activity levels of caspase-3 and cleaved caspase-3 (Fig. 3g) as well as cell apoptosis number (Fig. 3h) that was inhibited by VAL. All these results show that CHRF influenced the expression of TGF-β1 and cell apoptosis.
Effect of si-CHRF or pcDNA-CHRF on the expression of TGF-β1 and caspase-3 activity in primary myocardial cells. a, e Relative CHRF expression was determined by qRT-PCR; b, f Expression of the TGF-β1 protein was analyzed by western blot; c, g Caspase-3 activity was detected to assess cell apoptosis, and the cleaved caspase-3 was analyzed by western blot; d, h Cell apoptosis was examined by TUNEL. All data were expressed as the mean ± SEM of three independent experiments. β-actin was used as the internal control in the western blot, and GAPDH acted as the control in the qRT-PCR. *P < 0.05 versus control; #P < 0.05 versus DOX + si-control or DOX; &P < 0.05 versus DOX + VAL + pcDNA
Down-regulation of CHRF reversed the increased expression of TGF-β1 and downstream proteins induced by pcDNA-TGF-β1 in HL-1 myocardial cells
HL-1 cells were transfected or co-transfected with si-CHRF and pcDNA-TGF-β1, and the protein expressions of TGF-β1, p-Smad2, p-Smad3, and p-p38 were detected by western blot. In HL-1 cells transfected with si-CHRF, the levels of TGF-β1 (Fig. 4a), p-Smad2, p-Smad3, (Fig. 4b) and p-p38 (Fig. 4c) were significantly decreased compared with the si-control; however, expression of the abovementioned proteins was clearly reversed by pcDNA-TGF-β1, while the increased TGF-β1 (Fig. 4a), p-Smad2, p-Smad3 (Fig. 4b), and p-p38 (Fig. 4c) protein expressions in the pcDNA-TGF-β1 group were further reversed by si-CHRF (Fig. 4a, b, c). HL-1 cells were transfected with pcDNA, pcDNA-CHRF, pcDNA-CHRF + si-NC (si-negative control) or DMSO, pcDNA-CHRF + si-SMAD2/3, or SB202190 (p38 inhibitor, 5 μM, 24 h), then the cells were treated with 1 μM of VAL for 12 h, followed by 2 μM of DOX. The results showed that inhibiting of SMAD2/3 (Fig. 4d) or p38 (Fig. 4e) reversed the up-regulation of cell apoptosis induced by pcDNA-CHRF. These results demonstrate that CHRF regulated the expression of TGF-β1 and its target genes through TGF-β1/Smads and TGF-β1/p38 pathways.
CHRF regulated the TGF-β/Smads and TGF-β/p38 pathways to affect cell apoptosis and related protein expressions in HL-1 cells. a The TGF-β1 protein was analyzed by western blot; b The protein levels of p-Smad2 and p-Smad3 were analyzed by western blot; c The protein level of p-P38 was analyzed by western blot; d, e Cells were treated with si-Smad2 or SB202190, and cell apoptosis was examined by TUNEL. All data were expressed as the mean ± SEM of three independent experiments. β-actin was used as the control in the western blot and GAPDH acted as the control in the qRT-PCR. In Figures A-C, *P < 0.05 versus si-control; # P < 0.05 versus si-CHRF. In Figures D-E, *P < 0.05 versus pcDNA; #P < 0.05 versus pcDNA-CHRF + si-NC (si-negative control) or pcDNA-CHRF + DMSO
Over-expression of CHRF prevented the cardiac function improvement induced by VAL in vivo
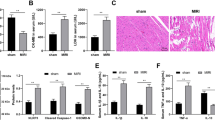
To explore whether CHRF’s overexpression moderated VAL’s effect on HF, the animals were randomly divided into four groups: control, VAL, VAL + Ad-control, and VAL + Ad-CHRF. Compared with the control, the levels of LVSP (Fig. 5a), + dp/dt max (Fig. 5c), and—dp/dt max (Fig. 5d) were elevated by VAL but reversed by Ad-CHRF injection, while the LVEDP level was sharply decreased by VAL but reversed by Ad-CHRF injection (Fig. 5b). These results suggest that while cardiac function clearly improved after treatment with VAL, those effects were reversed by Ad-CHRF. All the data suggest that overexpression of CHRF reversed VAL’s cardiac protective effects.
Overexpression of CHRF reversed the cardiac protective effect of VAL in vivo. Mice were randomly divided into four groups: control, VAL, VAL + Ad-control, and VAL + Ad-CHRF. After injection of adenovirus vectors, mice were treated with VAL by oral gavage. Four weeks later, LVSP (a), LVEDP (b), and ± dp/dt max (c, d) parameters were detected to assess the mice’s cardiac function. LVSP: left ventricular systolic pressure; LVDEP: left ventricular end-diastolic pressure; +dp/dtmax: the maximal rate of the increase of left ventricular pressure; -dp/dtmax: the maximal rate of the decrease of left ventricular pressure. All data were expressed as the mean ± SEM of three independent experiments. *P < 0.05 versus control; #P < 0.05 versus VAL + Ad-control
Discussion
In the present study, up-regulation of CHRF, TGF-β1, and increased cell apoptosis were observed in HF animals and primary myocardial cells treated with DOX. However, in mice or cells treated with VAL, the expression of CHRF and TGF-β1 decreased, and cell apoptosis reduced. Si-CHRF decreased the expression of TGF-β1 and inhibited myocardial cell apoptosis, while pcDNA-CHRF exhibited the opposite outcomes. Moreover, overexpression of CHRF inhibited VAL’s protective effects on cardiac function in vivo. Further, the results showed that CHRF regulated the expression of TGF-β1 and its target genes through TGF-β1/Smads and TGF-β/p38 pathways. Thus, VAL regulated TGF-β pathways through lncRNA CHRF to improve DOX-induced HF.
The functions of lncRNAs in heart diseases have been with the focus of increased researches. For example, microarray analysis has identified five significantly increased or decreased lncRNAs that are aberrantly expressed in patients, which may be used as novel biomarkers for prenatal diagnosis of fetuses with congenital heart defects (Gu et al. 2016). The circulating lncRNA LIPCAR level can identify patients developing cardiac remodeling and predict future death in HF patients (Kumarswamy et al. 2014). In this study, CHRF was up-regulated in DOX-induced HF and may influence the process of HF. Furthermore, overexpression of CHRF promoted myocardial cell apoptosis and dysregulated cardiac function. This study is the first to demonstrate the functional significance of CHRF in HF; it indicates that CHRF functions as a positive regulator to accelerate HF progression. However, more efforts are needed to develop CHRF as a novel therapy to treat HF.
TGF-β1 plays a critical role in cardiovascular systems. Expression of TGF-β1 leads to the activation of fibroblasts in the heart by promoting the differentiation of cardiac fibroblasts and the synthesis of extracellular matrix proteins (ECM), thereby regulating cardiac remodeling or HF (Kapur et al. 2012; Ieronimakis et al. 2013). Inhibiting the TGF-β1 pathway reduced left ventricular remodeling and systolic and diastolic dysfunction (Villar et al. 2013). Although the role of TGF-β1 in fibrotic cardiac remodeling has been established, its regulatory effects on HF have not been investigated. This study confirms that overexpressed TGF-β1 reverses the decreased expression of TGF-β1 and its target factors p-Smad2, p-Smad3, and p38 induced by si-CHRF in HL-1 cells. Other reports have indicated that TGF-β1 induces growth arrest and apoptosis in normal epithelial cells in vitro (Leight et al. 2012) and increases the resistance of NIH/3T3 fibroblasts toward apoptosis by activating the Smad2/3 and ERK1/2 pathways (Negmadjanov et al. 2016). The p38/MAPK signaling is activated by TGF-β1, and it regulates epithelial to mesenchymal transition (EMT)-related changes in pulmonary epithelial cells (Kolosova et al. 2011). Consistent with these findings, this study’s results showed that TGF-β1 and its downstream transcription factors p-Smad2, p-Smad3, and p38 are increased in HF, as is cell apoptosis. Similar levels of TGF-β1, caspase-3 activity, and numbers of cell apoptosis were observed after TGF-β1 overexpression. Moreover, the increased levels of TGF-β1, p-Smad2, p-Smad3, and p38 induced by overexpression of TGF-β1 were partly reversed by si-CHRF. These results indicate that the function mechanism of CHRF is involved in the regulation of TGF-β1 and apoptosis in HF progression.
This study also demonstrated that VAL is a key regulator of HF development. VAL mitigates DOX-induced HF by regulating TGF-β1 pathways. These findings are consistent with previous studies. One study found that high dosages of VAL significantly decreased TGF-β1 production and protected against peritoneal fibrosis induced by chlorhexidine digluconate (Subeq et al. 2011). Another showed that low dosages of VAL inhibited interstitial fibrosis by promoting apoptosis of the fibroblasts (Akashiba et al. 2008). Moreover, VAL’s renoprotective effects may be related to its potential in decreasing oxidative stress and the expressions of TGF-β1 in glomerular mesangial cells and glomerular epithelial cells (Jiao et al. 2011). Thus, identifying these target genes and determining the underlying pathways may shed new light on VAL’s roles in DOX-induced HF. Furthermore, this study found that while VAL improves cardiac dysfunction in HF mice, overexpression of CHRF may reverse the VAL’s cardiac protective effects.
In summary, VAL functions as a protective agent to regulate TGF-β/Smads and TGF-β/p38 pathways through CHRF to improve DOX-induced HF. The significant correlation among VAL, CHRF, and TGF-β1 pathways in HF was highlighted for the first time. However, further studies should further explore the mechanisms of HF to develop new therapies.
References
Akashiba A, Ono H, Ono Y, Ishimitsu T, Matsuoka H (2008) Valsartan improves L-NAME-exacerbated cardiac fibrosis with TGF-ß inhibition and apoptosis induction in spontaneously hypertensive rats. J Cardiol 52:239–246
Albini A, Pennesi G, Donatelli F, Cammarota R, Flora SD, Noonan DM (2010) Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst 102:14–25
Behnes M, Hoffmann U, Lang S, Weiss C, Ahmadnejad P, Neumaier M, Borggrefe M, Brueckmann M (2011) Transforming growth factor beta 1 (TGF-beta 1) in atrial fibrillation and acute congestive heart failure. Clin Res Cardiol 100:335–342
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147:358–369
Chaplin S (2016) Sacubitril/valsartan for the treatment of heart failure. Prescriber 27:56–57
Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, Nd DG, Kirshenbaum LA (2014) Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc Natl Acad Sci USA 111:5537–5544
Gu M, Zheng A, Tu W, Zhao J, Li L, Li M, Han S, Hu X, Zhu J, Pan Y (2016) Circulating LncRNAs as novel, non-invasive biomarkers for prenatal detection of fetal congenital heart defects. Cell Physiol Biochem 38:1459–1471
Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA (2012) Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111:131–150
Ieronimakis N, Hays AL, Janebodin K, Mahoney WM, Duffield JS, Majesky MW, Reyes M (2013) Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFβ1 signaling in the mdx mouse model of Duchenne muscular dystrophy. J Mol Cell Cardiol 63:122–134
Ikeuchi M, Tsutsui H, Shiomi T, Matsusaka H, Matsushima S, Wen J, Kubota T, Takeshita A (2004) Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res 64:526–535
Jain A, Kishore K (2013) Doxorubicin-induced dilated cardiomyopathy for modified radical mastectomy: a case managed under cervical epidural anaesthesia. Indian J Anaesth 57:185–187
Jasmin JF, Calderone A, Leung TK, Villeneuve L, Dupuis J (2003) Lung structural remodeling and pulmonary hypertension after myocardial infarction: complete reversal with irbesartan. Cardiovasc Res 58:621–631
Jiao B, Wang YS, Cheng YN, Gao JJ, Zhang QZ (2011) Valsartan attenuated oxidative stress, decreased MCP-1 and TGF-β1 expression in glomerular mesangial and epithelial cells induced by high-glucose levels. Biosci Trends 5:173–181
Kanduri C (2011) Kcnq1ot1: a chromatin regulatory RNA. Semin Cell Dev Biol 22:343–350
Kapur NK, Wilson S, Yunis AA, Qiao X, Mackey E, Paruchuri V, Baker C, Aronovitz MJ, Karumanchi SA, Letarte M (2012) Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation 125:2728–2738
Keating GM, McCormack PL (2016) Sacubitril/valsartan in chronic heart failure with reduced ejection fraction: a guide to its use. Drugs Ther Perspect 33:1–7
Kolosova I, Nethery D, Kern JA (2011) Role of Smad2/3 and p38 MAP kinase in TGF-beta1-induced epithelial-mesenchymal transition of pulmonary epithelial cells. J Cell Physiol 226:1248–1254
Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, De GP, Pinet F, Thum T (2014) Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res 114:1569–1575
Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS (2012) Matrix rigidity regulates a switch between TGF-β1–induced apoptosis and epithelial–mesenchymal transition. Mol Biol Cell 23:781–791
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF, Waye MM (2015) The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget 6:22513–22525
Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, Masson S, Cerè E, Tognoni G, Cohn JN (2005) Val-HeFT Investigators. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am Heart J 149:548–557
Majani G, Giardini A, Opasich C, Glazer R, Hester A, Tognoni G, Cohn JN, Tavazzi L (2005) Effect of valsartan on quality of life when added to usual therapy for heart failure: results from the Valsartan Heart Failure Trial. J Card Fail 11:253–259
Nakamae H, Tsumura K, Terada Y, Nakane T, Nakamae M, Ohta K, Yamane T, Hino M (2015) Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 104:2492–2498
Negmadjanov U, Holmuhamedov A, Emelyanova L, Xu H, Rizvi F, Ross GR, Tajik AJ, Shi Y, Holmuhamedov E, Jahangir A (2016) TGF-β1 increases resistance of NIH/3T3 fibroblasts toward apoptosis through activation of Smad2/3 and Erk1/2 pathways. J Patient Cent Res Rev 3:187–198
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Subeq YM, Ke CY, Lin NT, Lee CJ, Chiu YH, Hsu BG (2011) Valsartan decreases TGF-β1 production and protects against chlorhexidine digluconate-induced liver peritoneal fibrosis in rats. Cytokine 53:223–230
Sui X, Zheng J, Yao Q (2016) Obestatin improves doxorubicin-induced heart failure via the regulation of the Lncrna Mhrt on Nrf2. Lipid Cardiovasc Res 2:17–26
Sun M, Gadad SS, Kim DS, Kraus WL (2015) Discovery, annotation, and functional analysis of long noncoding RNAs controlling cell-cycle gene expression and proliferation in breast cancer cells. Mol Cell 59:698–711
Sun Z, Schriewer J, Tang M, Marlin J, Taylor F, Shohet RV, Konorev EA (2016) The TGF-β pathway mediates doxorubicin effects on cardiac endothelial cells. J Mol Cell Cardiol 90:129–138
Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J (2016) Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med 8:326ra22
Villar AV, García R, Llano M, Cobo M, Merino D, Lantero A, Tramullas M, Hurlé JM, Hurlé MA, Nistal JF (2013) BAMBI (BMP and activin membrane-bound inhibitor) protects the murine heart from pressure-overload biomechanical stress by restraining TGF-β signaling. Biochim Biophys Acta 1832:323–335
Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen M, Wang Y (2013) Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med 41:177–196
Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF (2014) The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res 114:1377–1388
Wang K, Liu CY, Zhou LY, Wang JX, Wang M, Zhao B, Zhao WK, Xu SJ, Fan LH, Zhang XJ, Feng C, Wang CQ, Zhao YF, Li PF (2015) APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun 6:6779
Wu Q, Han L, Yan W, Ji X, Han R, Yang J, Yuan J, Ni C (2016) miR-489 inhibits silica-induced pulmonary fibrosis by targeting MyD88 and Smad3 and is negatively regulated by lncRNA CHRF. Sci Rep 6:30921
Xu H, Liu C, Rao S, He L, Zhang T, Sun S, Wu B, Zou L, Wang S, Xue Y, Jia T, Zhao S, Li G, Liu S, Li G, Liang S (2016) LncRNA NONRATT021972 siRNA rescued decreased heart rate variability in diabetic rats in superior cervical ganglia. Auton Neurosci 201:1–7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Chen, L., Yan, KP., Liu, XC. et al. Valsartan regulates TGF-β/Smads and TGF-β/p38 pathways through lncRNA CHRF to improve doxorubicin-induced heart failure. Arch. Pharm. Res. 41, 101–109 (2018). https://doi.org/10.1007/s12272-017-0980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0980-4