Abstract
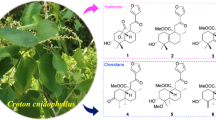
Phytochemical investigation of the methanol extract of Cyphostemma adenocaule liana (bark and wood) led to the isolation of two new ceanothane-type triterpenoids, cyphostemmic acid A 1 and cyphostemmic acid B 2, together with the known triterpenoids 3–7, β-sitosterol and its glucoside. The structures of the isolated compounds were established by 1D- and 2D-NMR spectroscopy. Ozonolysis of cyphostemmic acid A 1, epigouanic acid A 3 and betulin 6 yielded semisynthetic derivatives, cyphostemmic acid C 8, cyphostemmic acid D 9, and 3β,28-dihydroxy-30-norlupan-20-one 10 respectively. Compounds 1–4, 6, 8–10 were tested in vitro, for their antiplasmodial activity against Plasmodium falciparum 3D7 strain and showed weak activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyphostemma adenocaule (Steud. ex A.Rich.) Desc. ex Wild & R.B. Drumm (Vitaceae) has been reported to be used in traditional pharmacopoeia for the treatment of several illnesses such as malaria, urinary tract infections, syphilis, inflammatory pain and bloody diarrhoea (Burkill 2000; Katende et al. 1999; Kokwaro 1993). Phytochemical studies on the genus are scarce. However, the preliminary screening of the C. digitatum extracts showed that it exhibited inhibitory activity against Bacillus subtilis, good antioxidant activity and moderate antifungal potential (Khan et al. 2014). Phytochemical investigation of C. digitatum extracts revealed the presence of alkaloids, flavonoids, saponins, coumarins, steroids, triterpenes and tannins (Khan et al. 2014). Investigations of C. crotalarioides led to the isolation of antifungal oligostilbenes (Ducrot et al. 1998; Bala et al. 1999, 2000), whereas C. greveanum has been reported to contain sesquiterpenes and antiproliferative diterpenes (Cao et al. 2011). Our recent investigations on the extract of C. adenocaule reported that it has antioxidant properties (Assob et al. 2014). Further phytochemical research has been conducted with the extract of C. adenocaule, and resulted in the isolation, structure elucidation of two new ceanothane triterpenoids, cyphostemmic acid A 1 and cyphostemmic acid B 2, and two derivatives obtained by ozonolysis, cyphostemmic acid C 8 and cyphostemmic acid D 9. Several ceanothane type triterpenoids were found to possess antiplasmodial and antimycobacterial activities (Suksamrarn et al. 2006). For this purpose, the antiplasmodial activity of compounds 1–4, 6, 8–10 against Plasmodium falciparum 3D7 strain was evaluated.
Materials and methods
General experimental procedures
The EIMS spectra were recorded on a double focusing mass spectrometer (Varian MAT 311A). ESI-HRMS spectra were recorded on an Apex III (Bruker Daltonik) 7 Tesla (ESI-FT-ICR-MS). Optical rotations were recorded in MeOH or CH2Cl2 solution on a Jasco DIP-360 digital polarimeter. The 1H NMR spectra and 13C NMR spectra were recorded on a Bruker DRX spectrometer operating at 500 MHz and 125 MHz respectively, with TMS as an internal standard. Silica gel (Merck 60, 0.063–0.200 mm) was used for column chromatography. Percolated aluminum backed silica gel 60 F254 sheets were used for TLC. Spots were visualized under UV light (254 nm) and (365 nm) or using a solution of molybdate/Ce4+ reagent followed by heating.
Plant material
C. adenocaule (Steud. ex A.Rich.) Desc. ex Wild & R.B.Drumm (Vitaceae) was collected on November 2011 in Foumban (West-Region, Cameroon), and identified by Mr. Victor Nana at the Cameroon National Herbarium; Yaoundé, Cameroon, voucher specimen (NO 51976/HNC).
Extraction and isolation
The air-dried and ground bark and wood of C. adenocaule (3 kg) was extracted by maceration in 11 L of MeOH at room temperature during 72 h. The filtrate was evaporated under reduced pressure to dryness to afford 107 g of extract. The resulting MeOH extract (67 g) was submitted to repeated column chromatography over silica gel (0.063–0.200 mm) and eluted with mixtures of n-hexane–EtOAc and EtOAc–MeOH, with a gradient of increasing polarity. This resulted in 135 fractions of 150 mL each, which were combined on the basis of TLC analysis. Fractions 4–8 eluted at n-hexane–EtOAc (95:5) yielded β-sitosterol (85 mg), fractions 10–17 eluted at n-hexane–EtOAc (95:5 and 90:10) yielded lupeol 5 (715 mg). Fractions 31–33 eluted with n-hexane–EtOAc (85:15) afforded betulin 6 (63 mg) while fractions 38–40 (n-hexane–EtOAc 80:20) afforded betulinic acid 7 (13 mg). Fraction 43–49 obtained at n-hexane–EtOAc 75:25 and 70:30 afforded a mixture of 3 and 4. Compound 3 (126 mg) and compound 4 (6 mg) were separated after repeated column chromatography over silica gel under isocratic elution conditions (n-hexane–EtOAc 80:20). For fractions 76–82 (n-hexane–EtOAc 60:40 and 50:50), chromatographed once more on silica gel, isocratic elution n-hexane–EtOAc (65:35) afforded compound 2 (4 mg) and compound 1 (14 mg). The glucoside of β-sitosterol (32 mg) was obtained in fraction 109–126 (EtOAc and EtOAc–MeOH 98:2).
Ozonolysis procedure for compound 8–10
The ozonolysis reaction was carried out in 10 mL flask where 22.6 mg (compound 3) or 16 mg (compound 6) or 5 mg (compound 1) were dissolved in 5 mL of CH2Cl2/MeOH (50:50). The stirred solution was cooled to −78 °C (dry ice/acetone). O2 was bubbled through this solution, and after 10 min a steady stream of ozone was bubbled through this solution until the solution become pale blue in color. Then oxygen was again purged into the system until the solution become colorless. 0.5 mL dimethyl sulfide was then added to the resulting mixture at −78 °C, which was allowed warm to room temperature with stirring during 45 min. Saturated sodium hydrogen carbonate solution (5 mL) was then added to the reaction mixture and the aqueous layer was extracted with CH2Cl2. The dichloromethane extract was dry by evaporation which afforded the reaction product.
Cyphostemmic acid A (1)
White amorphous powder, [αD]20 +33.2 (c 0.50, MeOH); 13C NMR (Table 1); 1H NMR (Table 2). EI-MS m/z (rel. int.): 205 (100), 279 (18), 377 (20), 393 (31), 457 (28), 466 (37) and 484 [M-H2O] (28); ES-IMS [M+Na]+ m/z 525; HR-ESI-MS [2M+Na]+ m/z 1027.64746 [cald 1027.64810 (C30H46O6)2Na+].
Cyphostemmic acid B (2)
White amorphous powder, [αD]20 +32.6 (c 0.80, CH2Cl2); 13C NMR (Table 1); 1H NMR (Table 2). EI-MS m/z (rel. int.): 71 (100), 167 (10), 191 (8) and 211 (6). ESI-MS [M+Na]+ m/z 511, HR-ESI-MS [M+Na]+ m/z 511.33941 (cald 511.33940, C30H48O5Na+).
Cyphostemmic acid C (8)
Ozonolysis product of 1 (5 mg from 4.5 mg of 1, yield 90 %) White amorphous powder, [αD]20 +12.1 (c 0.85, MeOH); 13C NMR (Table 1); 1H NMR (Table 2). EI-MS m/z (rel. int.): 57 (100), 149 (76), 167 (25) and 279 (9). HR-ESI-MS [M-H]− m/z 503.3003 (cald 503.3010, C29H44O7).
Cyphostemmic acid D (9)
Ozonolysis product of epigouanic acid A 3 (22.6 mg from 21 mg of 3, yield 93 %). White amorphous powder, [αD]20 +42.8 (c 0.45, MeOH); 13C NMR (Table 1); 1H NMR (Table 2). EI-MS m/z (rel. int.): 149 (100), 167 (39) and 279 (13). HR-ESI-MS [M-H]− m/z 487.3054 (cald 487.3061, C29H44O6).
3β,28-Dihydroxy-30-norlupan-20-one 10 (15.5 mg) (Junko and Masatake 1997) is the ozonolysis product of betulin (6) (16 mg, yield 96 %). EI-MS m/z 444 (C29H48O3).
Antiplasmodial assay
In vitro antiplasmodial activity was evaluated by the Plasmodium lactate dehydrogenase (PLDH) immunodetection assay against P. falciparum 3D7 chloroquine sensitive strain, with a commercially available sandwich enzyme-linked immunosorbent assay (Advanced Practical Diagnostics BVBA, Turnhout, Belgium), as reported previously (Atchade et al. 2013). Assays were performed in a 96-well culture plate with cultures mostly at ring stages at 1 % parasitaemia (haematocrit, 2 %). Screening was realized at two different concentrations (100 and 10 µg/mL, 0.5 % DMSO). IC50 values were determined for compounds exhibiting an activity greater than or equal to 50 % of inhibition at 10 µg/mL. Parasite culture was indeed incubated with increasing concentrations of the tested compound (0.781–100 µg/mL, 8 concentrations, 2-fold dilutions) for 96 h at 37 °C under reduced oxygen condition (Candle jar). Positive control consisted of chloroquine diphosphate (Sigma–Aldrich, Saint Quentin Fallavier, France), ranging from 0.5 to 0.0039 µg/ml. Each experiment was performed in triplicate.
Results and discussion
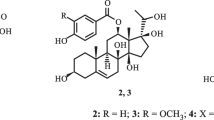
Air-dried and ground bark and wood of C. adenocaule was extracted at room temperature with MeOH. This extract was concentrated to dryness under reduced pressure and submitted to repeated column chromatography on silica gel and yielded cyphostemmic acid A 1, cyphostemmic acid B 2 and the known epigouanic acid A 3 (Ramos et al. 2010), zizyberanal acid 4 (Sheng Guo et al. 2009) (Fig. 1), lupeol 5, betulin 6, betulinic acid 7 (Junko and Masatake 1997), β-sitosterol and its glucoside (Moghaddam et al. 2007).
Compound 1 was obtained as a white powder. Its ESIMS showed the pseudo-molecular ion peak [M + Na]+ at m/z 525; in the HR-ESIMS the [2 M + Na]+ peak was observed at m/z 1027.64746 [calculated 1027.64810, for (C30H46O6)2Na+]. The 13C NMR spectrum of 1 (Table 1) displayed 30 carbon resonances, while the DEPT experiment revealed the presence of five methyls, ten methylenes, seven methines and eight quaternary carbons; in particular resonances of two carboxylic functions at δ 178.2 (C-27) and 177.1 (C-28), two sp2 carbons at δ 150.5 (C-20) and 108.9 (C-29), one oxymethylene carbon at δ 61.1 (C-2) and one methine carbon at δ 60.9 (C-1). These data (Table 1) revealed the backbone of ceanothane-type triterpenoid of the lupane skeleton (Sheela et al. 1993; Li et al. 1997; Shoei-Sheng et al. 1997; Tagadeesh et al. 2000; Ramos et al. 2010; Rambabu et al. 2011). The 1H NMR spectrum of 1 (Table 2) showed the signals of an isopropenyl group at δ 4.72 (1H, brs, H-29a), 4.59 (1H, brs, H-29b) and 1.70 (3H, s, H-30), an oxymethine proton at δ 3.97 (1H, brs, H-3), an oxymethylene group at δ 3.71 (1H, dd, J = 10.5; 4.1 Hz, H-2a) and 3.17 (1H, m, H-2b), as well as four additional methyl proton signals at δ 1.05 (6H, s, H-25, H-26), 1.02 (3H, s, H-24) and 0.89 (3H, s, H-23). Analysis of the 1H NMR data, the 1H-1H COSY cross signals and the HMBC correlations of compound 1 revealed some similarities with that of epigouanic acid A 3 or that of ceanothetric acid and 27-hydroxyceanothic acid (Ramos et al. 2010; Li et al. 1997) and further confirmed the previous assumption. The only difference between these data was indeed the presence of the signal of the oxymethine group at (H-3, δH 3.97/δC 84.8) in compound 1 and the disappearance of the signal of the methylene group of compound 3. The 1H-1H COSY spectrum of compound 1 showed a correlation between H-1 and H-2. Furthermore, its HMBC spectrum exhibited correlations between the proton H-3 and carbons atoms C-1, C-2, C-4 (δ 42.5), C-5 (δ 58.2), C-23 (δ 31.8), and C-24 (δ 18.9); between H-2 and carbons C-1, C-3, and between H-26 and C-7 (δ 37.0), C-8 (δ 40.4), C-9 (δ 42.9), C-14 (δ 59.8). The relative configurations of H-1 and H-3 were deduced from the NOESY spectrum where cross peaks observed between H-3/H-23, H-2b/H-5 and H-1/H-25 indicated the α-orientation of H-3 and β-orientation of H-1 (Fig. 2). The found NOE between H-2b and H-5 supports a preferred conformation of the five-membered A-ring with a dihedral angle H-1/C-1/C-3/H-3 not far from 90o, and correspond with the not resolved multiplet (“br s”) observed for H-3. This corroborated the stereochemistry proposed by Rambabu et al. (2011). From the above evidence, compound 1 was identifed as 2α-hydroxymethylene-3β-hydroxy-A(1)-norlup-20(29)-en-27,28-dioic acid, and trivially named cyphostemmic acid A.
Compound 2 was obtained as a white powder. Its ESIMS showed the pseudo-molecular ion peak [M + Na]+ at m/z 511 and the molecular composition C30H48O5 was confirmed by HR-ESIMS with the [M + Na]+ peak at m/z 511.33941 (calculated 511.33940, for C30H48O5Na+). Compound 2 has 14 mass units less than 1. Its 13C NMR spectra (Table 1) exhibited signal of one carboxyl group at δ 179.0 (C-28), two sp2 carbons at δ 150.5 (C-20) and 109.3 (C-29), and two oxymethylene carbon at δ 61.4 (C-2) and 60.3 (C-27). The 1H NMR spectrum of 2 (Table 2) showed the signals of an isopropenyl group at δ 4.69 (1H, brs, H-29a), 4.57 (1H, brs, H-29b) and 1.67 (3H, s, H-30), an oxymethine proton at δ 3.94 (1H, brs, H-3) and two oxymethylene groups at δ 3.70 (1H, dd, J = 10.3; 4.2 Hz, H-2a), 3.27 (1H, t, J = 10.8 Hz, H-2b), and at δ 4.11 (1H, d, J = 12.5 Hz, H-27a) and 3.76 (1H, d, J = 12.5 Hz, H-27b), as well as four additional methyl group signals at δ 0.88 (3H, s, H-23), 1.02 (6H, s, H-24, H-25), and 0.94 (3H, s, H-26). Analysis of the 1H NMR data, the 1H-1H COSY cross signals and the HMBC correlations of compound 2, revealed some similarities with compound 1. The only difference between these data was indeed the presence of the signal of the oxymethylene group at δ 60.3 (δH 4.11 and 3.76; H-27) in compound 2 and the lack of the signal of one carboxyl group (C-27) of compound 1. The proposed structure is supported by HMBC correlations from H-3 (δH 3.94) to C-1 (δ 61.1), C-2, C-4 (δ 42.6), C-5 (δ 58.2), C-23 (δ 32.3) and C-24 (δ 18.2); from H-2 to C-1 and C-3 (δ 85.0), from H-26 to C-7 (δ 35.0), C-8 (δ 42.0), C-9 (δ 43.7), C-14 (δ 46.6), and from H-27 to C-8, C-13 (δ 39.2), C-14, and C-15 (δ 23.4). The relative configuration of compound 2 was also deduced from NOESY spectrum where cross peaks were observed between H-3/H-23 and H-2/H-5 indicated the α-orientation of H-3 and β-orientation of H-1 (Fig. 2). From the above spectroscopic evidence, the structure of compound 2 was elucidated as 2α-hydroxymethylene-3β,27-dihydroxy-A(1)-norlup-20(29)-en-28-oic acid, and trivially named cyphostemmic acid B.
Ozonolysis of 1, 3 and 6 afforded compound 8, 9 and 3β,28-dihydroxy-30-norlupan-20-one 10 respectively. All these compounds were spectroscopically characterized in a similar manner as 1 and 2, using 1D- and 2D-NMR, and MS data.
In fact, the molecular formula of compounds 8 and 9 was assigned as C29H44O7 and C29H44O6 respectively, according to their HR-ESI-MS ([M-H]− m/z 503.3003, cald 503.3010) and ([M-H]− m/z 487.3054, cald 487.3061). The 1H and 13C NMR data for compounds 8 and 9 (Tables 1, 2) were almost similar to those of their precursors (1 and 3 respectively), except that the double bond Δ20,29 was replaced by a ketocarbonyl group, appearing at δ C 214.4 (C-20) for compound 8 and 214.7 (C-20) for compound 9. These semisynthetic analogues were trivially named cyphostemmic acid C 8 and cyphostemmic acid D 9, and they provided further confirmation of the location of the C20-C29 double bonds in their precursors.
Compounds 1–4, 6, 8–10 were tested in vitro, for their antiplasmodial activity against the P. falciparum 3D7 strain. All the tested compounds showed weak activity (Table 3). The most active compound 10 exhibited an IC50 value of 10.1 µg/mL against the chloroquine sensitive strain 3D7. The activity of compound 10 being higher than the one of its precursor 6 clearly indicated that the oxidation of the double bonds C20–C29 may increases the activity in a six-membered A-ring. However, in a five-membered A-ring, the activity obtained for compounds 8 and 9 are weaker than that of compounds 1 and 3 respectively. It can be concluded that, the C20–C29 double bonds is not the only organic function involved in the antiplasmodial activity.
The chemical investigation of bark and wood extract of C. adenocaule led to the isolation of seven triterpenoids of which two new ceanothane-type lupane derivatives. Some of these compounds and the ozonolysis derivatives exhibited weak antiplasmodial activity. The new compounds possess unusual lupane skeletons that according to our knowledge have not yet been found in Vitaceae. Such five-membered A-ring triterpenes have been reported in Rhamnaceae, Sapindaceae, Leguminosae, Phyllanthaceae, and Meliaceae (Grishko et al. 2015).
References
Assob NJC, Njouendou AJ, Nkeng-Efouet AP, Chouna JR, Badami SM, Verapur PV, Typpeswamy BD, Wanji S (2014) In vitro screening of antioxidant properties of ten Cameroonian medicinal plants. J Adv Biotech 3:171–182
Atchade PS, Doderer-Lang C, Chabi N, Perrotey S, Abdelrahman T, Akpovi CD, Anani L, Bigot A, Sanni A, Candolfi E (2013) Is a Plasmodium lactate dehydrogenase (PLDH) enzyme-linked immunosorbent (ELISA)-based assay a valid tool for detecting risky malaria blood donations in Africa? Malar J 12:279–289
Bala AE, Kollmann A, Ducrot P-H, Majira A, Kerhoas L, Delorme R, Einhorn J (1999) Antifungal activity of resveratrol oligomers from Cyphostemma crotalarioides. Pestic Sci 55:206–208
Bala AE, Kollmann A, Ducrot PH, Majira A, Kerhoas L, Leroux P, Delorme R, Einhorn J (2000) Cis-ɛ viniferin: a new antifungal reservatol dehydrodimer from Cyphostemma crotalarioides roots. J Phytopathol 148:29–32
Burkill HM (2000) The useful plants of West Tropical Africa, Vol 5, 2nd edn., Families S–Z, AddendaKew, Royal Botanic Gardens, p 686
Cao S, Hou Y, Brodie P, Miller JS, Randrianaivo R, Rakotobe E, Rasamison VE, Kingston DGI (2011) Antiproliferative compounds of Cyphostemma greveanum from a Madagascar dry forest. Chem. Biodiver 8:643–650
Ducrot P-H, Kollmann A, Bala AE, Majira A, Kerhoas L, Delorme R, Einhorn J (1998) Cyphostemmins A-B, two new antifungal oligostilbenes from Cyphostemma crotalarioides (Vitaceae). Tetrahedron Lett 39:9655–9658
Grishko VV, Tolmacheva IA, Pereslavtseva AV (2015) Triterpenoids with a five-membered A-ring: distribution in nature, transformations, synthesis, and biological activity. Chem Nat Compd 51:1–21
Junko I, Masatake N (1997) Triterpenoids from the cork of Vitis vinifera “Kyohou”. Nat Med 51:269–271
Katende AB, Ssegawa P, Birnie A (1999) Wild food plants and mushrooms of Uganda. Technical handbook No 19. Regional Land Management Unit/SIDA, Nairobi, p 490
Khan R, Saif AQ, Quradha MM, Ali J, Rauf A (2014) Phytochemical analysis, antimicrobial, antioxidant and urease inhibitory potential of Cyphostemma digitatum Lam. Nat Prod Res 26:1–3
Kokwaro JO (1993) Medicinal plants of East Africa, 2nd edn. Kenya Literature Bureau, Nairobi-Kenya, p 401
Li XC, Cai L, Wu CD (1997) Antimicrobial compounds from Ceanothus americanus against oral pathogens. Phytochemistry 46:97–102
Moghaddam MF, Farimani MM, Salahvarzi S, Amin F (2007) Chemical constituents of dichloromethane extract of cultivated Satureja khuzistanica. eCam 4:95–98
Rambabu P, Ramana VK, Ganapaty S (2011) Isolation and characterization of triterpenes from Zizyphus glabrata. Int J Chem Sci 9:1014–1024
Ramos LIC, Dos-Santos KRN, Itabaiana IJ, Ceva AOA, Porzel A, Wessjohann L, Machado KR (2010) Ceanothane and lupane-type triterpenes from Zizyphus joazeiro: an anti-staphylococcal evaluation. Planta Med 76:47–52
Sheela PN, Madhusudana JR (1993) Gouanic acid from the leaves of Gouania microcarpa. Phytochemistry 33:711–712
Sheng G, Yu PT, Jin AD, Shu LS, An WD (2009) Two new terpenoids from fruits of Ziziphus jujube. Chin Chem Lett 20:197–200
Shoei-Sheng L, Shew-Neu S, Karin CSL (1997) Triterpenes from Paliurus hemsleyanus. Phytochemisty 46:549–554
Suksamrarn S, Panseeta P, Kunchanawatta S, Distaporn T, Ruktasing S, Suksamrarn A (2006) Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana. Chem Pharm Bull 54:535–537
Tagadeesh SG, Krupadanam GLD, Srimannarayana G (2000) A new triterpenoid from Zizyphus xylopyrus stem wood. Indian J Chem 39B:396–398
Acknowledgments
The authors acknowledge the TWAS-DFG and the Alexander von Humboldt Foundation for awarding a fellowship to J. R. Chouna and B. N. Lenta, respectively, at Bielefeld University. They also acknowledge the financial support of the Alango Foundation (African Phytomedecine Center, Dschang, Cameroon).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The Authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Chouna, J.R., Nardella, F., Lenta, B.N. et al. Ceanothane-type triterpenoids from Cyphostemma adenocaule . Arch. Pharm. Res. (2016). https://doi.org/10.1007/s12272-016-0801-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12272-016-0801-1