Abstract
To explore the cardiovascular protective effects of Magnesium isoglycyrrhizinate (MI), especially the underlying cellular mechanisms related to L-type calcium channels and myocardial contractility, and to examine the effects of MI on hERG K+ current expressed in HEK293 cells. We used the whole-cell patch clamp technique, video-based edge detection and dual excitation fluorescence photomultiplier systems to explore the effect of MI on L-type Ca2+ currents (ICa-L) and cell contraction in rat cardiomyocytes. We also examined the rapidly activating delayed rectifier potassium current (IKr) expressed in HEK293 cells using a perforated patch clamp. MI inhibited ICa-L in a dose-dependent manner, with a half-maximal inhibitory concentration (IC50) of 0.22 mg/ml, and the maximal inhibitory effect was 61.10 ± 0.59%. MI at a concentration of 0.3 mg/ml reduced cell shortening by 24.12 ± 3.97% and the peak value of the Ca2+ transient by 36.54 ± 4.96%. MI had no significant influence on hERG K+ channels expressed in HEK293 cells at all test potentials. MI exerts protective effects on the heart via the inhibition of ICa-L and cell shortening in rat cardiomyocytes. However, MI had no significant influence on IKr; thus, MI may exert cardioprotective effects without causing drug-induced long QT syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The root of Glycyrrhiza uralensis Fisch (Glycyrrhiza glabra L. Leguminosae) is called licorice and is a native herbaceous perennial legume with a sweet flavor. It usually grows in southern Europe and parts of Asia. In addition, licorice is a traditional herbal remedy that has long been used worldwide in herbal and folk medications. Previous studies demonstrated the potential protection of licorice against ischemia–reperfusion injury (I–R) in the heart, and the results clearly demonstrated that licorice mediates protective effects against myocardial infarction through the amelioration of oxidative stress (Ojha et al. 2013). The active components of Radix Glycyrrhizae Preparata (Zhi Gancao, a licorice decoction) can markedly lower the incidence of cardiac triggered activity, protect the myocardium from injury and decrease the incidence of arrhythmia induced by I–R in rats (Li et al. 2003). A serum containing Radix Glycyrrhizae Preparata decreases the L-type Ca2+ currents (ICa-L) in isolated rabbit ventricular myocytes in a concentration-dependent manner, which may be the mechanism of action by which the preparation treats arrhythmia (Zhou et al. 2007).
Magnesium isoglycyrrhizinate (MI) is a saponin, which is alkali-catalyzed, isomerized, salified, and refined from glycyrrhizic acid extracted from the licorice (Xing et al. 2003). The structure of MI is shown in Fig. 1. MI has been shown to possess anti-inflammatory and hepatoprotective activity (Dong et al. 2006a). In addition, previous studies demonstrated that MI exerts positive effects on multiple types of organ damages and diseases, such as lung injury, alcoholic liver disease induced by paraquat poisoning (Xiao et al. 2014), and epithelial ovarian cancers (Chen et al. 2014). However, the cardiovascular protective effects of MI, especially the underlying cellular mechanisms, remain less studied.
Increasing the levels of intracellular Ca2+ ([Ca2+]i) can aggravate myocardial contraction and promote related pathological changes, such as apoptosis (Chen et al. 2005) and hypertrophy (Frey and Olson 2003). The main feature of the cardiomyocyte response to ischemic myocardial diseases is increased contractility (Bristow et al. 1985). Moreover, drugs that suppress cardiac contractility and L-type calcium channels (LTCCs) can effectively protect against myocardial ischemic injury. Previous studies suggested that the alteration of LTCC properties, in addition to rapidly activating delayed rectifier potassium current (IKr) antagonistic activity, should be considered when evaluating the proarrhythmic potential of drugs (Kim et al. 2016). In ventricular myocytes in the heart, the suppression of the cardiac delayed rectifier K+ current causes long QT syndrome (LQTS), which is a disease with a high risk of developing lethal ventricular tachyarrhythmias (Martin et al. 2012). However, calcium antagonists were reported to have the potential to block IKr (Chouabe et al. 1998), indicating that calcium antagonists may increase the risk of LQTS. Therefore, the assessing IKr inhibition is useful for the evaluation of drugs that are suspected of causing LQTS. We examined the effects of MI on ICa-L in rat cardiomyocytes and IKr expressed in HEK293 cells using the whole-cell patch-clamp technique. We observed contractility and Ca2+ transients in rat cardiomyocytes using video-based edge detection and dual excitation fluorescence photomultiplier systems.
Materials and methods
Drugs and reagents
MI was obtained from NIFDC (Batch: 100879-201302, Purity 90.8%, Beijing, China). MgCl2 was obtained from Sigma-Aldrich (Batch: WXBB1001V, Purity 98%, St. Louis, MO, USA). Collagenase type II was obtained from Worthington Biochemical (Batch: 45A15451, Lakewood, NJ, USA). Verapamil (Ver) was obtained from Cayman Chemical (Batch: AS402230, Purity ≥98%, Ann Arbor, MI, USA). Unless otherwise mentioned, all the other chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). All solvents used in this study were of analytical purity.
The Ca2+-free Tyrode’s solution included 135 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 0.33 mM NaH2PO4, 10 mM glucose and 10 mM HEPES, and the pH was adjusted to 7.4 with NaOH. The enzyme solution was made with the Ca2+-free Tyrode’s solution and 0.6 mg/ml of collagenase type II, 0.5 mg/ml of bovine serum albumin and 30 μM CaCl2. The Kreb’s buffer (KB) solution included 10 mM HEPES, 40 mM KCl, 25 mM KH2PO4, 80 mM KOH, 20 mM taurine, 50 mM glutamic acid, 1 mM EGTA, 10 mM glucose and 3 mM MgSO4 (pH adjusted to 7.2 with KOH). The intracellular pipette solution included 120 mM CsCl, 20 mM tetraethylammonium chloride, 10 mM HEPES, 5 mM Mg-ATP and 10 mM EGTA (pH adjusted to 7.2 with CsOH).
Animals
Sprague–Dawley rats weighing 200–240 g were obtained from the Experimental Animal Center (Hebei Medical University). The study was performed in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China.
Isolation of adult rat ventricular myocytes
Individual ventricular myocytes were separated from the Sprague–Dawley rat hearts via the collagenase perfusion method, as described previously (Gao et al. 2014). Each animal was anesthetized via an intraperitoneal injection of heparin (500 IU/kg) and ethylcarbamate (4 mg/kg). The heart was excised rapidly from the rats and cannulated onto a Langendorff apparatus for perfusion. Perfusion was performed via the aorta with an oxygenated calcium-free solution for 5 min to remove the blood. Subsequently, the heart was perfused with enzymatic solution for 18–25 min. After approximately 20 min, the heart was changed to 20 ml of Ca2+-free Tyrode’s solution to wash out the enzyme solution. After perfusion, the myocardial tissue was dissected into small pieces in KB solution. The cardiomyocytes were preserved in KB solution (bubbled with O2) at a temperature of 23–25 °C for up to 1–2 h before the experiment.
Cell culture
Human embryonic kidney 293 (HEK293) cells stably transfected with the human ether-à-go-go-related gene (hERG) were cultured in DMEM supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 600 μg/ml of G418 and 1% penicillin/streptomycin in a humidified incubator at 37 °C at 5% CO2. The cells were seeded on glass coverslips in a 24-multiwell plate. The assays were performed 48 h after plating.
Current recording
The experiments were performed at room temperature (22–25 °C). Whole-cell patch recordings were performed on ventricular myocytes to detect LTCCs, and the perforated patch configuration was used to record hERG K+ currents. A level puller (Sutter Instruments, Model P-97) was used to pull the borosilicate glass electrodes. When the cells first entered the solution, whole-cell patch recordings had a resistance of 2–5 MΩ and perforated patch recordings had a resistance of 1–2 MΩ. After gigaseal and membrane rupture to establish the whole-cell configuration, the ICa-L currents were recorded in voltage-clamp mode. To minimize the rundown of hERG K+ currents, 200 μg/ml of amphotericin B was added to the pipette solution in perforated patch recordings. The Axon patch 200B amplifier and the pClamp 10.0 software (Axon Instruments, Union City, CA, USA) were used to record the currents.
Measurement of myocyte contractions
A video-based edge-detection system (IonOptix, Milton, MA, USA) was used to detect cell shortening of myocardial myocytes. Cell contractions were elicited by the frequency of field stimulation at 0.5 Hz (2 ms duration). Cell activity could be observed by an inverted microscope and a detection system placed on the glass chamber. Myocytes with clear edges were chosen to measure cell shortening before and after MI administration.
Measurement of [Ca2+]i transients
The myocytes were incubated with fura-2/AM for 15 min in the dark at room temperature, as described previously (Gao et al. 2014). The fluorescence measurements were recorded using a dual-excitation fluorescence photomultiplier system (IonOptix). A 0.5 Hz field was used to stimulate myocyte contraction. Contractive myocytes were imaged through a Fluor 40 × oil objective and exposed to light, which was passed through a 340- or 380-nm filter (bandwidth measurements were ±15 nm). The emitted fluorescence was detected at 510 nm. The emitted fluorescence ratio was responded by two wavelengths (340/380) to determine an intracellular calcium concentration. Ca2+ transients were calibrated as described previously (Salem et al. 2010).
Data analysis
The electrophysiologic data were processed using Clampfit 10.0 software. The concentration–response curve was fitted with logistic equation: y = A 2 + (A 1 –A 2 )/[1 + (x/x 0 )p], where y is the response; A 1 and A 2 are the maximum and minimum response, respectively, x is the drug concentration, and p is the Hill coefficient. The current activation curves were generated by plotting the normalized tail current amplitudes against the step potentials and were fitted with a Boltzmann function: y = A/{1 + exp[(V h − V m )/k]}, where A is the maximal current amplitude, V h is the voltage for half-maximal activation, V m is the test potential and k is the slope factor. The data are presented as the mean ± S.E.M. Intergroup differences were assessed using the Student’s t test or a one-way ANOVA. The differences were considered significant to be at p < 0.05. Where ANOVA was used, a post hoc test (Bonferroni) was subsequently used if F achieved p < 0.05 and there was no significant variance inhomogeneity.
Results
Confirmation of ICa-L
ICa-L was elicited via the steady-state activation protocol in rat ventricular myocytes. Verapamil (100 µM) could almost completely block the currents, indicating that these currents were Ca2+ currents (Fig. 2) (p < 0.01).
Effects of MI on ICa-L
The peak of ICa-L was significantly reduced after exposure to 1 mg/ml of MI (p < 0.01). However, the ICa-L recovered after the MI was washed out with the external solution (Fig. 3a, b). These results indicate that the effect of MI on ICa-L was not because of rundown, and the effect of MI on ICa-L was partially reversible. Representative current recordings made with the activation protocol after the sequential administration of MI (0.01, 0.03, 0.1, 0.3 and 1 mg/ml, i.e., 0.01, 0.04, 0.12, 0.35 and 1.18 mM) are shown in Fig. 4a, b. The equation indicates that the half-maximal inhibitory concentration (IC50) of MI was 0.22 mg/ml. The rates of inhibition by MI at 0.01, 0.03, 0.1, 0.3 and 1 mg/ml were 9.88 ± 0.32%, 16.45 ± 0.27%, 24.07 ± 1.49%, 45.68 ± 0.92% and 61.10 ± 0.59%, respectively (Fig. 4c).
Reversible effects of MI on ICa-L of ventricular myocytes. Exemplary traces (a) and pooled data (b) of ICa-L were recorded under control conditions, during exposure to 1 mg/ml MI and during wash out. Data are presented as mean ± S.E.M. (n = 6 cells,** p < 0.01, compared with control, ## p < 0.01, compared with 1 mg/ml MI)
Effects of MI on ICa-L of ventricular myocytes. a Time course of ICa-L recorded under the control conditions, during exposure to 0.01, 0.03, 0.1, 0.3 and 1 mg/ml MI and 100 µM Ver. b Original current traces in response to 0.01, 0.03, 0.1, 0.3 and 1 mg/ml MI and 100 µM Ver. c Concentration–response curve of MI. Data are presented as mean ± S.E.M. (n = 6–8 cells)
Effects of MI on the I–V relationship of ICa-L
The effects of MI (0.01, 0.1, and 1 mg/ml) on the I–V relationship of the ICa-L are depicted in Fig. 5. Figure 5a shows representative trace recordings that shifted upward with a significant growth in the concentration of MI (0.01, 0.1, and 1 mg/ml). Figure 5b shows the current density–voltage relationship with controls for MI (0.01, 0.1, and 1 mg/ml) and verapamil (10−7 M). These results demonstrated that 0.01, 0.1 and 1 mg/ml MI could markedly depress the maximum current.
Effects of MI on current–voltage (I–V) relationship of ICa-L. Exemplary traces (a) and pooled data (b) show effects of MI at different concentrations on the I–V relationship. Control is marked with (square), MI at 0.01 mg/ml with (circle), MI at 0.1 mg/ml with (triangle) and MI at 1 mg/ml with (inverted triangle) and Ver at 100 µM with (). Data are presented as mean ± S.E.M. (n = 6 cells). The current activation curves were generated by plotting the normalized tail current amplitudes against the step potentials and were fitted with a Boltzmann function: y = A/{1 + exp [(V h − V m)/k]}, where A is the amplitude of relationship, V h is the voltage for half-maximal activation, V m is the test potential and k is the slope
Effects of MI on steady-state activation and inactivation of ICa-L
The voltage dependence of the steady-state activation and inactivation of ICa-L in the presence or absence of MI is shown in Fig. 6. The value at V1/2 and the slope factor (k) of the normalized activation conductance curves for the control and 0.01 mg/ml, 0.1 mg/ml, and 1 mg/ml MI were −1.46 ± 1.52 mV/9.80 ± 1.17; 0.14 ± 1.40 mV/9.56 ± 1.03; 1.04 ± 1.32 mV/9.66 ± 0.94; and −1.15 ± 1.22 mV/10.42 ± 0.92, respectively (p > 0.05, n = 6). The V1/2 and the k values of the steady-state inactivation curves for the control and 0.01 mg/ml, 0.1 mg/ml, and 1 mg/ml of MI were −26.55 ± 1.00 mV/4.27 ± 0.80; −25.56 ± 0.67 mV/4.57 ± 0.53; −25.51 ± 0.76 mV/4.63 ± 0.61; and −29.07 ± 0.28 mV/5.07 ± 0.27, respectively (p > 0.05, n = 6).
Effects of MI on steady-state activation and inactivation of ICa-L. (A) Activation kinetics of ICa-L. (B) Inactivation kinetics of ICa-L. Control is marked with (square), MI at 0.01 mg/ml with (circle), MI at 0.1 mg/ml with (triangle) and MI at 1 mg/ml with (inverted triangle). Data are presented as mean ± S.E.M. (n = 6 cells)
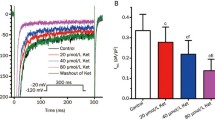
Effects of MI on cell shortening and Ca2+ transients
Figure 7 shows a time-based record of cell length from a representative rat ventricular myocyte before and after the administration of 0.3 mg/ml of MI. The results indicated that MI at a concentration of 0.3 mg/ml could significantly reduce cell shortening by 24.12 ± 3.97% (n = 6, p < 0.01) and decrease the amplitude of the Ca2+ transient by 36.54 ± 4.96% (n = 6, p < 0.01). Compared with the control group, MI at a concentration of 0.3 mg/ml had no significant effect on the time to reach 10% of the peak (Tp) and the time to 10% of the baseline (Tr) (p > 0.05).
Effects of 0.3 mg/ml MI on cell shortening, Ca2+ transient and time parameters of cell shortening. a Tracings demonstrating effects of 0.3 mg/ml MI in a myocyte. b Representative signals of cell shortening and fura-2 ratio (upper tracings). c Summary effects of 0.3 mg/ml MI on cell shortening and fura-2 ratio. Data are presented as mean ± S.E.M. (n = 6 cells, **p < 0.01, compared with control). d Summary results of the time to 10% of the peak (Tp) and the time to 10% of the baseline (Tr) for cell shortening before and after application of 0.3 mg/ml MI. Data are presented as mean ± S.E.M. (n = 6 cells, p > 0.05, compared with control)
Effects of MgCl2 on ICa-L, cell shortening and Ca2+ transients
The peak of ICa-L was slightly reduced after exposure to 0.1 and 0.3 mg/ml of MgCl2 ((n = 6, p < 0.01). The rates of inhibition by MgCl2 at 0.1, and 0.3 mg/ml were 5.12% ± 2.03% and 7.03% ± 3.22%, respectively (Fig. 8a).
Effects of MgCl2 on ICa-L, cell shortening and Ca2+ transient of ventricular myocytes. a Exemplary traces and pooled data of ICa-L were recorded under control conditions, during exposure to 0.1 and 0.3 mg/ml of MgCl2. Data are presented as mean ± S.E.M. (n = 6 cells,** p < 0.01, compared with control). b Representative signals of cell shortening and fura-2 ratio (upper tracings). c Summary effects of 0.3 mg/ml MI on cell shortening and fura-2 ratio. Data are presented as mean ± S.E.M. (n = 6 cells, p > 0.05, compared with control)
Figure 8b, c shows a time-based record of cell length from a representative rat ventricular myocyte before and after the administration of 0.3 mg/ml of MgCl2. Compared with the control group, MgCl2 at a concentration of 0.3 mg/ml had no significant effect on cell shortening and the Ca2+ transient (n = 6, p > 0.05).
Effects of MI on IKr
The effect of MI on IKr expressed in HEK293 cells was also examined. IKr was launched using 1-s test pulses between −60 mV and +60 mV with 20 mV steps from a holding potential of −80 mV, then back to −40 mV for 3-s at 0.05 Hz. Figure 9 shows the trace of IKr recorded in one cell in the absence or presence of 1 mg/ml of MI. MI at a concentration of 1 mg/ml remained stable on the expressed IKr (1.71 ± 0.43%) at all test potentials (p > 0.05, n = 6).
Effects of MI on IKr expressed in HEK293 cells. a The currents traces of IKr induced by the multiple depolarization steps were shown. Cells were exposed to 1 mg/ml MI and tefenadine. b Exemplary currents traces of IKr induced by 0 mV depolarization step were shown. The effect of 1 mg/ml MI was shown. c Summary data of (b). Data are presented as mean ± S.E.M. (n = 6 cells)
Discussion
Previous research indicated that licorice has positive effects on heart diseases, such as I–R injury, myocardial infarction and arrhythmia (Li et al. 2003; Zhou et al. 2007; Ojha et al. 2013). Because MI is one component of licorice extract, MI may exert protective effects against myocardial ischemia in a similar way.
However, the ion channel and cellular mechanisms of MI have not been clarified systematically. In our present study, we used the patch-clamp technique to confirm that MI suppresses the ICa-L of rat cardiomyocytes in a dose-dependent manner.
Ca2+ channels are found in all excitable cells and regulate many physiological activities, such as excitation-secretion coupling, excitation–contraction coupling and heart pace-making. An increase of the diastolic calcium concentration or cellular Ca2+ overload is a significant cause of many cardiovascular diseases, such as ischemic injury, hypertension, oxidant stress and glycuresis. These diseases can increase intracellular Ca2+ and trigger endo-calcium release in the sarcoplasmic reticulum (SR). Ca2+ overload is a main cause of myocardial ischemia reperfusion injury (Menown and Adgey 2001), and it is partially a result of the opening of LTCCs (Piper et al. 1998). Thus, during prolonged ischemia and reperfusion, blocking LTCCs has great significance (Woods 1991). Many studies have demonstrated that LTCCs play an important role in Ca2+ influx in cardiomyocytes, and modulating LTCC activity has been the focus of research interest (Bers 2002; Richard et al. 2006). In addition, LTCCs represent the main route of intracellular Ca2+ influx and have the properties of slow inactivation and a long opening duration (Guan et al. 2013). We found that MI could reduce the amplitude of the ICa-L in a dose-dependent manner at concentrations of 0.01, 0.03, 0.1, 0.3, and 1 mg/ml, and the inhibition was partially reversible (Fig. 3, 4). However, neither the current–voltage relationship nor the reversal potential of ICa-L was affected (Fig. 5). MI did not alter the steady-state activation and inactivation of ICa-L when the peak value of ICa-L. was restrained (Fig. 6). These results demonstrate that MI suppressed the ICa-L primarily by decreasing the Ca2+ current amplitude.
LTCCs play a major role in Ca2+ influx, as LTCCs can trigger Ca2+ release from SR Ca2+ stores. The reductions in the Ca2+ transient and the amplitude of contraction were caused by the blockade of ICa-L (Cannell and Lederer 1987; Cleemann and Morad 1991). In our study, MI had inhibitory effects on LTCCs, which resulted in cardiac negative inotropic effects. In the presence of MI, the Ca2+ transient and contractility was significantly reduced (Fig. 7a, b, c). In addition, we observe that MI seems to inhibit cell shortening less than ICa-L. This phenomenon is a result of the complex process of myocardial contraction, which is related to intracellular Ca2+ concentration and to intracellular proteins that are involved in contraction (actin and myosin) or regulation (troponin, tropomyosin, and tropomodulin) (Adamcova et al. 2006). The more detailed mechanisms of the effects of MI on myocardial contraction need to be explored in our future studies. The speed of cell contraction and cell relaxation are represented by Tp and Tr, respectively. In this study, MI at a concentration of 0.3 mg/ml had no significant effect on Tp and Tr (Fig. 7d). The reduction of the Ca2+ transient indicates a decrease in intracellular free Ca2+. MI may inhibit LTCCs to limit Ca2+ entry and inhibit Ca2+ release from the SR. This effect is likely responsible for the negative inotropic effect of MI because cardiac muscle contraction is modulated by the amplitude of the Ca2+ transient (Cannell and Lederer 1987; Cleemann and Morad 1991). In clinical treatment of myocardial ischemia, reduced myocardial contractility and myocardial oxygen consumption are the most important cellular mechanisms (Sonnenblick et al. 1965; Crystal et al. 2013). The activity of several ATP-consuming enzymes was accelerated by elevated [Ca2+]i; this phenomenon may consume cellular energy stores and increase the risk of ischemic damage (Balaban 2002). The benefits of Ca2+ antagonists have been shown to protect against cardiac injury that is induced by Ca2+ overload (Balaban 2002). Clinically, many drugs used to treat patients with myocardial ischemia are related to inhibition of the LTCCs (Poole-Wilson et al. 2004; Doggrell 2005), such as nifedipine, verapamil, diltiazem et al. Our research demonstrated that MI is a Ca2+ antagonist and could thus be expected to exert a cardioprotective effect against ischemic damage by inhibiting ICa-L and decreasing myocardial contractility.
It has been reported that higher concentration ranges of magnesium can inhibit the L-type Ca2+ channel in smooth muscle cells and isolated ventricles (Sharma et al. 2012; Zhao et al. 2015). The highest concentration MI used in the present experiments is 1 mg/ml (1.18 mM), which contains 0.11 mg/ml (1.18 mM) magnesium. To investigate the effects of the magnesium ion, we used MgCl2 up to a high concentration of 0.3 mg/ml (3.15 mM), which is much higher than magnesium contained in high concentration MI. The experimental results show that the inhibitory effect of 0.1 and 0.3 mg/ml MgCl2 on ICa-L is low, at 5 and 7%, respectively (Fig. 8a), which is much weaker than that of 1 mg/ml MI (1.18 mM), which was 61%. Compared with the control group, MgCl2 at concentration of 0.3 mg/ml had no significant effect on cell shortening and Ca2+ transients (p > 0.05, Fig. 8b, c). The effect of MI on ICa-L and cell shortening is considered to be mainly a result of the glycyrrhizic acid ingredient rather than the effect of the magnesium ion.
Drug-induced LQTS is commonly ascribed to the blockade of IKr because of a pharmacodynamic interaction between the drug and cardiac potassium channel proteins (ten Tusscher et al. 2004; ten Tusscher and Panfilov 2006). Drug-induced LQTS may culminate in ventricular fibrillation and polymorphic ventricular tachycardia, which cause syncope and sudden death (Holladay et al. 1997; Verkerk et al. 2005). Accordingly, compounds undergoing drug development and some commonly used calcium antagonists such as verapamil are routinely screened for their (undesired) ability to block IKr (Chouabe et al. 1998; Grandi et al. 2010). Previous studies showed that the characterizations of hERG K+ currents stably expressed in HEK293 cells are very similar to those of native IKr in cardiomyocytes (Dong et al. 2006b). Adult rat cardiomyocytes lack native IKr. Therefore, we examined the effects of MI on IKr expressed in HEK293 cells (Fig. 9) and found that MI did not significantly affect this current, indicating that the use of MI may not cause drug-induced LQTS. MI is currently superior to other calcium antagonists that affect IKr.
This study provided evidence that MI exerts its cardiovascular protective effects by inhibiting ICa-L and accordingly decreasing Ca2+ transients and myocardial contractility. As an LTCC blocker, MI may have a positive effect on protecting cardiomyocytes against ischemic damage and apoptosis induced by Ca2+ overload. Meanwhile, MI had no significant influence on IKr expressed in HEK293 cells at all test potentials; thus, MI may exert cardioprotective effects without causing drug-induced LQTS. Our study provides new ionic evidence to demonstrate that MI can decrease the ICa-L. Pretreatment with MI may have therapeutic benefits and may represent a promising approach to combating cardiovascular disease.
References
Adamcova M, Sterba M, Simunek T, Potacova A, Popelova O, Gersl V (2006) Myocardial regulatory proteins and heart failure. Eur J Heart Fail 8(4):333–342
Balaban RS (2002) Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 34(10):1259–1271
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 415(6868):198–205
Bristow MR, Kantrowitz NE, Ginsburg R, Fowler MB (1985) β-Adrenergic function in heart muscle disease and heart failure. J Mol Cell Cardiol 17(1):41–52
Cannell MB, Lederer W (1987) Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science 238(4832):1419–1423
Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR (2005) Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res 97(10):1009–1017
Chen KJ, Chen WY, Chen X, Jia YM, Peng GQ, Chen L (2014) Increased elimination of paclitaxel by magnesium isoglycyrrhizinate in epithelial ovarian cancer patients treated with paclitaxel plus cisplatin: a pilot clinical study. Eur J Drug Metab Pharmacokinet 39(1):25–31
Chouabe C, Drici MD, Romey G, Barhanin J, Lazdunski M (1998) hERG and KvLQT1/IsK, the cardiac K+ channels involved in long QT syndromes, are targets for calcium channel blockers. Mol Pharmacol 54(4):695–703
Cleemann L, Morad M (1991) Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. J Physiol 432(1):283
Crystal GJ, Silver JM, Salem MR (2013) Mechanisms of increased right and left ventricular oxygen uptake during inotropic stimulation. Life Sci 93(2–3):59–63
Doggrell SA (2005) Has the controversy over the use of calcium channel blockers in coronary artery disease been resolved? Expert Opin Pharmacother 6(5):831–834
Dong L, Yu F, Liu J, Mu X (2006a) Protective effect of magnesium isoglycyrrhizina on acute hepatic injury in mice. China Pharm 17(12):902–904
Dong MQ, Lau CP, Gao Z, Tseng GN, Li GR (2006b) Characterization of recombinant human cardiac KCNQ1/KCNE1 channels (IKs) stably expressed in HEK 293 cells. J Membr Biol 210(3):183–192
Frey N, Olson E (2003) Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65(1):45–79
Gao Y, Zhang K, Zhu F, Wu Z, Chu X, Zhang X, Zhang Y, Zhang J, Chu L (2014) Salvia miltiorrhiza (Danshen) inhibits L-type calcium current and attenuates calcium transient and contractility in rat ventricular myocytes. J Ethnopharmacol 158PA:397–403
Grandi E, Pasqualini FS, Bers DM (2010) A novel computational model of the human ventricular action potential and Ca transient. J Mol Cell Cardiol 48(1):112–121
Guan BC, Zhang HL, Li ZW (2013) Basic principles of cellular electrophysiology and patch clamp techniques, 1st edn. Science Press, Beijing
Holladay JW, Dewey MJ, Yoo SD (1997) Quantification of fluoxetine and norfluoxetine serum levels by reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl 704(1–2):259–263
Kim JG, Sung DJ, Kim HJ, Park SW, Won KJ, Kim B, Shin HC, Kim KS, Leem CH, Zhang YH (2016) Impaired inactivation of L-type Ca2+ current as a potential mechanism for variable arrhythmogenic liability of hERG K+ channel blocking drugs. PLoS ONE 11(3):e0149198
Li XG, Chen Q, Huang M, Ming BI, Chen L (2003) Effect of active components of Zhi Gancao decoction and their combination on triggered activity and myocardial damage in isolated ischemia-reperfusion rat heart. Tradit Chin Drug Res Clin Pharmacol 14(1):6–9
Martin CA, Matthews GD, Huang CL (2012) Sudden cardiac death and inherited channelopathy: the basic electrophysiology of the myocyte and myocardium in ion channel disease. Heart 98(7):536–543
Menown IB, Adgey AJ (2001) Cardioprotective therapy and sodium-hydrogen exchange inhibition: current concepts and future goals. J Am Coll Cardiol 38(6):1651–1653
Ojha S, Golechha M, Kumari S, Bhatia J, Arya DS (2013) Glycyrrhiza glabra protects from myocardial ischemia-reperfusion injury by improving hemodynamic, biochemical, histopathological and ventricular function. Exp Toxicol Pathol 65(1–2):219–227
Piper H, Garcña-Dorado D, Ovize M (1998) A fresh look at reperfusion injury. Cardiovasc Res 38(2):291–300
Poole-Wilson PA, Lubsen J, Kirwan BA, van Dalen FJ, Wagener G, Danchin N, Just H, Fox KA, Pocock SJ, Clayton TC, Motro M, Parker JD, Bourassa MG, Dart AM, Hildebrandt P, Hjalmarson A, Kragten JA, Molhoek GP, Otterstad JE, Seabra-Gomes R, Soler-Soler J, Weber S (2004) Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 364(9437):849–857
Richard S, Perrier E, Fauconnier J, Perrier R, Pereira L, Gõmez AM, Bénitah JP (2006) ‘Ca2+-induced Ca2+ entry’or how the L-type Ca2+ channel remodels its own signalling pathway in cardiac cells. Prog Biophys Mol Biol 90(1):118–135
Salem KA, Qureshi A, Ljubisavijevic M, Oz M, Isaev D, Hussain M, Howarth FC (2010) Alloxan reduces amplitude of ventricular myocyte shortening and intracellular Ca2+ without altering L-type Ca2+ current, sarcoplasmic reticulum Ca2+ content or myofilament sensitivity to Ca2+ in Wistar rats. Mol Cell Biochem 340(1–2):115–123
Sharma N, Cho DH, Kim SY, Bhattarai JP, Hwang PH, Han SK (2012) Magnesium sulfate suppresses L-type calcium currents on the basilar artery smooth muscle cells in rabbits. Neurol Res 34(3):291–296
Sonnenblick EH, Ross J Jr, Covell JW, Kaiser GA, Braunwald E (1965) Velocity of contraction as a determinant of myocardial oxygen consumption. Am J Physiol 209(5):919–927
ten Tusscher KH, Panfilov AV (2006) Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol 291(3):H1088–H1100
ten Tusscher KH, Noble D, Noble PJ, Panfilov AV (2004) A model for human ventricular tissue. Am J Physiol 286(4):H1573–H1589
Verkerk AO, Wilders R, Schulze-Bahr E, Beekman L, Bhuiyan ZA, Bertrand J, Eckardt L, Lin D, Borggrefe M, Breithardt G, Mannens MM, Tan HL, Wilde AA, Bezzina CR (2005) Role of sequence variations in the human ether-a-go-go-related gene (hERG, KCNH2) in the Brugada syndrome. Cardiovasc Res 68(3):441–453
Woods KL (1991) Possible pharmacological actions of magnesium in acute myocardial infarction. Br J Clin Pharmacol 32(1):3–10
Xiao ZW, Zhang W, Ma L, Qiu ZW (2014) Therapeutic effect of magnesium isoglycyrrhizinate in rats on lung injury induced by paraquat poisoning. Eur Rev Med Pharmacol Sci 18(3):311–320
Xing GX, Li N, Wang T, Yao MY (2003) Advances in studies on flavonoids of licorice. China J Chin Mater Med 28(7):593–597
Zhao M, Feng R, Shao D, Liu S, Lei M, Wang H, Sun X, Guo F, Hu H, Kameyama M, Hao L (2015) Mg2+- dependent facilitation and inactivation of L-type Ca2+ channels in guinea pig ventricular myocytes. J Pharmacol Sci 129(3):143–149
Zhou CZ, Zhang DL, Wang T (2007) Effect of serum containing Zhigancao decoction on L-type calcium current in isolated single rabbit’s ventricular myocyte. J Beijing Univ tradit Chin Med 28(7):468–471
Acknowledgements
This work was supported by the Research Foundation of Administration of Traditional Chinese Medicine of Hebei Province, China (Nos. 2017007 and 2015030), Research Foundation of Education Bureau of Hebei Province (QN20131046 to X.Z.), and Key Basic Applied Project of Hebei Provincial Department of Science & Technology (No. 15967730D to W.Z.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Yue Lin and Yuanyuan Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lin, Y., Zhang, Y., Song, Q. et al. Magnesium isoglycyrrhizinate inhibits L-type Ca2+ channels, Ca2+ transients, and contractility but not hERG K+ channels. Arch. Pharm. Res. 40, 1135–1145 (2017). https://doi.org/10.1007/s12272-017-0938-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0938-6