Abstract
5-Fluorouracil (5-FU) alone or in combination with other therapeutic drugs has been widely used for clinical treatment of various cancers. However, 5-FU-based chemotherapy has limited anticancer efficacy in clinic due to multidrug resistance and dose-limiting cytotoxicity. Some molecules and genes in cancer cells, such as nuclear factor kappa B, insulin-like growth factor-1 receptor, epidermal growth factor receptor, cyclooxygenase-2, signal transducer and activator of transcription 3, phosphatase and tensin homolog deleted on chromosome ten and Bcl-2 etc. are related to the chemoresistance and sensitivity of cancer cells to 5-FU. The activation of these molecules and genes expressions in cancer cells will be increased or decreased with long-term exposure of 5-FU. Curcumin has been found to be able to negatively regulate these processes. In order to overcome the problems of 5-FU, curcumin has been used to combine with 5-FU in cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
5-Fluorouracil (5-FU) is a fluoropyrimidine analogue and the main mechanistic actions of anticancer activity are via inhibiting the activity of thymidylate synthase (TS) and DNA synthesis and repair by directly incorporating its metabolites into DNA and RNA of cancer cell (Noordhuis et al. 2004). 5-FU was widely used alone or in combination with other anticancer drugs for the treatment of colorectal cancer (Rossi et al. 2007), breast cancer (Yao et al. 2014), liver cancer (Cao et al. 2014; Cheng et al. 2012), pancreatic cancer (André et al. 2004), esophageal cancer (Liu et al. 2015) and gastric cancer (Mayr et al. 2012) etc. However, the application and efficacy of 5-FU-based chemotherapy are severely limited in clinic due to dose-limiting toxicity to the patients and multidrug resistance (MDR) in cancer cells, which is related to the over-expressions of TS (Peters et al. 2002; Wang et al. 2007). nuclear transcription factor (NF-κB, Gupta et al. 2010), cyclooxygenase-2 (COX-2, Sobolewski et al. 2010), human epidermal growth factor receptor (EGFR, Molinari et al. 2009), insulin-like growth factor 1 receptor (IGF-1R, Pollak 2008), and MDR gene 1 encoding transporter P-glycoprotein (P-gp) etc. NF-κB is activated by some stimuli such as inflammatory cytokines, bacterial products, reactive oxygen species, phorbol esters and other molecules through the phosphorylation and degradation of IκB kinase (IKK) and then the activated NF-κB increases the transcription of the target genes (Beg and Baldwin 1993; Prasad et al. 2010). Numerous studies have indicated that NF-κB was a major downstream effector of chemoresistance of various therapeutic drugs and NF-κB activation was closely related with the development of MDR (Chen et al. 2010; Mongre et al. 2015; Bonavida and Baritaki 2011). In addition, NF-κB regulates the expressions of a number of gene products, such as survivin, Bcl-xL, Bcl-2, COX-2, CyclinD1, P53 and Fas, which are related to carcinogenesis and apoptosis of cancer cells (Bassères and Baldwin 1993; Cao et al. 2015; Li et al. 2015a; Tsai et al. 2015). When cancer cells are exposed to 5-FU for a long time, the expressions of P53, Bax and Bcl-2 are dysregulated, which also lead to MDR (Li et al. 2015d; Wang et al. 2013a; Manoochehri et al. 2014; Tang et al. 2016). COX-2 is an inducible enzyme and overexpressed in several cancer cells such as breast, colorectal, gastric, and prostate cancers compared to normal tissues and COX-2 overexpression is related to the development and progression of cancer (Singh et al. 2005; Sobolewski et al. 2010; Cheng and Fan 2013). EGFR and HER2 belonging to human epidermal growth factor receptor family mediate the proliferation, differentiation, migration and invasion of cancer cells through a variety of signaling pathways and have become important targets of anticancer drugs (Ciardiello and Tortora 2008; Molinari et al. 2009). Insulin-like growth factor 1 receptor (IGF-1R), a transmembrane glycoprotein with the activity of tyrosine kinase, is related to the development and progression of cancer (Pollak 2008, 2012). The abnormal activities of EGFR, HER2 and IGF-1R could lead to MDR (Chen et al. 2015a, 2000; Dallas et al. 2009). Activation of P-gp is an important cause for MDR (Li et al. 2015c). Some studies indicated that the anticancer efficacy of 5-FU was increased when its dose was increased. Unfortunately, the cytotoxicity was significantly increased to normal cells, thus, induced unacceptable toxicity to the patients (Tang et al. 2012; Polk et al. 2013). In order to overcome these problems, an ideal strategy is to combine 5-FU with other anticancer drugs with different mechanistic action.
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, C21H20O6, MW 368.37 g/mol] is a natural compound extracted from the rhizome of zingiberaceae plants. Curcumin had been used as a food additive for centuries (Yuan et al. 2012). Recently, numerous studies have proved its potential effects on prevention and treatment of many types of cancer (Perrone et al. 2015). In addition, it was found that the combination treatment of curcumin with anticancer drugs may overcome MDR to increase anticancer efficacy and reduce cytotoxicity such as 5-FU (Shakibaei et al. 2013), paclitaxel (Zhan et al. 2014; Dang et al. 2015), gemcitabine (Ali et al. 2010; Kanai et al. 2011), oxaliplatin (Howells et al. 2011; Ruiz de Porras et al. 2016), doxorubicin (Chen et al. 2013; Lv et al. 2016), cisplatin (Chen et al. 2015b; Waseem et al. 2014) and so on. On the other hand, curcumin had little toxicity itself and didn’t increase the toxicities of other anticancer drugs, therefore, making it a good candidate for combination therapy.
Anticancer activity of curcumin
Curcumin has exhibited potential preventive and therapeutic effects such as antiinflammation, antioxidant, antirheumatism, reduced cardiovascular events, liver protection, and especially anticancer (Deodhar et al. 1980; Kiso et al. 1983; Miriyala et al. 2007; Qadir et al. 2016; Chen et al. 2017b; Mise Yonar et al. 2017). Curcumin has been widely used in prevention and treatment of various cancers including colorectal, gastric, breast, liver, esophageal, prostate, lung, brain cancers, and leukemia in preclinical studies (Perrone et al. 2015; Taverna et al. 2015; Tong et al. 2016; Ali et al. 2017; Wang et al. 2017).
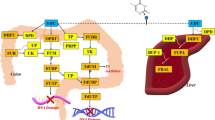
The anticancer activities of curcumin were closely related to a variety of mechanisms such as cancer cell development, proliferation, transformation, invasion, metastasis and angiogenesis by affecting transcription factors, cytokines, chemokines, reactive oxygen species (ROS), COX-2, NF-κB, tumor necrosis factors (TNF), matrix metalloproteinase (MMPs), signal transducer and activator of transcription (STAT) and protein kinase B (Akt) etc. (Lev-Ari et al. 2006; Anand et al. 2007; Shankar et al. 2008; Shehzad et al. 2010; Shiri et al. 2015) and the proposed scheme is illustrated in Fig. 1.
In addition, previous studies have shown that curcumin was able to reverse MDR through various mechanisms including antiproliferation, apoptosis induction, blocking cell cycle arrest, suppression of epithelial-mesenchymal transition (EMT) of cancer cells and so on (Zhou et al. 2011; Saha et al. 2012; Roy and Mukherjee 2014; Wang et al. 2014b; Rajitha et al. 2016). Curcumin also can reverse MDR by decreasing the expression and function of P-gp and promoting the activation of caspase-3 (Limtrakul et al. 2004; Tang et al. 2005; Lu et al. 2013; Oliveira et al. 2015). PI3K/Akt/mTOR, as one of the main cell signaling pathways, is involved in the proliferation, survival, metabolism and motility of cells and often activated in cancer cells (Fang et al. 2012; Gonzalez-Angulo and Blumenschein 2013). Its activation is related to the development of MDR in cancer cells (Sui et al. 2012). Activated PI3K can translate phosphatidylinositol 4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-triphosphate (PIP3) to phosphorylate Akt and further stimulate Akt-mediated activation of downstream targets including Bcl-2 family members, tuberous sclerosis complex 2 (TSC2) and mouse double minute 2 homolog (Mdm2, Massihnia et al. 2016). Furthermore, PTEN could negatively regulate this process by dephosphorylating PIP3 to inhibit the activation of PI3K/Akt/mTOR signaling pathway (Zhang et al. 2015). Studies also showed that curcumin could suppress the expression of P-gp via inhibition of the PI3K/Akt/NF-kB signaling pathway to reverse MDR (Choi et al. 2008). Cells undergoing EMT showed a similar feature to cancer stem cells (CSCs) with same drug resistance phenotypes (Singh and Settleman 2010; Wang et al. 2010). Studies showed that curcumin is an effective agent in treatment of esophageal cancer and exhibited potent cytotoxicity in a dose-dependent manner in all six tested cell lines, the inhibitory effect of curcumin not only eliminated cancer cells but also targeted CSCs against esophageal cancer cells (Almanaa et al. 2012). Study showed that curcumin upregulated EMT to reverse MDR (Suresh et al. 2016). Persistent activation of STAT3 was frequently detected in the majority of human cancer cells and tumor tissues (Bromberg et al. 1999; Bowman et al. 2000). Some studies showed that curcumin and its analog could reverse MDR via inhibiting the expression of STAT3 (Selvendiran et al. 2011; Kumar et al. 2014).
Extensive research from in vitro cell culture and in vivo animal models has shown that curcumin can sensitize various tumors to different chemotherapeutic agents such as 5-FU, paclitaxel, doxorubicin, vincristine, melphalan, butyrate, cisplatin, vinorelbine, gemcitabine, oxaliplatin, and etoposide etc. (Goel and Aggarwal 2010).
Taken together, curcumin enhances the therapeutic efficacy and reduces the toxicity of 5-FU, the combination of curcumin and 5-FU has been widely used to treat various cancer cells by enhancing the sensitivity and reverse the MDR induced by 5-FU. Therefore, this paper will review the synergistic combination of curcumin and 5-FU in the treatment of colorectal, breast, esophageal, gastric, liver, pancreatic and nasopharyngeal cancers and possible associated molecular mechanisms (as shown in Fig. 2) from the literature. So far, there are only two reports for randomized clinical trials of the combination of curcumin with 5-FU-based therapy (FOLFOX: 5-fluorouracil, folinic acid and oxaliplatin) in patients with colorectal cancer (James et al. 2015; Irving et al. 2015).
Schematic model of the molecular mechanisms of 5-FU combined with curcumin associated with anticancer effect. (When prolonged exposure to 5-FU, the activation of TS was enhanced, thereby leading to attenuate inhibit the death of cancer cell. The activated TS also could activate NF-κB to further inhibit the cell death. However, curcumin could inhibit the activation of TS, p-IKK and COX-2 to enhance the cancer cell death. Both 5-FU and curcumin could reduce the activation of STAT to enhance the cancer cell death. 5-FU could also activate PI3K, then, induced phosphorylation of AKT to stimulate the AKT-mediated activation of the Bcl-2 family members to inhibit apoptosis (cell death). Curcumin could reduce the activation of PI3K and AKT to enhance the cell death. In addition, the activation of PTEN could negatively regulate the activation of AKT to enhance the cell death
The synergistic combination of 5-FU and curcumin in cancer therapy
Colorectal cancer
Colorectal cancer is one of the most common cancers and the third leading cause of cancer death in the United States (Siegel et al. 2014). 5-FU alone or in combination with other chemotherapeutic drugs is widely used for the treatment of colorectal cancer (Inoue et al. 2006; Adamsen et al. 2007; Xu et al. 2016). However, MDR was developed in many patients with colorectal cancer during the course of chemotherapy with 5-FU-based regiments (Murakami et al. 2000; Körber et al. 2013). The problem was partly overcome by increasing the dose of 5-FU but high doses of 5-FU would lead to severe toxicities in patients and the treatment has to be discontinued (Eskandari et al. 2015; Vincenzi et al. 2015). Studies have found that the combination of 5-FU and curcumin may be an effective treatment regimen for the patients with colorectal cancer to overcome drug resistant and reduce cytotoxicity induced by 5-FU.
Direct genetic evidence showed that COX-2 plays a key role in tumorigenesis of colorectal cancer in heterozygous Apc Δ716 knockouts mice (Taketo 1998). Curcumin could reduce the risk of carcinogenesis and development of colorectal cancer by inhibiting COX-2 (Goel et al. 2001; Chen et al. 2006). Studies by Du et al. (2006) showed synergistic inhibitory effects of curcumin in combination with 5-FU on cell growth of human colon cancer HT-29 cells by significantly reducing the expression of COX-2 compared to each drug alone. Studies of curcumin in combined with FOLFOX (50 µM 5-FU and 1.25 µM oxaliplatin) significantly enhanced the cell growth inhibition of colon cancer HCT-116 and HT-29 cells to prevent the emergence of chemo-surviving cells by reducing the activation of EGFR, HER-2, IGF-1R and AKT and the expression of COX-2 and cyclin-D1 (shown in Figs. 3, 4) (Patel et al. 2010). Toden and colleagues carried out experiments with growth proliferation and apoptosis assays in parental and 5-FU resistant colorectal cancer cells and found that the combination of curcumin and 5-FU enhanced proliferation inhibition and cell apoptosis in both cells but 5-FU alone was ineffective against 5-FU resistant cells, the mechanism for curcumin sensitizing 5-FU-related chemoresistance in 5-FU resistant cells was through suppression of EMT (Toden et al. 2015). Moreover, the same group further demonstrated that curcumin sensitized 5-FU to inhibit tumor growth in vivo in a xenograft mouse models (Toden et al. 2015).

Reproduce with permission from Patel et al. (2010)
a Proportion of chemo-surviving HCT-116 cells following 48 h of treatment with FOLFOX (50 µM 5-FU and 1.25 µM oxaliplatin) by MTT assay. b Levels of activated (tyrosine phosphorylated) forms of EGFR, HER-2, IGF-1R, and downstream mediators AKT, and COX-2 in chemo-surviving HCT-116 cells by Western-blotting.

Reproduced with permission from (Patel et al. 2010)
a Growth inhibition of chemo-surviving HCT-116 and HT-29 cells treated with FOLFOX alone or in combination with curcumin (25 and 50 µM) for 96 h compared to media-treated cells (control). b The levels of activated growth factor receptors EGFR, HER-2, IGF-1R, and downstream mediators AKT, as well as COX-2, in chemo-surviving HCT-116 cells following treatment with FOLFOX alone or in combination with curcumin (25 and 50 µM), compared to media-treated cells (control). *P < 0.001 compared to control.
Shakibaei and colleagues showed that curcumin potentiated the chemosensitivity of 5-FU in 5-FU resistant colon cancer cells by down-regulating NF-κB activation and NF-κB-regulated gene products and enhanced 5-FU-induced expression cleavage of pro-apoptotic proteins (caspases 8, 9, and 3, PARP and Bax), and down-regulated anti-apoptotic (Bcl-xL) and proliferative (cyclin D1) proteins (Shakibaei et al. 2013, 2015). The same group also found that 5-FU activated NF-κB/PI-3K/Src pathway leading to the development of MDR to 5-FU, whereas curcumin down-regulated this activation to reverse MDR of 5-FU through inhibiting IKK activation and IκBα phosphorylation (Shakibaei et al. 2014). Studies showed that pre-treatment of curcumin was able to chemosensitize 5-FU and to reverse MDR in resistant MMR-deficient human colon cancer cells (Shakibaei et al. 2014). In addition, curcumin chemosensitized 5-FU by suppressing the expression of the multidrug resistance protein 1 (MRP1) and P-gp in resistant human colon cancer cells (Lu et al. 2013). Study showed that the anticancer effects of the combination of curcumin and 5-FU loaded thiolated chitosan nanoparticles were enhanced against on colon cancer cells in vitro and improved the bioavailability of the drugs in vivo than the free combination of curcumin and 5-FU (Anitha et al. 2014a. b).
Furthermore, a phase I clinical trial of curcumin in combination with FOLFOX in 12 colorectal cancer patients with inoperable liver metastases has been conducted, the dose escalation study showed that curcumin was safe and well-tolerated adjunct to FOLFOX chemotherapy in patients at doses up to 2 g daily (James et al. 2015). Another randomized control clinical trial has been conducted for the combination of curcumin with FOLFOX in 33 colorectal cancer patients with inoperable liver metastases to determine a target dose, side effects and antitumor efficacy, the results will be reported after completing the trial (Irving et al. 2015 ).
Breast cancer
Breast cancer is the most common cancer diagnosed among women and second most common cancer overall in the United States and worldwide (Torre et al. 2015). It is the second leading cause of cancer death in women only exceeded by lung cancer (DeSantis et al. 2014a). 5-FU alone or in combination with other chemotherapeutic drugs has been widely used for the treatment of patients with breast cancer clinically (Longley et al. 2003; Takahashi et al. 2014; Wu et al. 2015). However, 5-FU-based therapy has many limitations including drug resistance such as MDR and dose-limiting toxicity (Wang et al. 2013b). However, studies have found that the combination of 5-FU and curcumin may be an effective strategy for breast cancer treatment to overcome drug resistance and reduce toxicity induced by 5-FU.
In cancer therapy, MDR is developed in breast cancer cells when treating with chemotherapeutic drugs such as 5-FU because of the increase in the expressions of NF-κB, as the cancer cells usually reshape their survival signaling pathways. However, curcumin can inhibit NF-κB activity (Cao et al. 2015; Tsai et al. 2015), therefore, the combination of curcumin and 5-FU could reverse MDR of cancer cells to 5-FU treatment to increase therapeutic efficacy and reduce toxicity. Studies by Vinod et al. (2013) showed that the combination of curcumin (10 μM) and 5-FU (10 μM) could significantly increase the cell growth inhibition and enhance apoptosis compared to 5-FU alone in different breast cancer cells. Curcumin could sensitize the breast cancer cells to 5-FU through TS-dependent down-regulation of NF-κB, whereas 5-FU could up-regulate TS and NF-κB after long-term exposure (Vinod et al. 2013). In another study of curcumin combined with 5-FU against breast cancer cells and normal cells, curcumin enhanced cell growth inhibition of 5-FU against breast cancer cells but the LD50 value of 5-FU was increased from 28 µM to 200–300 µM with a 7 to 10-fold protection from 5-FU cytotoxicity by curcumin in normal breast cells, indicating that curcumin may enhance the chemotherapeutic effectiveness of 5-FU by protecting normal cells from reduced viability and thus permitting higher dosing or longer treatment duration (Ferguson and Orlando 2015). A recent study showed that the silk fibroin (SF) nanoparticles loaded with 5-FU and curcumin were more effective for inducing apoptosis of 4T1 murine breast cancer cells in vitro and antitumor activity in animal model in vivo compared to free curcumin and 5-FU via generation of cellular reactive oxygen species (ROS) (Li et al. 2016).
Esophageal squamous cell carcinoma
Esophageal cancer is the eighth most common cause of cancer-related death worldwide (Jemal et al. 2011; Torre et al. 2015). Esophageal squamous cell carcinoma (ESCC) is the most common histological type of esophageal cancer. 5-FU-based therapy has been widely used in the treatment of patients with ESCC but the efficacy of treatment has been limited by drug resistance and toxicity (Kuwano et al. 2008; Wang et al. 2016). However, the combination of 5-FU and curcumin had been proved to reverse drug resistant and reduce toxicity induced by 5-FU in the treatment of ESCC in preclinical studies.
Studies have shown that curcumin promoted apoptosis and enhanced cell sensitivity to 5-FU in ESCC cells through inhibiting the NF-κB signaling pathways and the sensitizing effect of curcumin was via inhibiting the phosphorylation of IκBα and downregulating the expressions of Bcl-2 and cyclin D1 (Tian et al. 2008, 2012a, b). Furthermore, the combination of curcumin and 5-FU significantly reduced the tumor growth compared to curcumin or 5-FU alone in in vivo animal experiments with a nude mouse model of human ESCC xenograft (shown in Fig. 5). The same group also found that the suppression of NF-κB by curcumin resulted in decreased expression of anti-apoptotic protein Bcl-2 and cell cycle arrest by inhibiting the expression of cyclin D1 in ESCC Eca109 and EC9706 cells in vitro (Tian et al. 2012a).
Gastric cancer
Gastric cancer is one of the most common malignancies and the second leading cause of cancer-related death worldwide (Torre et al. 2015). 5-FU alone or in combination with other chemotherapeutic drugs was widely used in the treatment of gastric cancer (Qu et al. 2013; Jung et al. 2016; Mori et al. 2013; Shirao et al. 2013). However, drug resistance and toxicity induced by 5-FU and/or other drugs were the main obstacles for the effective treatment of patients with gastric cancer. However, curcumin combined with 5-FU may overcome the obstacles of MDR and toxicity induced by 5-FU in the treatment of gastric cancer.
Zhou et al. (2013) investigated the combined effect of curcumin and FOLFOX (0.1 µM 5-FU and 5 µM oxaliplatin) on cell growth inhibition of gastric cancer BGC-823 cells, and found that the anticancer effect of the combination was better than that of curcumin or the FOLFOX alone. The molecular mechanisms were related to apoptosis by increasing the expressions of Bax and caspase-3 and decreasing the expression of Bcl-2. Furthermore, an in vivo studies has found that the combination of curcumin and FOLFOX showed a stronger tumor growth inhibition against BGC-823 xenograft tumors than curcumin or FOLFOX alone and the inhibitory effect was related to apoptotic pathway including Bcl-2, Bax, caspases 3, 8, and 9 (Zhou et al. 2016).
The combination of curcumin and 5-FU showed enhanced cell death and a synergistic inhibition of survivin and STAT3 compared to each drug alone in gastric cancer AGS cells, thus, the effect of overcoming the chemoresistance of AGS cells was via down-regulating survivin and STAT3 during treatment of gastric cancer (Pandey et al. 2015). Koo et al. (2004) showed that curcumin combined with 5-FU had a stronger inhibitory effect on the growth of human gastric cancer AGS cells compared to curcumin or 5-FU alone, the greater cell growth inhibition induced by the combination was associated with blocking AGS cells in G2/M phase by curcumin. Another study by Kang et al. (2016) showed that curcumin could reverse the MDR of 5-FU in human gastric cancer SGC-7901 cells by downregulating the NFκB signaling pathway.
Liver cancer
Liver cancer is one of the most common cancers and the second fatal malignancy after pancreatic cancer, whose 5-year survival rate is as low as 16% (DeSantis et al. 2014b; Torre et al. 2015). In the past two decades, the mortality of liver cancer increased by more than 50% in the United States. In China, the rates of incidence and mortality of liver cancer are the highest in the world, especially in the Western region (Wei et al. 2014). Therefore, it is urgently needed to find an effective strategy to decrease the incidence and mortality of liver cancer. 5-FU alone or in combination with other chemotherapeutic drugs was widely used in the treatment of patients with liver cancer (Li et al. 2015b). However, MDR and toxicity of 5-FU limited its therapeutic efficacy. The combination of curcumin and 5-FU may overcome, at least in part, these problems to improve the outcome in treatment of liver cancer.
A study of the effects of curcumin (5, 10, and 20 mg/ml) in combination with 5-FU (at IC50) on cell growth inhibition and apoptosis with 5-FU resistant liver cancer Bel7402/5-FU cells by Cao et al. (2012) showed that the combination achieved significantly greater growth inhibition (21.47 ± 1.49, 27.10 ± 2.32 and 59.37 ± 2.45%), and higher apoptosis induction (30.92 ± 2.10, 44.87 ± 2.24 and 50.36 ± 2.58%) compared to curcumin growth inhibition: 6.74 ± 0.13, 9.31 ± 0.20 and 14.45 ± 1.02%, apoptosis: 12.03 ± 0.46, 13.54 ± 0.60, 18.14 ± 1.32%) and 5-FU (growth inhibition: 12.56 ± 0.87% and apoptosis: 25.59 ± 1.52%) alone, indicating that curcumin could reverse the drug resistance of Bel7402/5-FU cells. A study by Jing and Kong (2016) found that the inhibitory effects of 5-FU (7.5 μM) combined with curcumin (20 μM) on proliferation of HepG2 cells were stronger than 5-FU (7.5 μM) alone. The results also indicated that the combination of 5-FU and curcumin increased the level of PTEN and TLR4 and reduce the level of AKT to negatively regulate the PI3K/AKT signal pathway. In addition, the study showed that curcumin improve the anticancer effect of 5-FU by enhancing the sensitivity of liver cancer cells to 5-FU. Another study showed that the combination of SLN-curcumin and LDH-5-FU has stronger synergetic effect on apoptosis induction than the free combination of curcumin and 5-FU for the treatment of liver cancer SMMC-7721 cells (Zhu et al. 2013).
Pancreatic cancer
Pancreatic cancer is the fourth leading cause of cancer-related death worldwide and the incidence of pancreatic cancer has increased three times in last 10 years (Jemal et al. 2010; Siegel et al. 2013). Many patients diagnosed with pancreatic cancer were in the end-stage and postoperative recurrence rate was high, so chemotherapy is very important for the treatment of patients with pancreatic cancer. 5-FU alone or in combination with other chemotherapeutic drugs has been widely used in the treatment of patients with pancreatic cancer (Berlin et al. 2002; Hong et al. 2012; Li et al. 2014). However, chemotherapy of pancreatic cancer often failed resulted from the development of MDR and toxicity induced by 5-FU and/or other drugs (Hagmann et al. 2009; Wang et al. 2014a).
The combination of 5-FU and curcumin may be an effective strategy to overcome MDR and reduce toxicity induced by 5-FU to treat pancreatic cancer. Multidrug resistance-associated protein 5 (MRP5) conferred resistance to 5-FU by active efflux of drugs from the cell curcumin could enhance the anticancer effect of 5-FU by inhibiting the expression of MRP5 to overcome the resistance of 5-FU in pancreatic cancer cells (Li et al. 2011). A phase II clinical trial of curcumin in patients with advanced pancreatic cancer showed that oral administration of curcumin to the patients at 8 g daily was well tolerated and exhibited biological activity in some patients (Dhillon et al. 2008).
Nasopharyngeal cancer
Nasopharyngeal cancer is a rare type of head and neck cancer located at the nasopharynx and nasopharyngeal carcinoma (NPC) is by far the most common malignant tumor of the nasopharynx. NPC has a typical epidemiological characteristics and a high incidence in Southern China (Cao et al. 2011). The common treatment for NPC is radiotherapy and chemotherapy and the mean 5-year disease-free survival rats were 60–68% (Wei and Kwong 2010; Chapman et al. 2017; Ma et al. 2017). 5-FU alone or in combination with other chemotherapeutic drugs was widely used in the treatment of NPC and exhibited some efficacy (Chen et al. 2017a; Peng et al. 2017). However, drug resistance and severe side events induced by 5-FU and/or other drugs limited the long-term benefit of the treatment. Curcumin induced G2/M phase arrest and apoptosis in human NPC cells via apoptosis inducing factor and caspase-3-dependent pathways in vitro and inhibited proliferation of NPC through altering expression of proteins in the extracellular regulated protein kinase (ERK)-1/2 signaling pathway in a mouse model of tumor xenograft (Kuo et al. 2011; Xie et al. 2014). Therefore, curcumin in combination with 5-FU may reverse MDR and reduce toxicity induced by 5-FU in treatment of NPC (Wu et al. 2014).
The combination of 5-FU and curcumin significantly induced apoptosis compared to 5-FU and curcumin alone in NPC CNE-2Z cells via inhibiting the activity of NF- kB and enhancing the expression of Bax while inhibiting the expression of Bcl-2 (Wu et al. 2014).
Conclusion
5-FU-based therapy has been commonly used in the treatment of various types of cancer clinically. However, its therapeutic efficacy is limited due to drug resistance and severe toxicity. Curcumin is a natural compound with various biological and therapeutic effects and has displayed potential effects on prevention and treatment of different types of cancer. Numerous preclinical studies have shown that the combination of curcumin and various anticancer drugs may overcome drugs resistance to increase anticancer efficacy and reduce host toxicity in vitro and in vivo. Recently, curcumin in combination with anticancer drugs such as 5-FU has emerged as a hot research topic. In this paper, we reviewed the synergistic effects of the combination of curcumin and 5-FU in various cancers and associated possible mechanisms including activation or inhibition of a series of cell signaling pathways to enhance the sensitivity and reverse MDR in cancer cells response to 5-FU from preclinical studies. We hope it may help to shed some light for future clinical studies of the combination of curcumin and 5-FU or other therapeutic drugs in cancer therapy.
The prospect of the combination of 5-FU and curcumin in clinical application for cancer therapy seems to be promising, but also faces challenge. Therefore, it is necessary to evaluate the effectiveness and toxicity of the combination to access its feasibility in clinical practice. The safety and tolerability of curcumin alone in cancer patients have been well established in clinical studies (Dhillon et al. 2008; Storka et al. 2015). To the best of our knowledge, there are only two reports of curcumin in combination with standard care FOLFOX in patients with colorectal cancer clinically up to date (James et al. 2015; Irving et al. 2015). The dose escalation studiy showed that curcumin at doses up to 2 g daily was safe and well-tolerated adjunct to FOLFOX chemotherapy (James et al. 2015). Clinical trials of curcumin in combination with other anticancer drugs such gemcitabine (pancreatic cancer patients, Epelbaum et al. 2010; Kanai et al. 2011), docetaxel (breast cancer patient, Bayet-Robert et al. 2010) or docetaxel and prednisone (castration-resistant prostate cancer patients, Mahammedi et al. 2016) have been reported. However, the clinical application of curcumin in combination with 5-FU in cancer therapy still needs to be validated for antitumor efficacy and toxicity through a large number of clinical trials.
References
Adamsen BL, Kravik KL, Clausen OP, De Angelis PM (2007) Apoptosis, cell cycle progression and gene expression in TP53-depleted HCT116 colon cancer cells in response to short-term 5-fluorouracil treatment. Int J Oncol 31:1491–1500
Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH (2010) Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res 70:3606–3617
Ali NM, Yeap SK, Abu N, Lim KL, Ky H, Pauzi AZM, Ho WY, Tan SW, Alan-Ong HK, Zareen S, Alitheen NB, Akhtar MN (2017) Synthetic curcumin derivative DK1 possessed G2/M arrest and induced apoptosis through accumulation of intracellular ROS in MCF-7 breast cancer cells. Cancer Cell Int 17:30
Almanaa TN, Geusz ME, Jamasbi RJ (2012) Effects of curcumin on stem-like cells in human esophageal squamous carcinoma cell lines. BMC Complement Altern Med 12:195
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818
André T, Noirclerc M, Hammel P, Meckenstock R, Landi B, Cattan S, Selle F, Codoul JF, Guerrier-Parmentier B, Mokhtar R, Louvet C, GERCOR (2004) Phase II study of leucovorin, 5-fluorouracil and gemcitabine for locally advanced and metastatic pancreatic cancer (FOLFUGEM 2). Gastroenterol Clin Biol 28:645–650
Anitha A, Deepa N, Chennazhi KP, Lakshmanan VK, Jayakumar R (2014a) Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochim Biophys Acta 1840:2730–2743
Anitha A, Sreeranganathan M, Chennazhi KP, Lakshmanan VK, Jayakumar R (2014b) In vitro combinatorial anticancer effects of 5-fluorouracil and curcumin loaded N, O-carboxymethyl chitosan nanoparticles toward colon cancer and in vivo pharmacokinetic studies. Eur J Pharm Biopharm 88:238–251
Bassères DS, Baldwin AS (1993) Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 25:6817–6830
Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, Mouret-Reynier MA, Durando X, Barthomeuf C, Chollet P (2010) Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 9:8–14
Beg AA, Baldwin AS Jr (1993) The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev 7:2064–2070
Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20:3270–3275
Bonavida B, Baritaki S (2011) Dual role of NO donors in the reversal of tumor cell resistance and EMT: downregulation of the NF-κB/Snail/YY1/RKIP circuitry. Nitric Oxide 24:1–7
Bowman T, Garcia R, Turkson J, Jove R (2000) STATs in oncogenesis. Oncogene 19:2474–2488
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr (1999) Stat3 as an oncogene. Cell 98:295–303
Cao SM, Simons MJ, Qian CN (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30:114–119
Cao SQ, Yin TY, Yang SL (2012) Reversing effects of curcumin on multi-drug resistance of Bel7402/5-fu cell line. Zhongguo Zhong Xi Yi Jie He Za Zhi 32:244–247
Cao Z, Zhang Z, Huang Z, Wang R, Yang A, Liao L, Du J (2014) Antitumor and immunomodulatory effects of low-dose 5-FU on hepatoma 22 tumor-bearing mice. Oncol Lett 7:1260–1264
Cao F, Liu T, Xu Y, Xu D, Feng S (2015) Curcumin inhibits cell proliferation and promotes apoptosis in human osteoclastoma cell through MMP-9, NF-κB and JNK signaling pathways. Int J Clin Exp Pathol 8:6037–6045
Chapman CH, Parvathaneni U, Yom SS (2017) Revisiting induction chemotherapy before radiotherapy for head and neck cancer, part II: nasopharyngeal carcinoma. Future Oncol 13:581–584
Chen X, Yeung TK, Wang Z (2000) Enhanced drug resistance in cells coexpressing ErbB2 with EGF receptor or ErbB3. Biochem Biophys Res Commun 277:757–763
Chen A, Xu J, Johnson AC (2006) Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 25:278–287
Chen Y, Wang Z, Chang P, Xiang L, Pan F, Li J, Jiang J, Zou L, Yang L, Bian Z, Liang H (2010) The effect of focal adhesion kinase gene silencing on 5-fluorouracil chemosensitivity involves an Akt/NF-kappaB signaling pathway in colorectal carcinomas. Int J Cancer 127:195–206
Chen WC, Lai YA, Lin YC, Ma JW, Huang LF, Yang NS, Ho CT, Kuo SC, Way TD (2013) Curcumin suppresses doxorubicin-induced epithelial-mesenchymal transition via the inhibition of TGF-β and PI3K/AKT signaling pathways in triple-negative breast cancer cells. J Agric Food Chem 61:11817–11824
Chen L, She X, Wang T, He L, Shigdar S, Duan W, Kong L (2015a) Overcoming acquired drug resistance in colorectal cancer cells by targeted delivery of 5-FU with EGF grafted hollow mesoporous silica nanoparticles. Nanoscale 7:14080–14092
Chen P, Li J, Jiang HG, Lan T, Chen YC (2015b) Curcumin reverses cisplatin resistance in cisplatin-resistant lung caner cells by inhibiting FA/BRCA pathway. Tumour Biol 36:3591–3599
Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, Chen Y, Xu TT, Li B, Long GX, Wang SY, Zheng BM, Guo Y, Sun Y, Mao YP, Tang LL, Chen YM, Liu MZ, Ma J (2017a) Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer 75:150–158
Chen L, Liu T, Wang Q, Liu J (2017b) Anti-inflammatory effect of combined tetramethylpyrazine, resveratrol and curcumin in vivo. BMC Complement Altern Med 17:233
Cheng J, Fan XM (2013) Role of cyclooxygenase-2 in gastric cancer development and progression. World J Gastroenterol 19:7361–7368
Cheng M, He B, Wan T, Zhu W, Han J, Zha B, Chen H, Yang F, Li Q, Wang W, Xu H, Ye T (2012) 5-Fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PLoS ONE 7:e47115
Choi BH, Kim CG, Lim Y, Shin SY, Lee YH (2008) Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett 259:111–118
Ciardiello F, Tortora G (2008) EGFR antagonists in cancer treatment. N Engl J Med 358:1160–1174
Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G 2nd, Samuel S, Kim MP, Lim SJ, Ellis LM (2009) Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res 69:1951–1957
Dang YP, Yuan XY, Tian R, Li DG, Liu W (2015) Curcumin improves the paclitaxel-induced apoptosis of HPV-positive human cervical cancer cells via the NF-κB-p53-caspase-3 pathway. Exp Ther Med 9:1470–1476
Deodhar SD, Sethi R, Srimal RC (1980) Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res 71:632–634
DeSantis C, Ma J, Bryan L, Jemal A (2014a) Breast cancer statistics, 2013. CA Cancer J Clin 64:52–62
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A (2014b) Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64:252–271
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Ab Kunnumakkara, Jl Abbruzzese, Ng CS, Badmaev V, Kurzrock R (2008) Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 14:4491–4499
Du B, Jiang L, Xia Q, Zhong L (2006) Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy 52:23–28
Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G (2010) Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer 62:1137–1141
Eskandari MR, Moghaddam F, Shahraki J, Pourahmad J (2015) A comparison of cardiomyocyte cytotoxic mechanisms for 5-fluorouracil and its pro-drug capecitabine. Xenobiotica 45:79–87
Fang Y, Xue JL, Shen Q, Chen J, Tian L (2012) MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology 55:1852–1862
Ferguson JE, Orlando RA (2015) Curcumin reduces cytotoxicity of 5-fluorouracil treatment in human breast cancer cell. J Med Food 18:497–502
Goel A, Aggarwal BB (2010) Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer 62:919–930
Goel A, Boland CR, Chauhan DP (2001) Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett 172:111–118
Gonzalez-Angulo AM, Blumenschein GR Jr (2013) Defining biomarkers to predict sensitivity to PI3K/Akt/mTOR pathway inhibitors in breast cancer. Cancer Treat Rev 39:313–320
Gupta SC, Kim JH, Prasad S, Aggarwal BB (2010) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29:405–434
Hagmann W, Jesnowski R, Faissner R, Guo C, Löhr JM (2009) ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology 9:136–144
Hong GB, Zhou JX, Sun HB, Li CY, Song LQ (2012) Continuous transarterial infusion chemotherapy with gemcitabine and 5-fluorouracil for advanced pancreatic carcinoma. Asian Pac J Cancer Prev 13:2669–2673
Howells LM, Sale S, Sriramareddy SN, Irving GR, Jones DJ, Ottley CJ, Pearson DG, Mann CD, Manson MM, Berry DP, Gescher A, Steward WP, Brown K (2011) Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo. Int J Cancer 129:476–486
Inoue Y, Tanaka K, Hiro J, Yoshiyama S, Toiyama Y, Eguchi T, Miki C, Kusunoki M (2006) In vitro synergistic antitumor activity of a combination of 5-fluorouracil and irinotecan in human colon cancer. Int J Oncol 28:479–486
Irving GR, Iwuji CO, Morgan B, Berry DP, Steward WP, Thomas A, Brown K, Howells LM (2015) Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): study protocol for a randomised control trial. Trials 16:110
James MI, Iwuji C, Irving G, Karmokar A, Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR, Morgan B, Dennison A, Metcalfe M, Garcea G, Lloyd DM, Berry DP, Steward WP, Howells LM, Brown K (2015) Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett 364:135–141
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Jing M, Kong L (2016) Effect of PI3K/AKT signaling pathway and related regulatory molecular of HepG2 cell intervened by curcumin combining with 5-fluorouracil. Chin J Clin Ration Drug Use 1:7–10
Jung JY, Ryu MH, Ryoo BY, Han B, Cho JW, Lim MS, Lim H, Kang HS, Kim MJ, Ha HI, Song H, Kim JH, Kim HS, Kang YK, Zang DY (2016) Second-line irinotecan, leucovorin, and 5-fluorouracil for gastric cancer patients after failed docetaxel and S-1. Gastroenterol Res Pract. https://doi.org/10.1155/2016/6857625
Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y, Yanagihara K, Yazumi S, Chiba T, Guha S, Aggarwal BB (2011) A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol 68:157–164
Kang Y, Hu W, Bai E, Zheng H, Liu Z, Wu J, Jin R, Zhao C, Liang G (2016) Curcumin sensitizes human gastric cancer cells to 5-fluorouracil through inhibition of the NFκB survival-signaling pathway. Onco Targets Ther 9:7373–7384
Kiso Y, Suzuki Y, Watanabe N, Oshima Y, Hikino H (1983) Antihepatotoxic principles of Curcuma longa rhizomes. Planta Med 49:185–187
Koo JY, Kim HJ, Jung KO, Park KY (2004) Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J Med Food 7:117–121
Körber MI, Klingenbrunner S, Bartsch R, Steger GG, Mader RM (2013) NF-κB addiction and resistance to 5-fluorouracil in a multi-stage colon carcinoma model. Int J Clin Pharmacol Ther 51:35–37
Kumar B, Yadav A, Hideg K, Kuppusamy P, Teknos TN, Kumar P (2014) A novel curcumin analog (H-4073) enhances the therapeutic efficacy of cisplatin treatment in head and neck cancer. PLoS ONE 9:e93208
Kuo CL, Wu SY, Ip SW, Wu PP, Yu CS, Yang JS, Chen PY, Wu SH, Chung JG (2011) Apoptotic death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma cells is mediated through the ROS, mitochondrial depolarization and caspase-3-dependent signaling responses. Int J Oncol 39:319–328
Kuwano H, Nishimura Y, Ohtsu A, Kato H, Kitagawa Y, Tamai S, Toh Y, Matsubara H (2008) Guidelines for diagnosis and treatment of carcinoma of the esophagus. Esophagus 5:117–132
Lev-Ari S, Starr A, Vexler A, Karaush V, Loew V, Greif J, Fenig E, Aderka D, Ben-Yosef R (2006) Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res 26:4423–4430
Li Y, Revalde JL, Reid G, Paxton JW (2011) Modulatory effects of curcumin on multi-drug resistance-associated protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol 68:603–610
Li Q, Yan H, Liu W, Zhen H, Yang Y, Cao B (2014) Efficacy and safety of gemcitabine-fluorouracil combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomized controlled trials. PLoS ONE 9:e104346
Li F, Zhang J, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Kumar AP, Ahn KS, Sethi G (2015a) NF-κB in cancer therapy. Arch Toxicol 89:711–731
Li JH, Xie XY, Zhang L, Le F, Ge NL, Li LX, Gan YH, Chen Y, Zhang JB, Xue TC, Chen RX, Xia JL, Zhang BH, Ye SL, Wang YH, Ren ZG (2015b) Oxaliplatin and 5-fluorouracil hepatic infusion with lipiodolized chemoembolization in large hepatocellular carcinoma. World J Gastroenterol 21:3970–3977
Li Q, Wang X, Shen A, Zhang Y, Chen Y, Sferra TJ, Lin J, Peng J (2015c) Hedyotis diffusa Willd overcomes 5-fluorouracil resistance in human colorectal cancer HCT-8/5-FU cells by downregulating the expression of P-glycoprotein and ATP-binding casette subfamily G member 2. Exp Ther Med 10:1845–1850
Li X, Yu J, Brock MV, Tao Q, Herman JG, Liang P, Guo M (2015d) Epigenetic silencing of BCL6B inactivates p53 signaling and causes human hepatocellular carcinoma cell resist to 5-FU. Oncotarget 6:11547–11560
Li H, Tian J, Wu A, Wang J, Ge C, Sun Z (2016) Self-assembled silk fibroin nanoparticles loaded with binary drugs in the treatment of breast carcinoma. Int J Nanomed 11:4373–4380
Limtrakul P, Anuchapreeda S, Buddhasukh D (2004) Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. BMC Cancer 4:13
Liu Y, Wang X, Wang Y, Zhang Y, Zheng K, Yan H, Zhang L, Chen W, Wang X, Liu Q, Wang S, Wang Y (2015) Combination of SNX-2112 with 5-FU exhibits antagonistic effect in esophageal cancer cells. Int J Oncol 46:299–307
Longley DB, Harkin DP, Johnston PG (2003) 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3:330–338
Lu WD, Qin Y, Yang C, Li L, Fu ZX (2013) Effect of curcumin on human colon cancer multidrug resistance in vitro and in vivo. Clinics (Sao Paulo) 68:694–701
Lv L, Qiu K, Yu X, Chen C, Qin F, Shi Y, Ou J, Zhang T, Zhu H, Wu J, Liu C, Li G (2016) Amphiphilic copolymeric micelles for doxorubicin and curcumin co-delivery to reverse multidrug resistance in breast cancer. J Biomed Nanotechnol 12:973–985
Ma WL, Liu R, Huang LH, Zou C, Huang J, Wang J, Chen SJ, Meng XG, Yang JK, Li H, Yang GP, Guo CX (2017) Impact of polymorphisms in angiogenesis-related genes on clinical outcomes of radiotherapy in patients with nasopharyngeal carcinoma. Clin Exp Pharmacol Physiol 44:539–548
Mahammedi H, Planchat E, Pouget M, Durando X, Curé H, Guy L, Van-Praagh I, Savareux L, Atger M, Bayet-Robert M, Gadea E, Abrial C, Thivat E, Chollet P, Eymard JC (2016) The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: a pilot phase II study. Oncology 90:69–78
Manoochehri M, Karbasi A, Bandehpour M, Kazemi B (2014) Down-regulation of BAX gene during carcinogenesis and acquisition of resistance to 5-FU in colorectal cancer. Pathol Oncol Res 20:301–307
Massihnia D, Galvano A, Fanale D, Perez A, Castiglia M, Incorvaia L, Listì A, Rizzo S, Cicero G, Bazan V, Castorina S, Russo A (2016) Triple negative breast cancer: shedding light onto the role of PI3K/akt/mtor pathway. Oncotarget 7:60712–60722
Mayr M, Becker K, Schulte N, Belle S, Hofheinz R, Krause A, Schmid RM, Röcken C, Ebert MP (2012) Phase I study of imatinib, cisplatin and 5-fluoruracil or capecitabine in advanced esophageal and gastric adenocarcinoma. BMC Cancer 12:587
Miriyala S, Panchatcharam M, Rengarajulu P (2007) Cardioprotective effects of curcumin. Adv Exp Med Biol 595:359–377
Mise Yonar S, Yonar ME, Ural MS (2017) Antioxidant effect of curcumin against exposure to malathion in Cyprinus carpio. Cell Mol Biol (Noisy-le-grand) 63:68–72
Molinari F, Martin V, Saletti P, De Dosso S, Spitale A, Camponovo A, Bordoni A, Crippa S, Mazzucchelli L, Frattini M (2009) Differing deregulation of EGFR and downstream proteins in primary colorectal cancer and related metastatic sites may be clinically relevant. Br J Cancer 100:1087–1094
Mongre RK, Sodhi SS, Ghosh M, Kim JH, Kim N, Park YH, Kim SJ, Heo YJ, Sharma N, Jeong DK (2015) The novel inhibitor BRM270 downregulates tumorigenesis by suppression of NF-κB signaling cascade in MDR-induced stem like cancer-initiating cells. Int J Oncol 46:2573–2585
Mori R, Yoshida K, Tanahashi T, Yawata K, Kato J, Okumura N, Tsutani Y, Okada M, Oue N, Yasui W (2013) Decreased FANCJ caused by 5FU contributes to the increased sensitivity to oxaliplatin in gastric cancer cells. Gastric Cancer 16:345–354
Murakami Y, Kazuno H, Emura T, Tsujimoto H, Suzuki N, Fukushima M (2000) Different mechanisms of acquired resistance to fluorinated pyrimidines in human colorectal cancer cells. Int J Oncol 17:277–283
Noordhuis P, Holwerda U, Van der Wilt CL, Van Groeningen CJ, Smid K, Meijer S, Pinedo HM, Peters GJ (2004) 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann Oncol 15:1025–1032
Oliveira AS, Sousa E, Vasconcelos MH, Pinto M (2015) Curcumin: a natural lead for potential new drug candidates. Curr Med Chem 22:4196–4232
Pandey A, Vishnoi K, Mahata S, Tripathi SC, Misra SP, Misra V, Mehrotra R, Dwivedi M, Bharti AC (2015) Berberine and curcumin target survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutr Cancer 67:1293–1304
Patel BB, Gupta D, Elliott AA, Sengupta V, Yu Y, Majumdar AP (2010) Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res 30:319–325
Peng H, Chen L, Li WF, Guo R, Mao YP, Zhang Y, Guo Y, Sun Y, Ma J (2017) Tumor response to neoadjuvant chemotherapy predicts long-term survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma: a secondary analysis of a randomized phase 3 clinical trial. Cancer 123:1643–1652
Perrone D, Ardito F, Giannatempo G, Dioguardi M, Troiano G, Lo Russo L, De Lillo A, Laino L, Lo Muzio L (2015) Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med 10:1615–1623
Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S, Jansen G, van Groeningen CJ, Pinedo HM (2002) Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta 1587:194–205
Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL (2013) Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 39:974–984
Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8:915–928
Pollak M (2012) The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12:159–169
Prasad S, Ravindran J, Aggarwal BB (2010) NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem 336:25–37
Qadir MI, Naqvi ST, Muhammad SA (2016) Curcumin: a polyphenol with molecular targets for cancer control. Asian Pac J Cancer Prev 17:2735–2739
Qu JL, Li X, Qu XJ, Zhu ZT, Zhou LZ, Teng YE, Zhang JD, Jin B, Zhao MF, Yu P, Liu YP (2013) Optimal duration of fluorouracil-based adjuvant chemotherapy for patients with resectable gastric cancer. PLoS ONE 8:e83196
Rajitha B, Belalcazar A, Nagaraju GP, Shaib WL, Snyder JP, Shoji M, Pattnaik S, Alam A, El-Rayes BF (2016) Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett 373:227–233
Rossi L, Bonmassar E, Faraoni I (2007) Modification of miR gene expression pattern in human colon cancer cells following exposure to 5-fluorouracil in vitro. Pharmacol Res 56:248–253
Roy M, Mukherjee S (2014) Reversal of resistance towards cisplatin by curcumin in cervical cancer cells. Asian Pac J Cancer Prev 15:1403–1410
Ruiz de Porras V, Bystrup S, Martínez-Cardús A, Pluvinet R, Sumoy L, Howells L, James MI, Iwuji C, Manzano JL, Layos L, Bugés C, Abad A, Martínez-Balibrea E (2016) Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci Rep 6:24675
Saha S, Adhikary A, Bhattacharyya P, Das T, Sa G (2012) Death by design: where curcumin sensitizes drug-resistant tumours. Anticancer Res 32:2567–2584
Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Rivera BK, Kálai T, Hideg K, Kuppusamy P (2011) HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian carcinoma, leading to therapeutic synergy through STAT3 inhibition. Cancer Biol Ther 12:837–845
Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A (2013) Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS ONE 8:e57218
Shakibaei M, Buhrmann C, Kraehe P, Shayan P, Lueders C, Goel A (2014) Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 9:e85397
Shakibaei M, Kraehe P, Popper B, Shayan P, Goel A, Buhrmann C (2015) Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 15:250
Shankar S, Ganapathy S, Chen Q, Srivastava RK (2008) Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer 7:16
Shehzad A, Wahid F, Lee YS (2010) Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 343:489–499
Shirao K, Boku N, Yamada Y, Yamaguchi K, Doi T, Goto M, Nasu J, Denda T, Hamamoto Y, Takashima A, Fukuda H, Ohtsu A, Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (2013) Randomized phase III study of 5-fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106). Jpn J Clin Oncol 43:972–980
Shiri S, Alizadeh AM, Baradaran B, Farhanghi B, Shanehbandi D, Khodayari S, Khodayari H, Tavassoli A (2015) Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac J Cancer Prev 16:3917–3922
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64:104–117
Singh A, Settleman J (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29:4741–4751
Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A (2005) COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol 26:1393–1399
Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M (2010) The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010:215158
Storka A, Vcelar B, Klickovic U, Gouya G, Weisshaar S, Aschauer S, Bolger G, Helson L, Wolzt M (2015) Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int J Clin Pharmacol Ther 53:54–65
Sui H, Fan ZZ, Li Q (2012) Signal transduction pathways and transcriptional mechanisms of ABCB1/Pgp-mediated multiple drug resistance in human cancer cells. J Int Med Res 40:426–435
Suresh R, Ali S, Ahmad A, Philip PA, Sarkar FH (2016) The role of cancer stem cells in recurrent and drug-resistant lung cancer. Adv Exp Med Biol 890:57–74
Takahashi M, Niwa K, Ishiyama S, Sugimoto K, Komiyama H, Yaginuma Y, Kojima Y, Goto M, Okuzawa A, Tomiki Y, Sakamoto K (2014) An effective 5-fluorouracil, levofolinate, and oxaliplatin therapy for recurrent breast cancer: a case report. J Med Case Rep 8:234
Taketo MM (1998) COX-2 and colon cancer. Inflamm Res 47(Suppl. 2):S112–S116
Tang XQ, Bi H, Feng JQ, Cao JG (2005) Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol Sin 26:1009–1016
Tang XL, Fu JH, Hyun PARK, Yang XY (2012) Efficacy and toxicity of 5-fluorouracil in colon cancer treatment of a mouse syngeneic model. J S China Agric Univ 33:535–538
Tang H, Zhang X, Cui S, Wang J, Ruan Q, Huang Y, Yang D (2016) Role and mechanism research on reversal of 5-fluorouracil resistance by epigallocatechin gallate in gastric cancer drug-resistance cells lines SGC-7901/5-FU. Zhonghua Wei Chang Wai Ke Za Zhi 19:1170–1175
Taverna S, Giallombardo M, Pucci M, Flugy A, Manno M, Raccosta S, Rolfo C, De Leo G, Alessandro R (2015) Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget 6:21918–21933
Tian F, Song M, Xu PR, Liu HT, Xue LX (2008) Curcumin promotes apoptosis of esophageal squamous carcinoma cell lines through inhibition of NF-kappaB signaling pathway. Ai Zheng 27:566–570
Tian F, Fan T, Zhang Y, Jiang Y, Zhang X (2012a) Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous carcinoma cells through downregulating the activation of NF-κB signaling pathway in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai) 44:847–855
Tian F, Zhang C, Tian W, Jiang Y, Zhang X (2012b) Comparison of the effect of p65 siRNA and curcumin in promoting apoptosis in esophageal squamous cell carcinoma cells and in nude mice. Oncol Rep 28:232–240
Toden S, Okugawa Y, Jascur T, Wodarz D, Komarova NL, Buhrmann C, Shakibaei M, Boland CR, Goel A (2015) Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 36:355–367
Tong W, Wang Q, Sun D, Suo J (2016) Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-κB, uPA activator and MMP9. Oncol Lett 12:4139–4146
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Tsai JR, Liu PL, Chen YH, Chou SH, Cheng YJ, Hwang JJ, Chong IW (2015) Curcumin inhibits non-small cell lung cancer cells metastasis through the adiponectin/NF-κb/MMPs signaling pathway. PLoS ONE 10:e0144462
Vincenzi B, Imperatori M, Picardi A, Vespasiani Gentilucci U, Gallo P, Fausti V, Spalato Ceruso M, Santini D, Tonini G (2015) Liver toxicity in colorectal cancer patients treated with first-line FOLFIRI-containing regimen: a single institution experience. Expert Rev Anticancer Ther 15:971–976
Vinod BS, Antony J, Nair HH, Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A, Anto RJ (2013) Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis 4:e505
Wang W, McLeod HL, Cassidy J, Collie-Duguid ES (2007) Mechanisms of acquired chemoresistance to 5-fluorouracil and tomudex: thymidylate synthase dependent and independent networks. Cancer Chemother Pharmacol 59:839–845
Wang Z, Li Y, Ahmad A, Azmi AS, Kong D, Banerjee S, Sarkar FH (2010) Targeting miRNAs involved in cancer stem cell and EMT regulation: an emerging concept in overcoming drug resistance. Drug Resist Updat 13:109–118
Wang JQ, Du ZW, Gao XF, Wu M, Zhang YC, Pan Y, Wang Q, Zhang GZ (2013a) The effect of Bcl-2 gene silencing on the sensitivity of cell line A549 to chemotherapeutic drugs. Zhonghua Jie He He Hu Xi Za Zhi 36:191–197
Wang M, Wang X, Yuan J, Guo L (2013b) Expression of the breast cancer resistance protein and 5-fluorouracil resistance in clinical breast cancer tissue specimens. Mol Clin Oncol 1:853–857
Wang WB, Yang Y, Zhao YP, Zhang TP, Liao Q, Shu H (2014a) Recent studies of 5-fluorouracil resistance in pancreatic cancer. World J Gastroenterol 20:15682–15690
Wang YT, Liu HS, Su CL (2014b) Curcumin-enhanced chemosensitivity of FDA-approved platinum (II)-based anti-cancer drugs involves downregulation of nuclear endonuclease G and NF-κB as well as induction of apoptosis and G2/M arrest. Int J Food Sci Nutr 65:368–374
Wang X, Wang X, Huang J (2016) Irinotecan plus fluorouracil-based regimen as second or third-line chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma. Thorac Cancer 7:246–250
Wang L, Chen X, Du Z, Li G, Chen M, Chen X, Liang G, Chen T (2017) Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase γ depletion disrupting cellular bioenergetics. J Exp Clin Cancer Res 36:47
Waseem M, Pandey P, Tomar B, Raisuddin S, Parvez S (2014) Ameliorative action of curcumin in cisplatin-mediated hepatotoxicity: an in vivo study in Wistar rats. Arch Med Res 45:462–468
Wei WI, Kwong DL (2010) Current management strategy of nasopharyngeal carcinoma. Clin Exp Otorhinolaryngol 3:1–12
Wei KR, Yu X, Zheng RS, Peng XB, Zhang SW, Ji MF, Liang ZH, Ou ZX, Chen WQ (2014) Incidence and mortality of liver cancer in China, 2010. Chin J Cancer 33:388–394
Wu Q, Zhang K, Yuan HJ, Li HJ (2014) Inhibitory effect of combined treatment with curcumin and 5-FU on nasopharyngeal carcinoma. J Harbin Med Univ 05:368–372
Wu BB, Gong YP, Wu XH, Chen YY, Chen FF, Jin LT, Cheng BR, Hu F, Xiong B (2015) Fourier transform infrared spectroscopy for the distinction of MCF-7 cells treated with different concentrations of 5-fluorouracil. J Transl Med 13:108
Xie YQ, Wu XB, Tang SQ (2014) Curcumin treatment alters ERK-1/2 signaling in vitro and inhibits nasopharyngeal carcinoma proliferation in mouse xenografts. Int J Clin Exp Med 7:108–114
Xu W, Kuang M, Gong Y, Cao C, Chen J, Tang C (2016) Survival benefit and safety of the combinations of FOLFOXIRI ± bevacizumab versus the combinations of FOLFIRI ± bevacizumab as first-line treatment for unresectable metastatic colorectal cancer: a meta-analysis. Onco Targets Ther 9:4833–4842
Yao Y, Chen S, Zhou X, Xie L, Chen A (2014) 5-FU and ixabepilone modify the microRNA expression profiles in MDA-MB-453 triple-negative breast cancer cells. Oncol Lett 7:541–547
Yuan P, Chen Y, Xiao F, Shen LR (2012) The bioactivities of curcumin and its application in foods. Sci Tech Food Ind 14:371–375
Zhan Y, Chen Y, Liu R, Zhang H, Zhang Y (2014) Potentiation of paclitaxel activity by curcumin in human breast cancer cell by modulating apoptosis and inhibiting EGFR signaling. Arch Pharm Res 37:1086–1095
Zhang LL, Mu GG, Ding QS, Li YX, Shi YB, Dai JF, Yu HG (2015) Phosphatase and tensin homolog (PTEN) represses colon cancer progression through inhibiting paxillin transcription via PI3K/AKT/NF-κB pathway. J Biol Chem 290:15018–15029
Zhou H, Beevers CS, Huang S (2011) Targets of curcumin. Curr Drug Targets 12:332–347
Zhou X, You T, Wang WM, Zheng ZQ (2013) Curcumin combined FOLFOX induced cell apoptosis of gastric cancer and its mechanism research. Zhongguo Zhong Xi Yi Jie He Za Zhi 33:810–813
Zhou X, Wang W, Li P, Zheng Z, Tu Y, Zhang Y, You T (2016) Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol Res 23:29–34
Zhu R, Wu X, Xiao Y, Gao B, Xie Q, Liu H, Wang S (2013) Synergetic effect of SLN-curcumin and LDH-5-Fu on SMMC-7721 liver cancer cell line. Cancer Biother Radiopharm 28:579–587
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (81341124, 81101678), Science and Technology Support Project of Sichuan Province (2017JQ0013), Sichuan Provincial Human Resource and Social Security Department (2016-183), the Joint Fund of Sichuan Province, Luzhou City and Southwest Medical University (14JC0134, 14ZC0026, 14ZC0066), the Joint Fund of Luzhou City and Southwest Medical University [2015LZCYD-S09 (4/8)] and the Distinguished Professor Research Startup Funding (S. Cao) from Southwest Medical University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors report no conflicts of interest in this work.
Rights and permissions
About this article
Cite this article
Wei, Y., Yang, P., Cao, S. et al. The combination of curcumin and 5-fluorouracil in cancer therapy. Arch. Pharm. Res. 41, 1–13 (2018). https://doi.org/10.1007/s12272-017-0979-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0979-x