Abstract
Pancreatic cancer is one of the leading causes of cancer, and it has the lowest 5-year survival rates. It is necessary to develop more potent anti-pancreatic cancer drugs to overcome the fast metastasis and resistance to surgery, radiotherapy, chemotherapy, and combinations of these. We have identified several diarylheptanoids as anti-pancreatic cancer agents from Alpinia officinarum (lesser galangal) and Alnus japonica. These diarylheptanoids suppressed cell proliferation and induced the cell cycle arrest of pancreatic cancer cells (PANC-1). Among them, the most potent compounds 1 and 7 inhibited the shh-Gli-FoxM1 pathway and their target gene expression in PANC-1 cells. Furthermore, they suppressed the expression of the cell cycle associated genes that were rescued by the overexpression of exogenous FoxM1. Taken together, (E)-7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (1) from Alpinia officinarum (lesser galangal) and platyphyllenone (7) from Alnus japonica inhibit PANC-1 cell proliferation by suppressing the shh-Gli-FoxM1 pathway, and they can be potential candidates for anti-pancreatic cancer drug development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is one of the leading causes of cancer-related mortality in developed countries, and it has the lowest 5-year survival rates (Bardeesy and DePinho 2002; Hidalgo 2010; Jemal et al. 2011). This low survival rate for pancreatic cancer is due to the fast metastasis and resistance to existing therapies, including surgery, radiotherapy, chemotherapy, and combinations of these (Siegel et al. 2011). Thus, the development of more potent anti-pancreatic drugs is necessary to ensure effective treatment.
The sonic hedgehog (shh)-Gli signaling pathway is essential in the development of tissues (Lum and Beachy 2004; Kalderon 2005; Rubin and de Sauvage 2006). In mammalian cells, the shh ligand binds to the 12-pass transmembrane receptor, Patched 1 (Ptch1), leading to activation of the seven-pass membrane protein, Smoothened (Smo) (Lum and Beachy 2004; Kalderon 2005). Activated Smo suppresses the Gli negative regulator, Sufu, to activate zinc finger transcription factor, Gli proteins, which control the phenotypes by increasing the target gene expression (Lum and Beachy 2004; Kalderon 2005). A loss in shh-Gli signaling control is a cause of tumor development, including pancreatic (Berman et al. 2003; Thayer et al. 2003; Lum and Beachy 2004; Kalderon 2005; Rubin and de Sauvage 2006; Morton et al. 2007; Yauch et al. 2008). The shh-Gli signaling pathway is highly active in human and mouse pancreatic cancer (Berman et al. 2003; Thayer et al. 2003; Rubin and de Sauvage 2006; Morton et al. 2007). The inhibition of the shh-Gli pathway suppressed pancreatic cancer cell proliferation and induced apoptosis in both in vitro and in vivo experiments (Sanchez et al. 2004; Rubin and de Sauvage 2006). Thus, the shh-Gli signaling pathway is one of targets of pancreatic cancer treatment.
Forkhead box protein M1 (FoxM1) is a transcription factor that belongs to the member of forkhead protein families. FoxM1 plays important roles in cell growth, proliferation, differentiation, invasion, migration, survival, and drug-resistance (Alvarez-Fernandez and Medema 2013; Halasi and Gartel 2013). FoxM1 was reported to be overexpressed in most cancers, including lung, breast, colorectal and pancreatic cancer (Xia et al. 2012; Alvarez-Fernandez and Medema 2013; Halasi and Gartel 2013; Quan et al. 2013; Huang et al. 2014). As an oncogenic transcriptional factor, FoxM1 promotes cancer cell proliferation, invasion, migration and survival through up-regulation of its target genes, c-Myc, cyclin D1, cyclin B, survivin (Xia et al. 2012; Alvarez-Fernandez and Medema 2013; Halasi and Gartel 2013; Quan et al. 2013; Huang et al. 2014). Recent studies have shown that FoxM1 is one of the downstream targets of shh-Gli signaling (Teh et al. 2002; Douard et al. 2006; Katoh and Katoh 2009).
Natural products play important roles in preventing and treating various disorders. Diarylheptanoids from medicinal plants have anti-inflammatory, anti-oxidant, anti-cancer, anti-diabetic, hepatoprotective and neuroprotective activities (Lee et al. 2006; Lv and She 2010; Rong et al. 2012; Du et al. 2013; Zhang et al. 2014; Devassy et al. 2015; Dong et al. 2015a; Ghosh et al. 2015). Curcumin, a representative diarylheptanoid from turmeric, has potential anti-cancer activity (Basnet and Skalko-Basnet 2011; Du et al. 2013; Zhang et al. 2014; Devassy et al. 2015; Kumar et al. 2016; Pulido-Moran et al. 2016). Curcumin suppressed cancer cell proliferation and survival by suppressing the shh-Gli-FoxM1 pathway both in vitro and in vivo (Du et al. 2013; Zhang et al. 2014). We recently reported on the anti-colon cancer activity of diarylheptanoids from medicinal plants as Wnt pathway inhibitors (Dong et al. 2015a). However, there is no report of the anti-pancreatic cancer effects of these diarylheptanoids. In this study, we investigated the anti-pancreatic cancer activity and their mechanism of diarylheptanoid from Alpinia officinarum (lesser galangal) and Alnus japonica.
Materials and methods
Reagents
Gli1/2, cyclin D1, lamin, c-Myc, and survivin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA); and horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (IgG) and anti-mouse IgG antibodies were purchased from Assay Designs (Ann Arbor, MI, USA). Luciferase assay systems were purchased from Promega (Madison, WI, USA); and the enhanced chemiluminescence (ECL) agent was purchased from GE Healthcare Life Science (Uppsala, Sweden). Lipofectamin3000, TRIzol reagent and SuperScript II first-strand cDNA synthesis kits were purchased from Life Technology (San Diego, CA, USA). Beta-actin antibody, hirsutenone (9), curcumin (14), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) and other chemicals were purchased from Sigma (St Louis, MO, USA). SiRNAs were purchased from Bioneer (Daejeon, Korea). The pcDNA-FoxM1 plasmid was a gift from Prof. Tae Jun Park (School of Medicine, Ajou University, Korea). Gli reporter plasmid and expression plasmid were gifts from Prof. Gyu-Un Bae (Sookmyung Women’s University) and Prof. Jong-Sun Kang (Sungkyunkwan University, Korea) (Tenzen et al. 2006; Zhang et al. 2006; Bae et al. 2011).
Extraction, isolation and synthesis of diarylheptanoids
Diarylheptanoids were isolated from Alpinia officinarum (lesser galangal) and Alnus japonica, and some of them were semi-synthesized as previously described (Dong et al. 2015a; Lee et al. 2006; Lee 2012) (Fig. 1).
Chemical structures of diarylheptanoids. Compounds 1–6 were isolated from Alpinia officinarum (lesser galangal); compounds 7–8 were isolated from Alnus japonica; compounds 10–13 were semi-synthesized as previously described (Dong et al. 2015a)
Cell culture and cell cycle analysis
The 293T cell line and human pancreatic ductal carcinoma cell line (PANC-1) were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 2 mM glutamine, 1 mM pyruvate, penicillin (100 U/ml), and streptomycin (10 μg/ml) at 37 °C. PANC-1 cells were treated with 20 μM of compounds 1 or 7 for 24 h. Then, the cells were harvested with trypsin–EDTA and fixed with 70% ethanol at 4 °C overnight. Fixed cells were stained with propidium iodide (PI), and the cell cycle analysis was performed by fluorescence-activated cell sorting (FACS) (BD Biosciences, San Jose, CA, USA).
Reporter gene assay
The cells were transfected with plasmid using Lipofectamine 3000 according to the manufacturer’s instructions. Gli3 expression plasmid was co-transfected as a negative control (Matise and Joyner 1999). Gli reporter plasmid transfected cells were treated with indicated concentrations of diarylheptanoids for 16 h, and the luciferase activity was measured using a luminometer (Promega, Madison, WI, USA) with a luciferase assay system. TOPFlash, a synthetic β-catenin/TCF-dependent luciferase reporter and human Frizzled-1 expression plasmids, and a negative control reporter FOPFlash (mutated β-catenin/TCF binding elements) were gifts from Prof. Sangtaek Oh (Kookmin University, Korea) (Park et al. 2006). The TOPFlash activity was normerized with FOPFlash, as described in a previous study (Dong et al. 2015b). All reporter gene activities were presented in terms of relative luciferase unit (RLU).
Western blot analysis
The Western blot analysis was performed as previously described (Dong et al. 2015a). Cells were treated with the indicated concentrations of diarylheptanoids for 16 h at 37 °C in 5% CO2. Then, the cells were lysed with RIPA buffer and run on SDS-PAGE gels. After transfer, the PVDF membrane was incubated with the indicated primary antibodies and HRP-conjugated secondary antibody. The protein bands of the cell lysates were detected using Fusion Solo (Vilber Lourmat, France) after incubation with ECL reagents. The band density was then analyzed with the Fusion Solo software (Vilber Lourmat, France).
Reverse-transcription polymerase chain reaction (RT-PCR)
The RT-PCR was performed as previously described (Dong et al. 2015a). The cells were treated with the indicated concentrations of diarylheptanoids for 16 h. Then, the total RNA was isolated using a TRIzol reagent and synthesized cDNA using the SuperScript II first-strand cDNA synthesis system. The cDNA was amplified using a PCR thermal cycler. The PCR primer sequences were as follows:
Beta-actin was amplified as an internal control, and the amplified DNA was separated on 2% agarose gels.
Statistical analysis
The IC50 values were calculated using the GraphPad Prism program (GraphPad Software, Inc., La Jolla, CA, USA), and the data are expressed as mean ± standard deviation. All experiments were performed 3–4 times, and the statistical analysis was performed using a two-tailed unpaired Student’s t test. A p-value less than 0.05 was considered to be significant.
Results
Anti-pancreatic activity screening of diarylheptanoids
Since the shh-Gli and β–catenin pathways are highly activated in pancreatic cancer, we tested the effect of diarylheptanoids on their pathways by using the Gli and β-catenin reporter gene assay in PANC-1 cells. Compounds 1, 2, 7, 9, 10 and 14 (Fig. 1) suppressed both of Gli reporter gene and β–catenin reporter gene activities in PANC-1 cells (Fig. 2a, b). To confirm these activities in pancreatic cancer cells, we tested the effects of diarylheptanoids on the PANC-1 cell viability. As expected, compounds 1, 2, 7, 9, 10 and 14 strongly suppressed PANC-1 cell viability at 100 μM (Fig. 2c). These parallel inhibitory patterns in the MTT and reporter gene assays indicate that the diarylheptanoids might exert anti-pancreatic cancer activity by suppressing the shh-Gli and β-catenin pathway in PANC-1 cells.
Effects of diarylheptanoids on Gli and β–catenin reporter genes activities and PANC-1 cell viability. Gli reporter gene transfected PANC-1 cells were treated with indicated concentrations of diarylheptanoids for 16 h to determine the Gli reporter gene activity as described in the materials and methods (a). After TOPFlash plasmid transfection, PANC-1 cells were treated with indicated concentrations of diarylheptanoids for 16 h to determine TOPFlash activity, as described in materials and methods (b). PANC-1 cells were treated with the indicated concentration of diarylheptanoids for 48 h to determine the cell viability by an MTT assay (c)
Furthermore, compounds 1, 7 and 9 showed a stronger suppressive activity than curcumin (14) in both MTT and Gli reporter gene activity assays (Fig. 3). Considering that compounds 1 and 7 show the strongest activity compared to the others, compounds 1 and 7 were used in the following experiments.
IC50 of diarylheptanoids on Gli reporter gene activity and PANC-1 cell viability. Gli reporter gene transfected PANC-1 cells were treated with indicated concentrations of diarylheptanoids for 16 h to determine the IC50 values on Gli reporter gene activity, as described in materials and methods (a). PANC-1 cells were treated with indicated concentrations of diarylheptanoids for 48 h to determine the cell viability by an MTT assay (b)
Diarylheptanoids suppress shh-Gli pathway and cell cycle in PANC-1 pancreatic cancer cells
The inhibitory effects of diarylheptanoids on PANC-1 cells were further confirmed through a cell cycle analysis. Compounds 1 and 7 increased the G2 phase and sub G1 phase of PANC-1 cells by a treatment of 20 μM for 24 h (Fig. 4). These data indicate that compounds 1 and 7 induced cell cycle arrest and cell death to suppress proliferation of the PANC-1 cells.
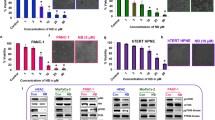
To investigate the mechanism of diarylheptanoids for the shh-Gli pathway inhibition, we tested their effects on the Gli protein levels in the PANC-1 cells by a Western blot analysis. Treatment of 20 μM diarylheptanoids 1 and 7 decreased Gli1 and Gli2 proteins in PANC-1 cells (Fig. 5a). Furthermore, compounds 1 and 7 suppressed the FoxM1 protein and mRNA expressions (Fig. 5b) and also suppressed the target gene levels of FoxM1, such as c-Myc, cyclin B1, cyclin D1 and survivin in PANC-1 cells (Fig. 5a). Since FoxM1 is the target gene of Gli (Teh et al. 2002), the knock-down of Gli1 and Gli2 by siRNAs decreased FoxM1 expression in PANC-1 cells (Fig. 5c). Cycloheximide was used to investigate the action mechanism of diarylheptanoids involved in the shh-Gli pathway. Compounds 1 and 7 reduced the protein levels of Gli1 and Gli2 in cycloheximide-treated cells (Fig. 5d). Furthermore, the inhibitory effects of diarylheptanoids on the Gli pathway were confirmed in the shh conditioned media-treated or Gli1 transfected 293T cells. Compounds 1 and 7 suppressed Gli reporter gene activities of 293T cells when treated with shh conditioned media (Fig. 5e) or transfected with Gli1 expression plasmid (Fig. 5f). The Gli reporter gene activity decreased when the cells were co-transfected with Gli3 as a negative control that was reported as a Gli1 repressor (Matise and Joyner 1999) (Fig. 5f). These data indicate that diarylheptanoids suppress pancreatic cancer cells by suppressing the shh-Gli-FoxM1 pathway and its target gene expression in PANC-1 cells.
Effects of diarylheptanoids on the shh-Gli-FoxM1 pathway. The PANC-1 cells were treated with 20 μM of compounds 1 or 7 for 16 h to determine the protein levels by Western blot analysis (a). The PANC-1 cells were treated with 20 μM of compounds 1 or 7 for 16 h to determine the level of FoxM1 mRNA by RT-PCR (b). The PANC-1 cells were transfected with siRNA against Gli1 or Gli2 to determine the expression levels of the Gli1 target gene, FoxM1 mRNA by RT-PCR (c). The PANC-1 cells were treated with 50 μg/ml of cycloheximide (CHX) and 20 μM of compounds 1 or 7 for 16 h to determine the protein levels by Western blot analysis (d). 293T cells were transfected with Gli reporter plasmid to measure the reporter gene activity after treatment of shh conditioned media with the indicated concentrations of compounds 1 or 7 for 16 h (e). The 293T cells were transfected with Gli1 and Gli reporter plasmid and were treated with indicated concentrations of compounds 1 or 7 for 16 h to measure the reporter gene activity (f)
Diarylheptanoids suppress shh-Gli pathway to suppress FoxM1
To investigate the involvement of FoxM1 in the effects of diarylheptanoids on PANC-1 cells, we overexpressed FoxM1 by FoxM1c plasmid (pcDNA3-FoxM1) transfection. Compounds 1 and 7 suppressed the levels of Gli1 and Gli2 in both empty and FoxM1 plasmid transfected cells. They could not decrease the levels of c-Myc, cyclin B1, cyclin D1 and survivin in FoxM1 overexpressed PANC-1 cells (Fig. 6a). These data indicate that diarylheptanoids suppress the level of Gli1 and Gli2 to inhibit the target gene expression of FoxM1 (Fig. 6b). Compounds 1 and 7 suppress the viability of pancreatic cancer PANC-1 cells by blocking the shh-Gli-FoxM1 pathway.
Effects of diarylheptanoids on FoxM1 target gene expression in FoxM1-overexpressed PANC-1 cell. The PANC-1 cells were transfected with pcDNA3-FoxM1c and treated with 20 μM of compounds 1 or 7 for 16 h to determine the related proteins by Western blot analysis (a). The proposed action mechanism of diarylheptanoids on shh-Gli-FoxM1 pathway (b)
Discussion
With an increasing incidence rate, pancreatic cancer still shows the lowest 5-year survival rate among various cancers (Bardeesy and DePinho 2002; Hidalgo 2010; Jemal et al. 2011). Since shh-Gli signaling is over-activated in pancreatic cancer, it is considered to be a promising target for anti-pancreatic cancer drug development (Berman et al. 2003; Thayer et al. 2003; Rubin and de Sauvage 2006; Morton et al. 2007). FoxM1, one of the target genes of the shh-Gli pathway, is also considered as another promising target (Xia et al. 2012; Alvarez-Fernandez and Medema 2013; Halasi and Gartel 2013; Quan et al. 2013; Huang et al. 2014). In this study, diarylheptanoids suppress pancreatic cancer cell viability and induce cell cycle arrest (Fig. 4) by suppressing the shh-Gli-FoxM1 pathway and their target gene expression (Fig. 5a). The overexpression of exogenous FoxM1 abrogated the effects of diarylheptanoids on the cell cycle associated gene expression (Fig. 6a). Diarylheptanoids block the shh-Gli-FoxM1 pathway by suppressing the Gli1 and Gli2 protein levels, as was confirmed in the cycloheximide-treated experiment (Fig. 5d). The results of the FoxM1 overexpressed PANC-1 cells confirmed that the diarylheptanoids block the pathway upstream of FoxM1 (Fig. 6b). The exact mechanism through which the diarylheptanoids control the Gli1 and Gli2 levels in cancer could be determined with further research.
Compound 1 has been reported to suppress the cell proliferation of melanoma cells (Matsuda et al. 2009), and compounds 1, 2, 7 and 10 suppressed the proliferation of colon cancer cells (Dong et al. 2015a). Compound 9 also has anti-cancer activities, including against colon cancer (Dong et al. 2015a), prostate cancer (Kang et al. 2015), non-small cell lung carcinoma (Novaković et al. 2014) and ovarian cancer (Lee et al. 2012). Compound 14, curcumin, the well-known diarylheptanoid from turmeric, has potential anti-cancer activity against most cancers (Basnet and Skalko-Basnet 2011; Du et al. 2013; Zhang et al. 2014; Devassy et al. 2015; Kumar et al. 2016; Pulido-Moran et al. 2016). We recently reported the anti-colon cancer activity of diarylheptanoids as inhibitors of the Wnt/β-catenin pathway. They suppressed the nuclear translocation of β-catenin by suppressing the interaction of β-catenin and galectin-3 (Dong et al. 2015a). In this study, diarylheptanoids also inhibit the Wnt/β-catenin pathway in pancreatic cancer cells (Fig. 2b). Their inhibitory effects on the Gli pathway in PANC-1 cells has the same pattern as that of the Wnt/β-catenin pathway in colon cancer cells with respect to the chemical structure activity relationship. The enone group in the linker between the two aryl groups is critical for the activity (compounds 1 vs. 12, 2 vs. 4, 7 vs. 8) and the p-hydroxy group in the aromatic ring is also important (compounds 1 vs. 11, 7 vs. 2, 10 vs. 11) (Figs. 1, 2, 3).
Several studies have reported on the inhibitors of the shh-Gli pathway from natural products to suppress the proliferation of various cancer cells (Matsuda et al. 2009; Novaković et al. 2014; Kang et al. 2015; Kumar et al. 2016). Curcumin suppress shh-Gli pathway in various cancers, including both in vitro and in vivo pancreatic cancer (Matsuda et al. 2009; Novaković et al. 2014; Kumar et al. 2016). Compound 1 (IC50 = 9.3 μM) and 7 (IC50 = 6.1 μM) show higher anti-proliferative potencies than curcumin (IC50 = 13.0 μM). Our data indicate that diarylheptanoids 1 from Alpinia officinarum and 7 from Alnus japonica can be potential candidates to develop anti-pancreatic cancer drugs.
In conclusion, we purified several diarylheptanoids from medicinal plants as inhibitors of the shh-Gli-FoxM1 pathway. In particular, (E)-7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (compound 1) from Alpinia officinarum (lesser galangal) and platyphyllenone (compound 7) from Alnus japonica suppressed the proliferation of pancreatic cancer PANC-1 cells by suppressing FoxM1 and its target gene expression through destabilizing Gli1 and Gli2 proteins. Diarylheptanoids from Alpinia officinarum and Alnus japonica are potential inhibitors of the shh-Gli-FoxM1 pathway, and they might be useful to treat pancreatic cancer.
References
Alvarez-Fernandez M, Medema MH (2013) Novel functions of FoxM1: from molecular mechanisms to cancer therapy. Front Oncol 3:1–5
Bae GU, Domene S, Roessler E, Schachter K, Kang JS, Muenke M, Krauss RS (2011) Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am J Hum Genet 89:231–240
Bardeesy N, DePinho RA (2002) Pancreatic cancer biology and genetics. Nat Rev Cancer 2:897–909
Basnet P, Skalko-Basnet N (2011) Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 16:4567–4598
Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA (2003) Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425:846–851
Devassy JG, Nwachukwu ID, Jones PJ (2015) Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev 73:155–165
Dong GZ, Lee SY, Zhao HY, Lee Y, Jeong JH, Jeon R, Lee HJ, Ryu JH (2015a) Diarylheptanoids from lesser galangal suppress human colon cancer cell growth through modulating Wnt/β-catenin pathway. J Funct Foods 18:47–57
Dong GZ, Shim AR, Hyeon JS, Lee HJ, Ryu JH (2015b) Inhibition of Wnt/beta-catenin pathway by dehydrocostus lactone and costunolide in colon cancer cells. Phytother Res 29:680–686
Douard R, Moutereau S, Pernet P, Chimingqi M, Allory Y, Manivet P, Conti M, Vaubourdolle M, Cugnenc PH, Loric S (2006) Sonic Hedgehog-dependent proliferation in a series of patients with colorectal cancer. Surgery 139:665–670
Du WZ, Feng Y, Wang XF, Piao XY, Cui YQ, Chen LC, Lei XH, Sun X, Liu X, Wang BH, Li XF, Yang DB, Sun Y, Zhao ZF, Jiang T, Li YL, Jiang CL (2013) Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI1 signaling pathway in vitro and vivo. CNS Neurosci Ther 19:926–936
Ghosh S, Banerjee S, Sil PC (2015) The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol 83:111–124
Halasi M, Gartel AL (2013) Targeting FOXM1 in cancer. Biochem Pharmacol 85:644–652
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362:1605–1617
Huang C, Du J, Xie K (2014) FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim Biophys Acta 1845:104–116
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Kalderon D (2005) The mechanism of hedgehog signal transduction. Biochem Soc Trans 33:1509–1512
Kang S, Kim JE, Li Y, Jung SK, Song NR, Thimmegowda NR, Kim BY, Lee HJ, Bode AM, Dong D, Lee KW (2015) Hirsutenone in Alnus extract inhibits akt activity and suppresses prostate cancer cell proliferation. Mol Carcinog 54:1354–1362
Katoh Y, Katoh M (2009) Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med 9:873–886
Kumar G, Mittal S, Sak K, Tuli HS (2016) Molecular mechanisms underlying chemopreventive potential of curcumin: current challenges and future perspectives. Life Sci 148:313–328
Lee Y (2012) Modulators of Wnt/β-catenin and hedgehog signaling pathway from Alnus japonica. Dissertation, Sookmyung Women’s University, Seoul, Korea
Lee HJ, Kim JS, Ryu JH (2006) Suppression of inducible nitric oxide synthase expression by diarylheptanoids from Alpinia officinarum. Planta Med 72:68–71
Lee CS, Jang ER, Kim YJ, Myung SC, Kim W, Lee MW (2012) Diarylheptanoid hirsutenone enhances apoptotic effect of TRAIL on epithelial ovarian carcinoma cell lines via activation of death receptor and mitochondrial pathway. Invest New Drugs 30:548–557
Lum L, Beachy PA (2004) The Hedgehog response network: sensors, switches, and routers. Science 304:1755–1759
Lv H, She G (2010) Naturally occurring diarylheptanoids. Nat Prod Commun 5:1687–1708
Matise MP, Joyner AL (1999) Gli genes in development and cancer. Oncogene 18:7852–7859
Matsuda H, Nakashima S, Oda Y, Nakamura S, Yoshikawa M (2009) Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg Med Chem 17:6048–6053
Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M, Lewis BC (2007) Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA 104:5103–5108
Novaković M, Pešić M, Trifunović S, Vučković I, Todorović N, Podolski-Renić A, Dinić J, Stojković S, Tešević V, Vajs V, Milosavljević S (2014) Diarylheptanoids from the bark of black alder inhibit the growth of sensitive and multi-drug resistant non-small cell lung carcinoma cells. Phytochem 97:46–54
Park S, Gwak J, Cho M, Song T, Won J, Kim DE, Shin JG, Oh S (2006) Hexachlorophene inhibits Wnt/beta-catenin pathway by promoting Siah-mediated beta-catenin degradation. Mol Pharmacol 70:960–966
Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M (2016) Curcumin and health. Molecules 21:264
Quan M, Wang P, Cui J, Gao Y, Xie K (2013) The roles of FOXM1 in pancreatic stem cells and carcinogenesis. Mol Cancer 12:159
Rong S, Zhao Y, Bao W, Xiao X, Wang D, Nussler AK, Yan H, Yao P, Liu L (2012) Curcumin prevents chronic alcohol-induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomed 19:545–550
Rubin LL, de Sauvage FJ (2006) Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov 5:1026–1033
Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta W, Datta S, Ruiz i Altaba A (2004) Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA 101:12561–12566
Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212–236
Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG (2002) FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res 62:4773–4780
Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP (2006) The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell 10:647–656
Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M (2003) Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425:851–856
Xia JT, Wang H, Liang LJ, Peng BG, Wu ZF, Chen LZ, Xue L, Li Z, Li W (2012) Overexpression of FOXM1 is associated with poor prognosis and clinicopathologic stage of pancreatic ductal adenocarcinoma. Pancreas 41:629–635
Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ (2008) A paracrine requirement for hedgehog signalling in cancer. Nature 455:406–410
Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS (2006) Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell 10:657–665
Zhang JR, Lu F, Lu T, Dong WH, Li P, Liu N, Ma DX, Ji CY (2014) Inactivation of FoxM1 transcription factor contributes to curcumin-induced inhibition of survival, angiogenesis, and chemosensitivity in acute myeloid leukemia cells. J Mol Med (Berl) 92:1319–1330
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (No. 2011-0030074, No. 2010-0009582).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dong, Gz., Jeong, J.H., Lee, Yi. et al. Diarylheptanoids suppress proliferation of pancreatic cancer PANC-1 cells through modulating shh-Gli-FoxM1 pathway. Arch. Pharm. Res. 40, 509–517 (2017). https://doi.org/10.1007/s12272-017-0905-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0905-2